ABSTRACT
In this work, broad-specific monoclonal antibody based immunoaffinity columns (IACs) coupled to ultra-high performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) for purification and determination of T-2 and HT-2 toxin in maize and cherry samples. Based on broad-specific anti-T-2 monoclonal antibody, the prepared IACs could be used for both T-2 and HT-2 purification with cross reactivity values at 100% for T-2 and 108% for HT-2. Column capacity was 3.14 μg mL−1 gel for T-2 toxin, and 3.20 μg mL−1 gel for HT-2 toxin. After 10 cycles of usage at intervals of 2 days, column capacity decreased to 0.85 μg mL−1 gel for T-2 and 1.22 μg mL−1 gel for HT-2 toxin. The developed method exhibited high sensitive results with limit of detection (LOD) lower than 0.35 μg kg−1 and 1.42 μg kg−1.
1. Introduction
Naturally occurring mycotoxins contaminated in cereals and fruits bring sever health risks to animals and even human beings. T-2 toxin and it major metabolite HT-2 toxin (structure shown in ), belong type A trichothecenes, are mainly produced by various species including F. soprotrichioides, F. poae, and F. acuinatum (Brezina et al., Citation2014; McCormick et al., Citation2015). It is reported that humid and cool conditions were associated with increased fusarium mycotoxin accumulation in harvested cereals, fruits and even seafoods (Hjelkrem et al., Citation2018; Li, Wu, et al., Citation2017; Lu et al., Citation2016). T-2 toxin and HT-2 toxin are most important fusarium mycotoxins with severe acutely toxic mycotoxins. T-2 toxin was reported to exhibit protein synthesis and mitochondrial function, and they were also identified to show immunosuppression and general cytotoxicity toxic effects both in vivo and in vitro(Li, Zou, et al., Citation2017; Lin et al., Citation2019; Zhang, Li, Xu, Pan, & Sun, Citation2019). As the major metabolite, the toxic effects of HT-2 toxin were comparable as T-2 toxin itself (Maruniakova, Kadasi, Sirotkin, Bulla, & Kolesarova, Citation2014, Citation2015; Zhang et al., Citation2019). It seems that the toxicity of T-2 toxin might be induced by the sum contamination of HT-2 toxin as well. Considering their high toxic effects, T-2 toxin and HT-2 toxin received lots of concerns in recent years (Wei et al., Citation2019; Wu et al., Citation2019; Zhang, Zhang, Liu, Wu, & Zhang, Citation2018). In 2011, the Panel on Contaminants in the Food Chain (CONTAM) from the European Food Safety Authority (EFSA) has established a group tolerable daily intake (TDI) for the sum of T-2 and HT-2 as low as 0.1 μg kg−1·B·W per day.
Considering the severe toxic effects of T-2 and HT-2 toxin, reliable and sensitive analytical methods are needed for the determination of these mycotoxins to control their contamination in foods. Immunoassays, such as enzyme-linked immuno sorbent assay (ELISA) (Arola, Tullila, Nathanail, & Nevanen, Citation2017) and immobilized activated beads (Edupuganti, Edupuganti, O'Kennedy, Defrancq, & Boullanger, Citation2013), can allow specific recognition without complicated sample preparation. These methods are fast and high-throughput, and can be applied for the screening of T-2 and HT-2. Compared to the immunoassays, instrument methods are much more reliable for the application of confirmation methods. Literature about analysis of T-2 and HT-2 as well as other mycotoxins has been reported in the recent years. In general, gas chromatography (GC) coupled with electron capture detector (ECD) (Majerus, Hain, & Scheer, Citation2008) or mass spectrometric detection (MS) (Rodriguez-Carrasco, Molto, Manes, & Berrada, Citation2014) has been applied. Moreover, high-performance liquid chromatography (HPLC) and ultra-high-performance liquid chromatography (UHPLC) coupled with UV were the most frequently applied techniques rencently (Grajewski, Kosicki, Twaruzek, & Blajet-Kosicka, Citation2019). Based on the structures, T-2 and HT-2 are less sensitive via ultraviolet (UV) or FLD detector. Therefore, extra derivatization procedure is necessary before UV or FLD detector is applied (Visconti, Lattanzio, Pascale, & Haidukowski, Citation2005). In recent years, high-performance liquid chromatography tandem mass spectrometry (LC-MS/MS) is the most popular technique than others because of its sensitivity and the simplified sample preparation without derivatization. Apart from this, LC-MS/MS is also applicable for simultaneous detection of multiple mycotoxins in a single analysis.
However, as most previous reports, food matrices are complex and contain substances which might inhibit ionization in LC-MS/MS results. Therefore, sample preparation is the most important step prior to the instrument analysis steps. During the past decades, solid phase extraction (SPE) procedures including reverse-phase C18, Oasis HLB (Gentili et al., Citation2007; Stecher et al., Citation2007), and MycoSep 227 cartridges (Haubl et al., Citation2007) were mostly reported for T-2 and HT-2 clean-up. These purification protocols were mainly based on non-specific, hydrophobic interactions between SPE adsorbents and analytes, which might lead to the co-extraction of interferences as well as target analytes and result in matrix effects in the following LC-MS/MS analysis.
Recently, immunoaffinity columns (IACs) based on high-specific antigen and antibody recognization were proved to be an efficient tool with high selectivity and affinity characters for isolation, purification, and concentration of target analytes from complex matrix (Li et al., Citation2008). Compared to traditional SPE methods, IACs showed higher efficiency for targets purification in diverse sample matrices (Li, Wang, et al. Citation2012). However, reports about T-2 toxin immune-affinity purification were either focused on T-2 toxin itself (Pascale, Haidukowski, & Visconti, Citation2003) or coupled with fluorescence or ECD detector with lower sensitivity than LC-MS/MS (Kong, Zhang, Shen, Ou-Yang, & Yang, Citation2012; Visconti et al., Citation2005). What’s more, most of these literature mainly focused on cereals samples, neglecting other exposure pathway in fruit foods. In this research, new broad-specificity anti-T-2 monoclonal antibody (mAb) was used to develop IACs for the T-2 toxin and HT-2 toxin purification coupled an ultra-high performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method for high sensitive determination of T-2 and HT-2 toxin in maize and cherry samples.
2. Materials and methods
2.1. Chemicals and reagents
T-2 and HT-2 standard were obtained from Pribolab (Qingdao, China). Anti-T-2 mAb (NO. 021T-0101) was obtained from Zeyang Biotechnology (Beijing, China). CNBr-activated Sephrose 4B gel was purchased from Amersham Biotech (Uppsala, Sweden). HPLC grade acetonitrile and methanol were purchased from Fisher Scientific Inc. (Pittsburgh, PA, USA). Other chemicals and solvents were analytical grade and obtained from Beijing Chemical Reagent Co. (Beijing, China). Deionized water was prepared using a Milli-Q water purification system (Millipore, Bedford, MA, USA).
2.2. Apparatus
Ultraviolet–visible detector was from Shanghai Analytical Instrument (Shanghai, China). Acquity UPLC system with Acquity BEH C18 column (50 mm × 2.1 mm i.d., 1.7 μm particle size), and Micromass Quattro Premier XE triple quadrupole mass spectrometer were from Waters (MA, USA). Vortex mixer (model HQ-60) was obtained from North TZ-Biotech Develop Co. Ltd. (Beijing, China). Nitrogen evaporator (N-EVAP 112) was from Organomation Associates (MA, USA).
2.3. Standard solutions and buffers
T-2 and HT-2 standard solutions were prepared by dissolving 1 mg of each standard to a final volume of 1 mL with methanol, respectively (1 mg/mL for each). Posphate buffered saline (PBS, pH 7.4), HCl solution (1 mM), NaHCO3 solution (0.1M, pH 8.4), Tris-HCl buffer (0.1 M, pH 8.0), and Acetate buffer (0.1 M, pH 4.0) were prepared in this work as follows.
PBS (0.01M, pH 7.4) was prepared with 0.2 g of KH2PO4, 0.2 g of KCl, 2.9 g of Na2HPO4·12 H2O, and 8.0 g of NaCl dissolved to a final volume of 1 L with deionized water. HCl solution (1 mM) was prepared by diluting 84 μL of HCl (37%) to a final volume of 1 L with deionized water. NaHCO3 solution (0.1M, pH 8.4) was prepared with 8.4 g of NaHCO3 and 29.3 g of NaCl dissolved to a final volume of 1 L with deionized water. Tris-HCl buffer (0.1 M, pH 8.0) was prepared with 12.1 g of Tris, 29.3 g of NaCl, and 2.4 mL of HCl (37%) dissolved to a final volume of 1 L with deionized water. Acetate buffer (0.1 M, pH 4.0) was prepared with 2.5 g of CH3COONa·3H2O, 29.3 g of NaCl, and 4.7 mL of glacial acetic acid dissolved to a final volume of 1 L with deionized water.
2.4. Preparation of immunoaffinity columns
For immunoaffinity columns (IACs) preparation, the first and most important step is the immunosorbents preparation. In this work, immunosorbents were prepared according to the previous protocol with some minor revisions (Yao et al., Citation2020). Details were as follows: 1 g of CNBr Sepharose 4B was dissolved to a final volume of 5 mL with HCl for activation. The activated gel was transferred to a sintered-glass funnel and washed with 200 mL of 1 mM HCl. After that, the active gel was washed with 600 mL of NaHCO3 solution and then mixed with 30 mg of anti-T-2 mAb (NO. 021T-0101). The coupling was processed by stirring at 4°C for 20 h. Then, unbound mAb was removed by adding 50 mL of PBS for washing. The concentration of mAb in elute was tested via a UV-Vis spectrometry to determine the coupling efficiency. Excess binding sites of activated gel were blocked by adding 10 mL of Tris-HCl buffer and stirred at 4°C for 2 h. After washing with 20 mL of acetate buffer (0.1 M, pH 4.0) and 20 mL of Tris-HCl buffer in turn for 3 cycles, 1 mL of prepared gel was transferred to a glass column (3 cc) with a filter plate (10 mm × 0.8 mm, i.d.). The prepared IACs were stored in PBS at 4°C until use.
2.5. Column capacity determination for T-2 toxin and HT-2 toxin
Column capacities for T-2 toxin and HT-2 toxin were tested with 10 μg of each standard dissolved in 10 mL of methanol/PBS (10/90, v/v). First, IACs were conditioned with 10 mL of PBS, and then the standard analyte was loaded onto each column by gravity. Each column was washed with 10 mL of methanol/water (10/90, v/v) and 10 mL of water. Target analyte was eluted with 3 mL of methanol and dried under a stream of nitrogen at 65°C. The residues were redissolved with 1 mL of acetonitrile/water (50/50, v/v) and then filtered through a 0.22 μm filter for analysis. For regeneration of the IACs after using, each column was washed with 50 mL of PBS and then stored in PBS at 4°C for the next use.
2.6. Sample preparation
Before analysis, maize samples were crushed and sift through 50 mesh sieve and cherry samples were kernel removed. An amount of 1 g (±0.01 g) of negative cereal or fruit samples (maize and cherry) were weight and transferred to a 15 mL of polypropylene centrifuge tube. Samples were spiked at three groups at concentration of 10, 40, and 100 μg kg−1 with five replicates each (n = 5). One unfortified sample was set as negative control. Spiked group samples were vortexed and stay at room temperature for 15 min for incubation. Then, 10 mL of methanol/water (60/40, v/v) were added and vortexed for 3 min for extraction. Each sample was centrifuged at 10,000 × g for 15 min, and 5 mL of each supernatant was transferred to another polypropylene tube. Then, 25 mL of PBS was added to each tube for sample dilution to decrease the percentage of methanol as low as 10% before purification. Immunoaffinity purification protocol was the same as described in column capacity determination.
2.7. UPLC-MS/MS method
A Waters UPLC system was selected in this research for separation using an Acquity BEH C18 column with column temperature at 30°C. Mobile phase was consisted of 5 μM ammonia water (A) and 5 μM ammonia acetonitrile (B). Flow rate was settled at 0.3 mL min−1 with injection volume at 10 μL. Gradient elute programme was adopted as follows: 0∼1 min 100%∼90% A, 1∼6 min 90%∼20% A, 6∼6.5 min 20∼100% A. Detection of T-2 and HT-2 was performed in positive ionization mode with desolvation temperature at 300°C, source temperature at 80°C, and capillary voltage at 2.8 kV. Nitrogen was performed as desolvation gas at a flow rate of 650 L/h, with a cone gas flow rate of 30 L/h. Argon was performed as collision gas at a pressure of 2.5 × 10−3 mbar in the collision cell. Multiple reaction mode (MRM) was adopted for qualitative and quantitative analysis (Parameters were shown in ).
Table 1. MRM parameters of T-2 toxin and HT-2 toxin.
3. Results and discussion
3.1. Performance and optimization of IAC conditions
CNBr-actived Sepharose 4B is suitable for immunosorbent preparation due to its easy derivation charicater (Li, Wang, et al. Citation2012). For conjugation, 5∼10 mg of mAb are recommended for each millilitre gel. CNBr-actived Sepharose 4B is adopted in this experiment and 6 mg mAb were employed for each millilitre of gel in this research. Coupling efficiency is monitored over 95% with 5.5 mg of mAb per millilitre gel.
After that, IACs purification steps for T-2 and HT-2 toxin were optimized in this work. On the basis of sample preparation procedure, loading solvent containing no more than 10% of methanol led to satisfactory results for both T-2 toxin and HT-2 toxin. Then, 10 mL of deionized water was used to remove the ions in PBS to minimize ionization effects and 10 mL of 10% methanol was used to remove non-specific binding to reduce matrix interferences. Finally, target analytes were eluted with 3 mL of methanol for antibody–antigen dissociation. Methanol is a mild elution medium without causing irreversible denaturation in order to allow the re-usage of these prepared IACs. What’s more, methanol performed as the elution solvent is easy to concentrate without extra salt ions effect in MS/MS analysis, which is superior to acid or other buffer solutions (Vera-Avila, Vazquez-Lira, Garcia, & Covarrubia, Citation2005).
Specificity of this mAb was tested by cross reactivity (CR) value according to previous report (Li, Zhang, et al. Citation2012). This mAb showed broad-specific character and can recognize both T-2 and HT-2 with negligible CR value (<0.01) for other analogues, including structure related DON and DOM-1 (). Therefore, the prepared IACs could be used for T-2 and HT-2 purification. In order to test the column capacity, 10 μg of T-2 toxin and 10 μg of HT-2 toxins were passed through the prepared IACs, respectively. After loading, washing, and eluting procedure of IAC treatment, concentrations of T-2 toxin and HT-2 toxin were detected with LC-MS/MS method. The column capacity was 3.14 μg mL−1 gel for T-2 toxin, and 3.20 μg mL−1 gel for HT-2 toxin.
Table 2. Performances of IC50, cross-reactivity (%CR) of T-2 toxin analogues (DON, DOM-1, HT-2, NIV, T-2 tetrael, T-2 toxin, T-2 triol).
Reusability depends on activity of the immobilized antibodies and the chemical stability of the support is an important IACs parameter. The column capacity of repeat usage was evaluated in 20 days at 2 days intervals (shown in ). The column capacity decreased following the increasing cycles of usage. During 10 times of usages, the column capacity was 0.85∼3.14 μg mL−1 gel for T-2 toxin and 1.22∼3.2 μg mL−1 gel for HT-2 toxin. Considering the determination of mycotoxins is trace analysis, it is suitable for T-2 and HT-2 toxin purification with these prepared IACs for at least ten cycles.
3.2. UPLC-MS/MS method
In order to minimize potential interruption and obtain satisfactory sensitivity and peak shape, gradient UPLC elution programme was settled and optimized for isolation within 5 min (). MRM mode was selected for T-2 toxin and HT-2 toxin in ESI positive mode by injecting standard solution with the tandem mass spectrometer directly. The most abundant precursor ion for both T-2 and HT-2 are [M+NH4]+. MS/MS product ions were tested at different collision energy and two abundant product ions were used for qualitative and quantitative ions (). According to the identification criteria of Commission Decision 2002/657/EC, this development UPLC-MS/MS method is suitable for qualitative and quantitative of T-2 and HT-2 toxin.
3.3. Method validation
3.3.1. Linearity
Linearity was evaluated based on matrix-spiked calibration curves at 7 spiked levels with concentrations ranged from 1 to 500 μg kg−1 for T-2 toxin and 5 to 500 μg kg−1 for HT-2 toxin. Calibration curves for maize and cherry samples were shown in . From the table, the liner range obtained is wide to cover most nature contaminated concentrations. Samples of especially high concentrations of T-2 or HT-2 could be diluted before detection.
Table 3. Calibration curve, LOD, and LOQ of T-2 and HT-2.
3.3.2. LOD and LOD
LOD (limit of detection) and LOQ (Limit of Quantification) are determined on the basis of S/N (Signal to Noise) ratio. LOD in different samples depend on S/N ratio over 3 was determined lower than 0.35 μg kg−1 and 1.42 μg kg−1 for T-2 toxin and HT-2 toxin, respectively. LOQ depend on S/N ratio over 10 were lower than 1.17 μg kg−1 and 4.73 μg kg−1, respectively ().
3.3.3. Accuracy and precision
Accuracy and precision were determined based on recoveries of spiked samples at three different spiked concentrations. Recoveries of samples were determined by calculated and spiked concentrations. Intra–day and inter–day RSD (Relative Standard Deviation) were determined on the basis of 5 replicates at different spiked levels of 3 consecutive days (results shown in ). From the table, mean recoveries ranged from 89.6∼96.6% for T-2 toxin and 89.2∼96.2% for HT-2 with intra-day and inter-day RSD less than 7.33% and 6.49% in maize samples. In cherry samples, mean recoveries ranged from 89.9∼98.8% for T-2 toxin and 88.9∼98.7% for HT-2 with intra-day and inter-day RSD less than 7.88% and 6.95%. From the results, it can be concluded that the developed method could be applied for T-2 and HT-2 toxin determination in maize and cherry samples.
Table 4. Accuracy and precision of mycotoxins in maize and cherry.
The result showed this developed IACs purification coupled UPLC-MS/MS protocol exhibited higher sensitivity results with lower LODs than published ELISA (Kawamura et al., Citation1990; Kierek Jaszczuk et al., Citation1995) or UPLC-MSMS methods (Kafouris, Christofidou, Christodoulou, Christou, & Ioannou-Kakouri, Citation2017). What’s more, this developed protocol is the first report for T-2 and HT-2 toxin detection in cherry samples.
3.4. Contamxination exposure of T-2 toxin and HT-2 toxin in commercial samples
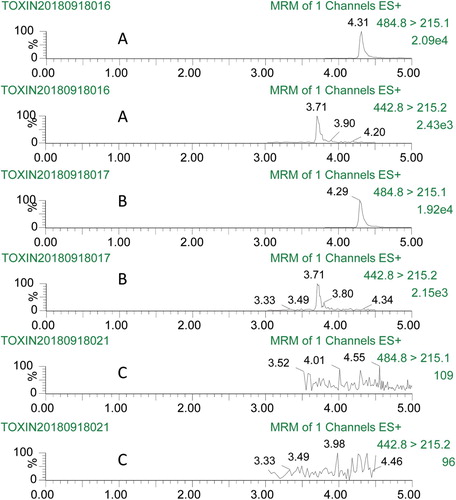
Totally 30 commercial samples (15 maize samples and 15 cherry samples) were obtained from local farmer's market in Shandong Province to investigate the exposure of T-2 toxin and HT-2 toxin. Samples were processed according to the developed IAC-UPLC-MS/MS methods, and concentrations of targets in each sample were determined on the basis of each calibration curves. Finally, 12 samples were detected T-2 toxin positive, in which 5 samples were also detected HT-2 toxin positive in maize samples with positive rate at 80% in maize. In cherry samples, only 2 samples were detected T-2 toxin positive, and none HT-2 positive samples detected (positive rate 13.3%) ().
Table 5. T-2 and HT-2 contamination exposure in commercial maize and cherry samples in Shandong Province.
4. Conclusions
In this work, a broad-specific mAb based IACs were prepared and coupled to UPLC-MS/MS detection method was developed for T-2 and HT-2 toxin purification and determination in maize and cherry samples. The mAb used in this work showed broad-specific CR values to both T-2 and HT-2 toxin. Negligible CR values were obtained with structure related mycotoxins in this work. Under the optimized conditions, this IAC-UPLC-MS/MS protocol could be used for T-2 and HT-2 determination in both maize and cherry samples. With high sensitive UPLC-MS/MS conditions, lower LOD of T-2 and HT-2 were adopted at 0.28 μg kg−1 and 1.25 μg kg−1 for maize samples and 0.35 μg kg−1 and 1.42 μg kg−1 for cherry samples. Applying to commercial samples, 12 samples were detected T-2 toxin positive with 5 detected HT-2 toxin positive in maize samples (positive rate 80.0%). In cherry samples, only 2 samples were detected T-2 positive (positive rate 13.3%). The proposed protocol meets the requirement for sample preparation and highly sensitive identification of multiple T-2 and HT-2 in agricultural product and food safety.
Disclosure statement
Shaoxia Lin and Yanshen Li are equally contributors. No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Arola, H. O., Tullila, A., Nathanail, A., & Nevanen, T. K. (2017). A simple and specific noncompetitive ELISA method for HT-2 toxin detection. Toxins (Basel), 9(4), 145. doi: 10.3390/toxins9040145
- Brezina, U., Rempe, I., Kersten, S., Valenta, H., Humpf, H. U., & Danicke, S. (2014). Diagnosis of intoxications of piglets fed with Fusarium toxin-contaminated maize by the analysis of mycotoxin residues in serum, liquor and urine with LC-MS/MS. Archives of Animal Nutrition, 68(6), 425–447. doi: 10.1080/1745039X.2014.973227
- Edupuganti, S. R., Edupuganti, O. P., O'Kennedy, R., Defrancq, E., & Boullanger, S. (2013). Use of T-2 toxin-immobilized amine-activated beads as an efficient affinity purification matrix for the isolation of specific IgY. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 923–924, 98–101. doi: 10.1016/j.jchromb.2013.02.007
- Gentili, A., Caretti, F., Ascenzo, G., Rocca, L. M., Marchese, S., Materazzi, S., & Perret, D. (2007). Simultaneous determination of trichothecenes A, B, and D in maize food products by LC-MS-MS. Chromatographia, 66, 669–676. doi: 10.1365/s10337-007-0411-7
- Grajewski, J., Kosicki, R., Twaruzek, M., & Blajet-Kosicka, A. (2019). Occurrence and risk assessment of mycotoxins through polish beer consumption. Toxins (Basel), 11(5), 254. doi: 10.3390/toxins11050254
- Haubl, G., Berthiller, F., Hametner, C., Rechthaler, J., Jaunecker, G., Freudenschuss, M., … Schuhmacher, R. (2007). Characterization of (13C24) T-2 toxin and its use as an internal standard for the quantification of T-2 toxin in cereals with HPLC-MS/MS. Analytical and Bioanalytical Chemistry, 389, 931–940. doi: 10.1007/s00216-007-1493-7
- Hjelkrem, A. R., Aamot, H. U., Brodal, G., Strand, E. C., Torp, T., Edwards, S. G., … Hofgaard, I. S. (2018). HT-2 and T-2 toxins in Norwegian oat grains related to weather conditions at different growth stages. European Journal of Plant Pathology, 151, 501–514. doi: 10.1007/s10658-017-1394-3
- Kafouris, D., Christofidou, M., Christodoulou, M., Christou, E., & Ioannou-Kakouri, E. (2017). A validated UPLC-MS/MS multi-mycotoxin method for nuts and cereals: Results of the official control in Cyprus within the EU requirements. Food and Agricultural Immunology, 28, 90–108. doi: 10.1080/09540105.2016.1228834
- Kawamura, O., Nagayama, S., Sato, S., Ohtani, K., Sugiura, Y., Tanaka, T., & Ueno, Y. (1990). Survey of T-2 toxin in cereals by an indirect enzyme-linked immunosorbent assay. Food and Agricultural Immunology, 2, 173–180. doi: 10.1080/09540109009354718
- Kierek Jaszczuk, D., Marquardt, R. R., Frohlich, A. A., Clarke, J., Xiao, H., & Abramson, D. (1995). Detection and quantification of the T-2 mycotoxin by ELISA utilizing toxin-specific polyclonal antibodies raised in chickens. Food and Agricultural Immunology, 7, 243–252. doi: 10.1080/09540109509354882
- Kong, W., Zhang, X., Shen, H., Ou-Yang, Z., & Yang, M. (2012). Validation of a gas chromatography-electron capture detection of T-2 and HT-2 toxins in Chinese herbal medicines and related products after immunoaffinity column clean-up and pre-column derivatization. Food Chemistry, 132(1), 574–581. doi: 10.1016/j.foodchem.2011.10.073
- Li, C., Wang, Z., Cao, X., Beier, R. C., Zhang, S., Ding, S., … Shen, J. (2008). Development of an immunoaffinity column method using broad-specificity monoclonal antibodies for simultaneous extraction and cleanup of quinolone and sulfonamide antibiotics in animal muscle tissues. Journal of Chromatography A, 1209, 1–9. doi: 10.1016/j.chroma.2008.08.116
- Li, Y., Wang, Z., De Saeger, S., Shi, W., Li, C., Zhang, S., … Shen, J. (2012). Determination of deoxynivalenol in cereals by immunoaffinity clean-up and ultra-high performance liquid chromatography tandem mass spectrometry. Methods, 56, 192–197. doi: 10.1016/j.ymeth.2011.10.009
- Li, T., Wu, Q., Wang, Y., John, A., Qu, H., Gong, L., … Jiang, Y. (2017). Application of proteomics for the investigation of the effect of initial pH on pathogenic mechanisms of fusarium proliferatum on banana fruit. Frontiers in Microbiology, 8, 2327. doi: 10.3389/fmicb.2017.02327
- Li, Y., Zhang, Y., Shi, W., Wang, Z., Shen, J., & Zhang, S. (2012). Determination of T-2 toxin and HT-2 toxin in milk: A comparison of three formats of immunoassays. Analytical Letters, 45, 2425–2435. doi: 10.1080/00032719.2012.686134
- Li, Y., Zou, N., Wang, J., Wang, K. W., Li, F. Y., Chen, F. X., … Sun, D. J. (2017). TGF-beta1/Smad3 signaling pathway mediates T-2 toxin-induced decrease of Type II collagen in cultured rat chondrocytes. Toxins (Basel), 9(11), 359. doi: 10.3390/toxins9110359
- Lin, R., Sun, Y., Ye, W., Zheng, T., Wen, J., & Deng, Y. (2019). T-2 toxin inhibits the production of mucin via activating the IRE1/XBP1 pathway. Toxicology, 424, 152230. doi: 10.1016/j.tox.2019.06.001
- Lu, P., Wu, C., Shi, Q., Wang, Y., Sun, L., Liao, J., … Liu, Y. (2016). A sensitive and validated method for determination of T-2 and HT-2 toxin residues in shrimp tissues by LC-MS/MS. Food Analytical Methods, 9, 1580–1594. doi: 10.1007/s12161-015-0336-y
- Majerus, P., Hain, J., & Scheer, M. (2008). T-2 and HT-2 toxin analysis in cereals and cereal products following IAC cleanup and determination via GC-ECD after derivatization. Mycotoxin Research, 24, 24–30. doi: 10.1007/BF02985267
- Maruniakova, N., Kadasi, A., Sirotkin, A., Bulla, J., & Kolesarova, A. (2014). T-2 toxin and its metabolite HT-2 toxin combined with insulin-like growth factor-I modify progesterone secretion by porcine ovarian granulosa cells. Journal of Environmental Science and Health Part A-Toxic/Hazardous Substances & Environmental Engineering, 49, 404–409.
- Maruniakova, N., Kadasi, A., Sirotkin, A., Lesniak, A., Ferreira, A. M., Bulla, J., & Kolesarova, A. (2015). Assessment of T-2 toxin effect and its metabolite HT-2 toxin combined with insulin-like growth factor I, leptin and ghrelin on progesterone secretion by rabbit ovarian fragments. Journal of Environmental Science and Health Part B-Pesticides Food Contaminants and Agricultural Wastes, 50, 128–134.
- McCormick, S. P., Kato, T., Maragos, C. M., Busman, M., Lattanzio, V. M., Galaverna, G., … Kurtzman, C. P. (2015). Anomericity of T-2 toxin-glucoside: Masked mycotoxin in cereal crops. Journal of Agricultural and Food Chemistry, 63, 731–738. doi: 10.1021/jf504737f
- Pascale, M., Haidukowski, M., & Visconti, A. (2003). Determination of T-2 toxin in cereal grains by liquid chromatography with fluorescence detection after immunoaffinity column clean-up and derivatization with 1-anthroylnitrile. Journal of Chromatography A, 989, 257–264. doi: 10.1016/S0021-9673(03)00081-5
- Rodriguez-Carrasco, Y., Molto, J. C., Manes, J., & Berrada, H. (2014). Exposure assessment approach through mycotoxin/creatinine ratio evaluation in urine by GC-MS/MS. Food and Chemical Toxicology, 72, 69–75. doi: 10.1016/j.fct.2014.07.014
- Stecher, G., Jarukamjorn, K., Zaborski, P., Bakry, R., Huck, C. W., & Bonn, G. K. (2007). Evaluation of extraction methods for the simultaneous analysis of simple and macrocyclic trichothecenes. Talanta, 73, 251–257. doi: 10.1016/j.talanta.2007.03.028
- Vera-Avila, L. E., Vazquez-Lira, J. C., Garcia, D. L. M., & Covarrubia, R. (2005). Sol-gel immunosorbents doped with polyclonal antibodies for the selective extraction of malathion and triazines from aqueous samples. Environmental Science & Technology, 39, 5421–5426. doi: 10.1021/es048000c
- Visconti, A., Lattanzio, V. M. T., Pascale, M., & Haidukowski, M. (2005). Analysis of T-2 and HT-2 toxins in cereal grains by immunoaffinity clean-up and liquid chromatography with fluorescence detection. Journal of Chromatography A, 1075, 151–158. doi: 10.1016/j.chroma.2005.04.009
- Wei, J. T., Wu, K. T., Sun, H., Khalil, M. M., Dai, J. F., Liu, Y., … Sun, L. H. (2019). A novel modified hydrated sodium calcium aluminosilicate (HSCAS) adsorbent can effectively reduce T-2 toxin-induced toxicity in growth performance, nutrient digestibility, serum biochemistry, and small intestinal morphology in chicks. Toxins (Basel), 11, 199. doi: 10.3390/toxins11040199
- Wu, J., Zhou, Y., Yuan, Z., Yi, J., Chen, J., Wang, N., & Tian, Y. (2019). Autophagy and apoptosis interact to modulate T-2 toxin-induced toxicity in liver cells. Toxins (Basel), 11, 45. doi: 10.3390/toxins11010045
- Yao, K., Wang, J., Ren, Z., Zhang, Y., Wen, K., Shao, B., & Jiang, H. (2020). Development of a novel monoclonal antibody–based indirect competitive ELISA with immunoaffinity cleanup for the detection of triclosan in chickens. Food Analytical Methods, 13, 382–389. doi: 10.1007/s12161-019-01644-y
- Zhang, L., Li, L., Xu, J., Pan, M. H., & Sun, S. C. (2019). HT-2 toxin exposure induces mitochondria dysfunction and DNA damage during mouse early embryo development. Reproductive Toxicology, 85, 104–109. doi: 10.1016/j.reprotox.2019.02.011
- Zhang, J., Zhang, H., Liu, S., Wu, W., & Zhang, H. (2018). Comparison of anorectic potencies of type A trichothecenes T-2 toxin, HT-2 toxin, diacetoxyscirpenol, and neosolaniol. Toxins (Basel), 10(5), 179. doi: 10.3390/toxins10050179