ABSTRACT
This study was conducted to investigate the effects of composited functional bioactive substances on the growth performance and immune function of growth retardation (GR) pigs. Twenty-four 42-day-old GR pigs were fed with basal diet containing 0 or 0.5% composited functional bioactive substances package (FP) for 21 days. The results showed that dietary supplementation with FP decreased feed:gain ratio, increased the relative and absolute weight of the liver (P < 0.05). Supplementation with FP decreased serum alkaline phosphatase and malondialdehyde, but increased aspartic acid, cysteine, arginine, proline and taurine concentrations, as well as serum superoxide dismutase and catalase activities (p < 0.05). FP treatment increased all determined cytokines (IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, GM-CSF, IFN-γ, TGF-β1, TNF-α) concentrations in serum compared with the control treatment (p < 0.05). These findings indicate that functional bioactive substances can improve feed conversion rate, serum anti-oxidant capacity and immunity and can be as effective growth promoter in the GR piglets.
Introduction
Slow growth and decreased feed efficiency occur frequently in the pig breeding production. About 10% of piglets are retardative in growth, resulting in significant economic losses to pig farmers (Harada, Shizuyama, Ihara, & Takeuchi, Citation2003). In the previous study, small intestinal transcriptome analysis has revealed changes of genes involved in nutrition metabolism and immune responses in growth retardation (GR) of piglets (Qi et al., Citation2019a). GR was associated with the impaired intestinal mucosal barrier function, gut hormone profiles, immune and antioxidant function (Qi et al., Citation2019b). The causes resulting in growth retardation (GR) of piglets are very complicated, including intrauterine growth retardation, diseases, parasites, and so on. However, metabolism and immune response should be impaired for any reasons. Therefore, integrated regulating nutrient metabolism and immune functions may be useful to alleviate growth retardation of pigs. Many strategies have been developed to improve growth performance of piglets, including plant extracts, enzyme and probiotics interventions (Garry, Fogarty, Curran, O'Connell, & O'Doherty, Citation2007; Ilsley, Miller, Greathead, & Kamel, Citation2003; Shim, Verstegen, Kim, Kwon, & Verdonk, Citation2005).
Complex-probiotic-preparation could promote the absorption of nutrients and maintain the intestinal health by regulating the host mucosal and systemic immune function (Ren, Wang, Liu, Shen, & Yu, Citation2019; Valeriano, Balolong, & Kang, Citation2017). Probiotics has demonstrated to improve the growth performance and nutrient digestibility of livestock and poultry (Liu, Devi, Park, & Kim, Citation2017). Probiotics have anti-inflammatory activity and can activate dendritic cells, improve the production of cytokines, and stimulate B cells to secrete antibodies (Michele et al., Citation2017). Herb complex extract also has been widely used as a substitute for antibiotics in feed additives (Yan, Meng, & Kim, Citation2012; Zhou, Zhang, & Kim, Citation2013). It also confirmed that the addition of herbal extracts to diets could improve the growth performance and immune function of weaned piglets (Zhao, Zhang, Zhou, Dong, & Zhang, Citation2019). Multi-enzyme complex also can promote the digestion and absorption of nutrients and, effectively inhibit the growth of pathogenic bacteria in the intestinal tract of livestock and poultry (Mohammadi Gheisar, Hosseindoust, & Kim, Citation2016; Zhang, Yang, Wang, Yang, & Zhou, Citation2014). The combination of complex complex-probiotic-preparation and multi-enzyme complex has achieved beneficial effects in poultry production (Momtazan, Moravej, & Taheri, Citation2011; Wealleans, Walsh, Romero, & Ravindran, Citation2017)
Therefore, we hypothesised that comprehensive utilisation of complex-probiotic-preparation, herb complex extract and multi-enzyme complex could alleviate the GR induced by various complex reasons in pigs. The effects of dietary supplementation with bioactive compounds on growth performance, serum biochemical profile, antioxidant capacity and immune functions in GR pigs were investigated in the present study.
Materials and methods
This animal test was conducted in accordance with the “Guidelines for Animal Welfare in China” and approved by the Animal Protection and Use Committee of Hunan Agricultural University.
Experimental animals and diets
A total of twenty-four 42-day-old Landracre×Large Yorkshire crossbred postnatal growth retardation piglets (with a BW of < 70% of average BW of herd and no obvious characteristics of the disease or injury) were randomly assigned to 2 treatments with 6 pens/treatment and 2 piglets/pen. Piglets were fed the basal diet or the basal diet plus 0.5% functional package (FP). The diets were formulated to meet the nutrient requirements of National Research Council (Citation2012) for weanling piglets, which have shown in the previous study (Yuan et al., Citation2017). The FP was obtained from a commercial company (Xinqidian Biotechnology Co., Ltd, Changsha, Hunan, China) that consisted mainly of complex-probiotic-preparation, herb complex extract and multi-enzyme complex.
Animal management and sample collection
The piglets were housed individually in an environmentally controlled nursery with hard-plastic slatted flooring. All piglets had free access to diets and water. After a 3-day adaptation period, piglets were fed with their respective diets 3 times per day at 8:00, 13:00, and 18:00 for a 21-day period. Body weights of piglets and feed consumption were recorded per replicate on the morning of 0 and 21 d. On day 21 after the initiation of treatment, 6 piglets (1 pig/pen) were randomly selected and 5 mL of blood was collected aseptically in tubes from a jugular vein 2 h after feeding, centrifuged at 2000 × g for 10 min at 4°C to obtain serum samples, and stored at –80°C for further analysis. On d 21, piglets were anesthetised with sodium pentobarbital (20 mg/kg body weight) and killed by jugular puncture. The liver, kidney, spleen and heart were obtained and weighed. The relative weight of each organ was calculated as the organ weight divided by the BW (g/kg).
Serum biochemical profile and amino acid concentrations
Serum concentrations of total protein, albumin, aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), urea nitrogen (UN), glucose, total cholesterol (CHOL), high-density lipoprotein (HDL), low-density lipoprotein (LDL), immunoglobulin M (IgM), immunoglobulin G (IgG) were analysed using the corresponding kits (Nanjing jiancheng, Nanjing, China) and measured by an instrument (Biochemical Analytical Instrument, cobas c311, Roche Inc., Basel). Another 800 μL was added an equal volume of sulfosalicylic acid to the serum samples, centrifuged at 12000 × g for 10 min at 4°C to obtain the supernatant then measured free amino acid concentration using Agilent 1260 Liquid chromatograph (Agilent Technologies Inc., California).
Serum antioxidant capacity
Serum concentrations of malondialdehyde (MDA) and glutathione (GSH), as well as the activities of superoxide dismutase (SOD) andcatalase (CAT), were measured using methods of 2-thiobarbituric acid, reduced glutathione, xanthine oxidase-xanthine reaction and CAT-H2O2 reaction, and, respectively. Their corresponding assay kits were purchased form Nanjing Jiancheng Co., Ltd (Nanjing, China). All procedures were performed according to manufacturer instructions and all samples were measured by UV/visible spectrophotometer (UV-2450, Shimadzu, Kyoto, Japan).
Fluorescence intensity of serum cytokines
Serum interleukin (IL)-1β, IL-4, IL-6, IL-8, IL-10, IL-12, Granulocyte-macrophage colony-stimulating factor (GM-CSF), transforming growth factor-β1 (TGF-β1), tumour necrosis factor-α (TNF-α), and interferon gamma (IFN-γ) were measured using a porcine cytokine array QAP-CYT-1 (RayBiotech Inc., Guangzhou, China). Quantibody® Array is a multi-sandwich ELISA quantitative array platform for quantitative measurement of multiple cytokines according to the manufacturer's protocol. Briefly, 100 μL of sample dilution was added to each well and incubated for 30 min at room temperature to block the slide decantation buffer from each well. 100 μL of standard cytokine or sample was added to each well and incubated overnight at 4 °C. The samples were decanted from each well and washed 5 and 2 times with Wash Buffers I and II, respectively. 80 μL of sample dilution was added to each well and incubated for 30 min at room temperature to block the slide decantation buffer from each well. 80 μL Cy3 dye equivalent dye-conjugated streptavidin was added to each well and incubated for 1 h in a dark room. After being washed 5 times, the slides were placed in the slide washer/dryer and gently washed with Wash Buffers I and II for 15 and 5 min, respectively. The signal was visualised at the Cy3 wavelength of excitation frequency = 532 nm with an InnoScan 300 Microarray Scanner (Innopsys, Parc d'Activité'AActivestre, Carbonne, France).
Statistical analysis
All data analysis was performed by unpaired t-test using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Probability values less than 0.05 were considered statistically significant.
Result
Growth performance and weight of visceral organs
The body weight, average daily gain, average daily feed intake, and feed: gain ratio are shown in . There were no differences (P > 0.05) in initial BW, final BW, average daily gain and daily feed intake between pigs of control and FP treatments. The feed: gain ratio was decreased (P < 0.05) by functional bioactive substance supplementation. There were no differences (P > 0.05) in the relative weights of the kidney, heart, or spleen between two treatments except that FP treatment increased (P < 0.05) the relative and absolute weight of the liver ().
Table 1. Effects of functional bioactive substance supplement on growth performance of growth retardation pigsa,b.
Table 2. Effects of functional bioactive substance supplement on organ weight of growth retardation pigs.a,b
Serum biochemical parameters
Compared with the control treatment, pigs in FP treatment had decreases in serum concentrations of aspartate aminotransferase (P < 0.05), but there had no differences (P > 0.05) in serum concentrations of total protein, albumin, aspartate aminotransferase, alanine aminotransferase, urea nitrogen, glucose, total cholesterol, high-density lipoprotein, low-density lipoprotein ().
Table 3. Effects of functional bioactive substance supplement on serum biochemical parameters of growth retardation pigs.a,b
Serum free amino acid concentrations
According to the , compared with control treatment, supplementation with functional bioactive substance significantly increased the serum concentrations of aspartic acid, cysteine, arginine, proline and taurine in pigs (P < 0.05). On the contrary, the serum lysine content were significantly reduced (P < 0.05) in FP treated pigs.
Table 4. Effects of functional bioactive substance supplement on serum free amino acid concentration of growth retardation pigs.a,b
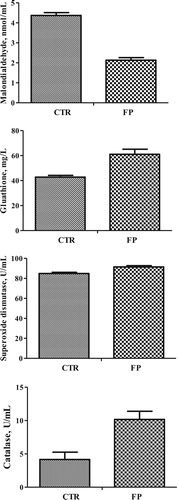
Serum antioxidant parameters
The serum concentrations of MDA and GSH as well as SOD and CAT activities are presented in . Supplementation with functional bioactive substances increased the serum GSH concentration and of the activities of SOD and CAT but decreased the serum MDA content (P < 0.05).
Serum profiles of cytokines and immunoglobulin concentration
Semi-quantitative data of serum cytokine fluorescence are shown in . Compared with the control treatment, dietary supplementation with FP significantly increased the serum concentrations of all determined cytokines (IL-1β, IL-4, IL-8, IL-6, IL-10, IL-12, GM-CSF, TGF-β1, IFN-γ and TNF-α) as well as IgM (P < 0.05).
Table 5. Effects of functional bioactive substance supplement on fluorescence intensity of serum cytokines and immunoglobulinin growth retardation pigs.a,b
Discussion
Postnatal growth retardation is associated with lifelong consequences beyond reduced weight, including metabolic disturbance and impaired immune and anti-oxidant functions in pigs has been confirmed in the previous studies (Qi et al., Citation2019a, Citation2019b). Composited functional bioactive substance packages including complex-probiotic-preparation, herb complex extract and multi-enzyme complex were used to alleviate the growth retardation s induced by complicated factors in the postnatal days of pigs in the present study. The growth-promoting effects of these bioactive substances have been well documented in animals (Ghaedi, Nasr, Kheiri, Rahimian, & Miri, Citation2014; Li et al., Citation2017; Morteza, Ahmad, Abdolmansour, Abolghasem, & Hassan, Citation2018). The present results showed that dietary supplementation with composited functional packages improved metabolic status and anti-oxidant capacity and activated immune response.
A significant feature of growth retardation pigs is the extremely low feed conversion rate. Suffering from variety of challenges and severe stress, young pigs are experienced damage of parenchymal organs, a sub-health status and poor digestion or conversion (Zhang, Li, & Wang, Citation2015). In addition, the previous study has demonstrated that the relative weight of the liver was decreased and serum alanine transaminase was increased in PGR pigs (Qi et al., Citation2019b). Although the FP administration didn't improve the average daily gain, the feed:gain ratio was significantly reduced that accompanied with the increased serum concentrations of aspartic acid, cysteine, arginine, proline and taurine. The main ingredients of the herbal complex used in the present study were Angelica water extract, Ligustrum lucidum, Gentian extract and black pepper that have indicated to improve the growth performance of broilers and piglets (Li et al., Citation2017; Morteza et al., Citation2018). For example, the multi-enzyme complex functional enzymes such as amylase, cellulase, lactase and so on can degrade the indigestible part of the nutrients in the feed and promote the absorption of nutrients, thereby improving the feed conversion rate (Omogbenigun, Nyachoti, & Slominski, Citation2004). Black pepper is rich in glutathione peroxidase and glucose-6-phosphate dehydrogenase, and its active ingredient, piperine, has been demonstrated to significantly increase absorption of selenium, vitamin B complex, beta-carotene and curcumin as well as other nutrients (Srinivasan, Citation2007). Gentiopicroside, the main component of gentian extract, can alleviate liver injury induced by carbon tetrachloride, decrease the serum concentration of alkaline phosphatase in rat (Mihailovi et al., Citation2014). The present results showed that the weight of the liver was increased and the serum concentration of alkaline phosphatase was decreased in FP-treated pigs. In addition, the increased serum amino acids concentrations indicated that FP addition stimulated the digestion of dietary protein and the absorption of resultant amino acids, therefore improving growth performance (Wu et al., Citation2007). The previous studies have showed that complex probiotic, multi-enzyme preparations and herbal compounds improved the digestive and absorptive function and regulated amino acids metabolism (Kong et al., Citation2009; Liu et al., Citation2017; Omogbenigun et al., Citation2004; Yin et al., Citation2009). Additionally, increasing the circulatory aspartic acid, cysteine, arginine, proline and taurine are beneficial to immune status and anti-oxidant capacity (Wu et al., Citation2007,Zhang, Jin, Peng, Guo, & Wang, Citation2019).
Immature immune system and oxidative imbalance in pigs may be responsible for their growth retardation (Qi et al., Citation2019a, Citation2019b). The PGR pigs exhibited higher plasma concentrations of IgG and some inflammatory cytokines indicating toxic or oxidative damage in organisms (Qi et al., Citation2019b). But supplementation with functional bioactive substances increased the serum GSH concentration, SOD and CAT activities. Functional bioactive substance directly inhibited ROS production or protect against antioxidant defenses to inhibit ROS formation (Hussain, Tan, Liu, Oladele, & Yin, Citation2016). For example, the active ingredients of pepper extract, piperine and pepper oleoresin, have also been shown to have antioxidant activity, and the extracts’ percentage inhibition were 88.75 and 90.31% for DPPH and hydrogen peroxide, respectively (Olalere, Abdurahman, & Alara, Citation2017). Previous studies reported that Lactobacillus paracasei and Lactobacillus plantarum had potential anti-inflammatory effects (Yin et al., Citation2018; Zagato et al., Citation2014). Pepper and ligustrum lucidum also exhibited anti-inflammatory effects (Hazekawa et al., Citation2017; Wu et al., Citation2010). The supplementation of functional bioactive substances increased serum concentrations of anti-inflammatory cytokines IL-4 and IL-10, and also increased serum concentrations of pro-inflammatory cytokines IL-1β, IL-8 and IL-6. This can be explained by the negative feedback regulating mechanism among cytokines (Dambuza et al., Citation2017). In addition, we observed significantly higher serum concentrations of TGF-β1, TNF-α, IFN-γ and GM-CSF in FP than the control group, possibly because the increased concentrations of TNF-α, IFN-γ and GM-CSF promoted the production of TGF-β1, while TGF-β1 inhibited the production of TNF-α, IFN-γ and GM-CSF (Seckin, Kalayci, Turan, Yilmaz, & Yilmaz, Citation2018; Zhao et al., Citation2020). The down-regulated cytokines showing immunosuppression were observed in the PGR piglets and IUGR piglet neonates (Qi et al., Citation2019b ; Dong et al., Citation2014), but supplementation with functional bioactive substances could regulate the activity of serum cytokines and stimulate the immune function of growth retardation piglets.
In summary, dietary supplementation with functional bioactive substances can improve feed conversion rate, serum amino acids concentrations, antioxidant capacity and immune function of growth retardation piglets. The comprehensive utilisation of complex-probiotic-preparation, herb complex extract and multi-enzyme complex is an effective way to alleviate the growth retardation induced by various complex reasons in pigs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dambuza, I. M., He, C., Choi, J. K., Yu, C. R., Wang, R., Mattapallil, M. J., … Egwuagu, C. E. (2017). Il-12p35 induces expansion of il-10 and il-35-expressing regulatory b cells and ameliorates autoimmune disease. Nature Communications, 8(1), 719. doi: 10.1038/s41467-017-00838-4
- Dong, L., Zhong, X., Ahmad, H., Li, W., Wang, Y., Zhang, L., & Wang, T. (2014). Intrauterine growth restriction impairs small intestinal mucosal immunity in neonatal piglets. Journal of Histochemistry & Cytochemistry, 62(7), 510–518. doi: 10.1369/0022155414532655
- Garry, B. P., Fogarty, M., Curran, T. P., O'Connell, M. J., & O'Doherty, J. V. (2007). The effect of cereal type and enzyme addition on pig performance, intestinal microflora, and ammonia and odour emissions. Animal, 1(05), 751. doi: 10.1017/s1751731107720190
- Ghaedi, H., Nasr, J., Kheiri, F., Rahimian, Y., & Miri, Y. (2014). The effect of virginiamycin and black pepper (piper nigrum l.) extract on performance of broiler chicks. Research Opinions in Animal and Veterinary Sciences, 4(2), 91–95.
- Harada, E., Shizuyama, M., Ihara, N., & Takeuchi, T. (2003). Impaired pancreatic endocrine and exocrine responses in growth-retarded piglets. Journal of Veterinary Medicine Series A, 50(9), 433–441. doi: 10.1046/j.1439-0442.2003.00574.x
- Hazekawa, M., Hideshima, Y., Ono, K., Nishinakagawa, T., Kawakubo-Yasukochi, T., Takatani-Nakase, T., & Nakashima, M. (2017). Anti-inflammatory effects of water extract from bell pepper (capsicum annuum l. var. grossum) leaves in vitro. Experimental and Therapeutic Medicine, doi: 10.3892/etm.2017.5106
- Hussain, T., Tan, B., Liu, G., Oladele, O. A., & Yin, Y. (2016). Health-promoting properties of eucommia ulmoides: A review. Evidence-based Complementary and Alternative Medicine, 2016(12), 1–9. doi: 10.1155/2016/5202908
- Ilsley, S. E., Miller, H. M., Greathead, H. M. R., & Kamel, C. (2003). Plant extracts as supplements for lactating sows: Effects on piglet performance, sow food intake and diet digestibility. Animal Science, 77(2), 247–254. doi: 10.1017/S1357729800058987
- Kong, X. F., Yin, Y. L., He, Q. H., Yin, F. G., Liu, H. J., Li, T. J., … Wu, G. (2008). Dietary supplementation with Chinese herbal powder enhances ileal digestibilities and serum concentrations of amino acids in young pigs. Amino Acids, 37(4), 573–582. doi: 10.1007/s00726-008-0176-9
- Li, X. L., He, W. L., Yang, M. L., Yan, Y. M., Xue, Y. H., & Zhao, S. T. (2017). Effect of dietary supplementation of ligustrum lucidum on performance, egg quality and blood biochemical parameters of hy-line brown hens during the late laying period. Animal, 1–6. doi: 10.1017/S1751731117000532
- Liu, W., Devi, S., Park, J., & Kim, I. (2017). Effects of complex probiotic supplementation in growing pig diets with and without palm kernel expellers on growth performance, nutrient digestibility, blood parameters, fecal microbial shedding and noxious gas emission. Animal Science Journal, doi: 10.1111/asj.12965
- Michele, B., Luca, L., Adriana, C., Sabrina, C., Eleonora, D., Silvia, M., … Fiorucci, S. (2017). Metabolic variability of a multispecies probiotic preparation impacts on the anti-inflammatory activity. Frontiers in Pharmacology, 8, 505. doi: 10.3389/fphar.2017.00505
- Mihailovi, V., Katani, J., MiI, D., Stankovi, V., Mihailovi, M., Uskokovi, A., … Stanković, N. (2014). Hepatoprotective effects of secoiridoid-rich extracts from gentiana cruciata l. Against carbon tetrachloride induced liver damage in rats. Food & Function, 5(8), 1795. doi: 10.1039/c4fo00088a
- Mohammadi Gheisar, M., Hosseindoust, A., & Kim, I. H. (2016). Effects of thermo-resistant non-starch polysaccharide degrading multi-enzyme on growth performance, meat quality, relative weights of body organs and blood profile in broiler chickens. Journal of Animal Physiology and Animal Nutrition, 100(3), 499–505. doi: 10.1111/jpn.12387
- Momtazan, R., Moravej, H., & Taheri, M. Z. R. (2011). A note on the effects of a combination of an enzyme complex and probiotic in the diet on performance of broiler chickens. Irish Journal of Agricultural and Food Research, 50(2), 249–254. doi: 10.2307/41549255
- Morteza, E. S., Ahmad, H., Abdolmansour, T., Abolghasem, G., & Hassan, N. M. (2018). The effect of hydroalcoholic extract of angelica (heracleum persicum), fruit on performance, immune responses, small intestine histology, haematological parameters and carcass characteristics of broiler chickens. Journal of Applied Animal Research, 46(1), 1336–1343. doi: 10.1080/09712119.2018.1505621
- NRC. (2012). Nutrient requirements of swine. Washington DC: National Academy Press.
- Olalere, O. A., Abdurahman, N. H., & Alara, O. R. (2017). Extraction, radical scavenging activities and physicochemical fingerprints of black pepper (piper nigrum) extract. Journal of Food Measurement and Characterization, doi: 10.1007/s11694-017-9604-4
- Omogbenigun, F. O., Nyachoti, C. M., & Slominski, B. A. (2004). Dietary supplementation with multienzyme preparations improves nutrient utilization and growth performance in weaned pigs. Journal of Animal Science, 82(4), 1053–1061. doi: 10.2527/2004.8241053x
- Qi, M., Tan, B., Wang, J., Li, J., Liao, S., Yan, J., … Yin, Y. (2019a). Small intestinal transcriptome analysis revealed changes of genes involved in nutrition metabolism and immune responses in growth retardation piglets. Journal of Animal Science, 97, 3795–3808. doi: 10.1093/jas/skz205
- Qi, M., Tan, B., Wang, J., Liao, S., Li, J., Liu, Y., & Yin, Y. (2019b). Postnatal growth retardation associated with impaired gut hormone profiles, immune and antioxidant function in pigs. Frontiers in Endocrinology, 10, 660. doi: 10.3389/fendo.2019.00660
- Ren, D., Wang, D., Liu, H., Shen, M., & Yu, H. (2019). Two strains of probiotic lactobacillus enhance immune response and promote naive t cell polarization to th1. Food and Agricultural Immunology, 30(1), 281–295. doi: 10.1080/09540105.2019.1579785
- Seckin, C., Kalayci, G. A., Turan, N., Yilmaz, A., & Yilmaz, H. (2018). Immunomodulatory effects of echinacea and pelargonium on the innate and adoptive immunity in calves. Food and Agricultural Immunology, 6, 1–18. doi: 10.1080/09540105.2018.1444738
- Shim, S. B., Verstegen, M. W. A., Kim, I. H., Kwon, O. S., & Verdonk, J. M. A. J. (2005). Effects of feeding antibiotic-free creep feed supplemented with oligofructose, probiotics or synbiotics to suckling piglets increases the preweaning weight gain and composition of intestinal microbiota. Archives of Animal Nutrition, 59(6), 419–427. doi: 10.1080/17450390500353234
- Srinivasan, K. (2007). Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Critical Reviews in Food Science and Nutrition, 47(8), 735–748. doi: 10.1080/10408390601062054
- Valeriano, V. D. V., Balolong, M. P., & Kang, D. K. (2017). Probiotic roles of lactobacillus spp. in swine: Insights from gut microbiota. Journal of Applied Microbiology, 122(3), 554. doi: 10.1111/jam.13364
- Wealleans, A. L., Walsh, M. C., Romero, L. F., & Ravindran, V. (2017). Comparative effects of two multi-enzyme combinations and a bacillus probiotic on growth performance, digestibility of energy and nutrients, disappearance of non-starch polysaccharides, and gut microflora in broiler chickens. Poultry Science, doi: 10.3382/ps/pex226
- Wu, G. Y., Bazer, F. W., Davis, T. A., Jaeger, L. A., Johnson, G. A., Kim, S. W., … Yin, Y. L. (2007). Important roles for the arginine family of amino acids in swine nutrition and production. Livestock Science, 112, 8–22. doi: 10.1016/j.livsci.2007.07.003
- Wu, C. R., Hseu, Y. C., Lien, J. C., Lin, L. W., Lin, Y. T., & Ching, H. (2010). Triterpenoid contents and anti-inflammatory properties of the methanol extracts of ligustrum species leaves. Molecules, 16(1), 1–15. doi: 10.3390/molecules16010001
- Yan, L., Meng, Q. W., & Kim, I. H. (2012). Effect of an herb extract mixture on growth performance, nutrient digestibility, blood characteristics, and fecal microbial shedding in weanling pigs. Livestock Science, 145(1-3), 189–195. doi: 10.1016/j.livsci.2012.02.001
- Yin, X., Heeney, D., Srisengfa, Y., Golomb, B., Griffey, S., & Marco, M. (2018). Bacteriocin biosynthesis contributes to the anti-inflammatory capacities of probiotic lactobacillus plantarum. Beneficial Microbes, doi: info:doi/10.3920/BM2017.0096
- Yin, F. G., Liu, Y. L., Yin, Y. L., Kong, X. F., Huang, R. L., Li, T. J., … Hou, Y. (2009). Dietary supplementation with astragalus polysaccharide enhances ileal digestibilities and serum concentrations of amino acids in early weaned piglets. Amino Acids, 37(2), 263–270. doi: 10.1007/s00726-008-0142-6
- Yuan, D., Hussain, T., Tan, B., Liu, Y., Ji, P., & Yin, Y. (2017). The evaluation of antioxidant and anti-inflammatory effects of Eucommia ulmoides flavones using diquat-challenged piglet models. Oxidative Medicine and Cellular Longevity, 8140962. doi:10.1155/2017/8140962
- Zagato, E., Mileti, E., Massimiliano, L., Fasano, F., Budelli, A., Penna, G., & Rescigno, M. (2014). Lactobacillus paracasei cba l74 metabolic products and fermented milk for infant formula have anti-inflammatory activity on dendritic cells in vitro and protective effects against colitis and an enteric pathogen in vivo. PLOS ONE, 9. doi: 10.1371/journal.pone.0087615
- Zhang, H., Jin, Y., Peng, A., Guo, S., & Wang, H. (2019). L-arginine protects ovine intestinal epithelial cells from lipopolysaccharide-induced intestinal barrier injury. Food and Agricultural Immunology, 30(1), 1067–1084. doi: 10.1080/09540105.2019.1664417
- Zhang, H., Li, Y., & Wang, T. (2015). Antioxidant capacity and concentration of redox-active trace mineral in fully weaned intra-uterine growth retardation piglets. Journal of Animal Science and Biotechnology, 6(1), 48. doi: 10.1186/s40104-015-0047-7
- Zhang, G. G., Yang, Z. B., Wang, Y., Yang, W. R., & Zhou, H. J. (2014). Effects of dietary supplementation of multi-enzyme on growth performance, nutrient digestibility, small intestinal digestive enzyme activities, and large intestinal selected microbiota in weanling pigs. Journal of Animal Science, 92(5), 2063–2069. doi: 10.2527/jas.2013-6672
- Zhao, L., Rao, S., Zhu, X., Liu, S., Tao, Q., Yang, X., … Hu, J. (2020). Coicis Semen formula treating monosodium glutamate-induced obesity in mice by alleviating hypothalamic injury. Food and Agricultural Immunology, 31(1), 84–99. doi: 10.1080/09540105.2019.1703911
- Zhao, J., Zhang, G., Zhou, X., Dong, W., & Zhang, S. (2019). Effect of dandelion root extract on growth performance, immune function and bacterial community in weaned pigs. Food and Agricultural Immunology, 30(1), 95–111. doi: 10.1080/09540105.2018.1548578
- Zhou, T. X., Zhang, Z. F., & Kim, I. H. (2013). Effects of dietary coptis chinensis herb extract on growth performance, nutrient digestibility, blood characteristics and meat quality in growing-finishing pigs. Asian-australasian Journal of Animal Sciences, 26(1), 108–115. doi: 10.5713/ajas.2011.11400