ABSTRACT
In the present study, recombinant L. lactis NZ9000 displaying anchored dendritic cell-targeting peptide (DCpep) and E. tenella 3-1E protein was constructed. Chickens were orally immunized with the recombinant lactococci for three times at 2-week intervals. After three immunizations, chickens were challenged with E. tenella sporulated oocyst, and the immune response and protective efficacy were evaluated. The finding from animal experiments showed recombinant lactococci expressing anchored DCpep-3-1E protein elicited higher levels of 3-1E specific serum IgG and secretory IgA (sIgA) in caecal lavage, higher proportions of CD4+ and CD8α+ cells in peripheral blood, and higher mRNA expression levels of IL-2 and IFN-γ in the spleen compared to 3-1E-expressing lactococci, and hence offered obvious protective efficacy against homologous challenge. The present work demonstrated that cell surface-displayed target protein and immune enhancers in lactococci may be a promising approach to enhance immunity and immune efficacy against pathogen infection.
Introduction
Avian coccidiosis, a kind of intestinal disease caused by apicomplexan parasites belonging to the genus Eimeria, leads to severe economic losses for the global poultry industry (Shirley, Smith, & Tomley, Citation2005). Up to date, a variety of strategies are applied to reduce the economic losses by Eimeria infection including prophylaxis with anticoccidial drugs and commercial attenuated live vaccines (Lillehoj & Lillehoj, Citation2000). Although the widespread application of the above strategies has contributed to prevent Eimeria infections, the emergence of drug-resistant Eimeria strains, the unintended infection under the immunosuppressive conditions to some extent make coccidiosis prevention more complicated. Meanwhile, the drug residue in animal food poses a hidden danger to human health and safety (Dalloul & Lillehoj, Citation2006). Therefore, researchers gradually focus on the development of next-generation vaccines based on the expression of adjuvanted recombinant proteins in several live vector systems (Blake & Tomley, Citation2014).
It was generally accepted that cell-mediated immunity plays a vital role against Eimeria infection (Lillehoj & Trout, Citation1996). However, several reports showed Eimeria-specific antibodies inhibited Eimeria sporozoites invasion into host cells (Jiang et al., Citation2012; Sathish et al., Citation2011; Wallach, Citation2010). Early research reported that a variety of cells, including macrophages, dendritic cells, mast cells, natural killer cells, basophils, and eosinophils, are involved in the immune responses following Eimeria infection (Rose & Hesketh, Citation1979). Among the above-mentioned immune cells, dendritic cells (DC) are one of the professional antigen-presenting cells (APC) existing in the subepithelial lamina propria of the intestine and play a key role in eliciting intestinal immune responses (Chirdo, Millington, Beacock-Sharp, & Mowat, Citation2005). Previous research demonstrated that dendritic cell-targeting peptide (DCpep) effectively enhanced the immune responses induced by target antigen delivered by Lactobacillus acidophilus (Mohamadzadeh, Duong, Sandwick, Hoover, & Klaenhammer, Citation2009). Recently, we and others reported that the delivery of Eimeria protein by lactic acid bacteria could induce immune responses and provide protection against Eimeria challenge (Li et al., Citation2018; Ma et al., Citation2013; Ma, Zhang, Gao, & Ma, Citation2017; Yang et al., Citation2017). Based on our previous studies, we postulate that the recombinant L. lactis NZ9000 expressing DCpep fused with Eimeria protein could enhance the immunogenicity of the target protein. To investigate this possibility, we constructed recombinant L. lactis NZ9000 displaying DCpep and E. tenella 3-1E protein on the surface of bacteria. Then the immune response and efficacy were evaluated through animal immunizations and challenging experiments.
Materials and methods
Strains and plasmids
The strains and plasmids used in the present study are listed in . Lactococcus lactis NZ9000 were cultured at 30°C without shaking in M17 medium (Luqiao, Beijing) containing 5 g/l glucose (GM17) and 10 μg/ml chloramphenicol.
Table 1. Strains and plasmids used in this study.
Generation of recombinant lactococci co-expressing DCpep and 3-1E
Construction of plasmid pTX8048-SP-DCpep-3-1E-CWA harbouring fusion protein gene DCpep-3-1E was showed in . The synthesized fusion fragment SP-DCpep-linker-TrxA-His6 consists of signal peptide (SP) of L. lactis secretion protein Usp45, dendritic cell-targeting peptides (DCpep) (Curiel et al., Citation2004), linker sequence (G4S)2, coding sequence of trxA protein and His6 amino acid was cloned into Nco I/Bam H I sites of pTX8048-SP-3-1E-CWA (Ma et al., Citation2017) to generate pTX8048-SP-DCpep-3-1E-CWA. The plasmid pTX8048-SP-DCpep-3-1E-CWA, pTX8048-SP-3-1E-CWA and pTX8048 (Douillard, Mahony, Campanacci, Cambillau, & van Sinderen, Citation2011) was, respectively, electrotransformed into L. lactis NZ9000 using Gene Pulser apparatus (Bio-Rad, Hercules, CA, USA) to generate three recombinant bacteria L. lactis NZ9000/pTX8048-SP-DCpep-3-1E-CWA, L. lactis NZ9000/pTX8048-SP-3-1E-CWA and L. lactis NZ9000/pTX8048 according to the methods described in our previous report (Ma et al., Citation2017).
Figure 1. Schematic illustration of plasmids pTX8048-SP-DCpep-3-1E-CWA. The synthesized fusion fragment SP-DCpep-linker-TrxA-His6 consists of signal peptide (SP) of L. lactis secretion protein Usp45, dendritic cell-targeting peptides (DCpep) (Curiel et al., Citation2004), linker sequence (G4S)2, coding sequence of trxA protein and His6 amino acid was cloned into Nco I/Bam H I sites of pTX8048-SP-3-1E-CWA plasmid (A) (Ma et al., Citation2017) to generate pTX8048-SP-DCpep-3-1E-CWA (B).
Western blot analysis of fusion protein DCpep-3-1E
The cell wall-anchored fusion protein DCpep-3-1E was prepared as described in our previous report (Ma et al., Citation2017). Briefly, three kinds of target bacterial cultures with OD600 values of 0.5 were induced by the addition of nisin (Sigma-Aldrich) to the final concentration of 5 ng/ml. After incubation for 4 h, the induced cultures were harvested and centrifuged. The pellets were washed and resuspended in TES (10 mM Tris–HCl pH 8.0, 1 mM EDTA, 25% sucrose). The generated cell walls were then digested by the buffer TES-LMR (TES containing 1 mg/ml lysozyme, 0.1 mg/ml mutanolysin, 0.1 mg/ml RNase). The digested solution was then centrifuged, and the supernatant containing target cell wall-anchored proteins were precipitated with 16% TCA. The collected target protein DCpep-3-1E was washed, dried, and resuspended in 50 mM NaOH.
The prepared protein samples were separated by 12% SDS-PAGE, and then electrophoretically transferred to nitrocellulose membranes. The above membranes were probed with rabbit anti-3-1E specific antibodies (Ma et al., Citation2011) at a dilution of 1:1000 for 1.5 h. After washing three times with TTBS (Tris–HCl 20 mmol/l, pH 7.5, NaCl 100 mmol/l, 0.1% Tween-20), the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibodies (Sigma, USA). Then the target bands were detected with ECL chemiluminescence detection kit according to the manufacturer’s instructions.
Experimental design and immunizing procedure
Specific-pathogen-free (SPF) White Leghorn chickens purchased from Harbin Veterinary Research Institute were housed in individual cages under coccidia-free conditions. All chickens were randomly divided into five groups of 35 chickens each (). Chickens in group 1 were orally inoculated with L. lactis NZ9000/pTX8048-SP-DCpep-3-1E-CWA, Group 2 with L. lactis NZ9000/pTX8048-SP-3-1E-CWA, Group 3 with L. lactis NZ9000/pTX8048, each with a 100 μl volume containing 1 × 1010 CFU recombinant bacteria for three consecutive days at 2-week intervals for a total of three immunizations. Chickens in group 4 (unchallenged control group) and group 5 (challenged control group) were both orally fed with 100 μl PBS (pH 7.2). During the stage of immunizations, groups 4 and 5 were together as the PBS control group. At 54 days of age, all chickens except those in group 4 were challenged orally with 2 × 104 E. tenella sporulated oocysts. The experimental protocol (SRM-12) was accepted by the international recommendations for animal welfare and the Ethical Committee for animal sciences of Heilongjiang Province, PR China.
Table 2. Experimental design of immunizations and challenge.
Detection of IgG in serum and IgA in caecal lavage fluid
At 2 weeks post-tertiary oral immunization, the specific serum IgG levels and secreted IgA (sIgA) levels in caecal lavage fluid from chickens in each group (n = 5) were respectively detected by indirect Enzyme-linked Immunosorbent Assay (ELISA) according to the protocol as previously described (Ma et al., Citation2017). Briefly, 100 μl (1.0 μg) E. tenella 3-1E protein was coated onto the individual wells of the plate and incubated overnight at 4°C. Each well was blocked with 5% skim milk in PBS containing 0.05% Tween-20 (PBST) followed by a reaction with 100 μl of prepared diluted serum (1:128), caecal lavage fluid (1:100). The HRP-conjugated goat anti-chicken IgG and goat anti-chicken IgA (Sigma-Aldrich, USA) were, respectively, used as the secondary antibody. The optical density was measured at 450 nm by a microplate reader (Bio-Rad, Hercules, CA, USA). Each sample was tested in triplicate.
Cellular immunity induced by lactococci expressing DCpep-3-1E
On day 14 post-tertiary immunization, lymphocytes in peripheral blood from five chickens in each group were, respectively, isolated as previously described (Ma et al., Citation2017). 100 μl of the isolated lymphocytes (1 × 106 cells) were, respectively, incubated with fluorescein-isothiocyanate (FITC)-conjugated mouse anti-chicken CD4+ antibody (0.5 mg/ml) and phycoerythrin (PE)-conjugated mouse anti-chicken CD8α+ antibody (0.5 mg/ml) (Southern Biotech) for 30 min at 4°C in the dark. After washing with PBS (pH 7.4), cells were resuspended in 500 μl of PBS (pH 7.4), and the relative values of CD4+ and CD8α+ T lymphocytes subtypes were measured by flow cytometry (Epics X MCL, Beckman Coulter).
mRNA expression levels of ChIL-2 and ChIFN-γ in the spleen
At 2 weeks post-tertiary immunization, chicken IL-2 (ChIL-2) and IFN-γ (ChIFN-γ) mRNA expression levels in the spleen were quantified by real-time PCR as described in the previous report (Wang et al., Citation2018). The mRNA levels of ChIL-2 and ChIFN-γ were determined by the 2−ΔΔCt method (Livak & Schmittgen, Citation2001).
Evaluation of immune protection
To evaluate the protective efficacy of recombinant Dcpep-3-1E-expressing lactococci, body weight gain (BWG), lesion scores in caeca, and oocyst decrease ratio from groups of chickens were recorded. Ten chickens randomly selected from each group were weighed both prior to challenge and on day 7 post challenge to determine body weight gain (BWG). Lesion scores from ten chickens in each group were observed and recorded according to the reported method (Johnson & Reid, Citation1970). Faeces from ten chickens housed in ten individual cages in each group were respectively collected between days 7 and 11 post infection (PI), oocyst counting was determined via McMaster’s counting technique, and oocyst decrease ratio was calculated according to the previously reported method (Ma et al., Citation2011). On day 7 post challenge, caecal samples from chickens in each group were, respectively, fixed in 10% neutral buffered formalin, embedded by paraffin, sectioned, and stained with haematoxylin and eosin (HE) to assess histopathological changes.
Statistical analysis
Data were expressed as means ± SD and was statistically analysed by one-way analysis of variance(ANOVA). Differences between all groups were compared by ANOVA Duncan's multiple-comparison procedures through SPSS statistical package (SPSS Inc. Champaign, IL, USA). Results were considered significant at p < 0.05 and highly significant at p < 0.01.
Results
Protein expression assays and immunoblotting analysis
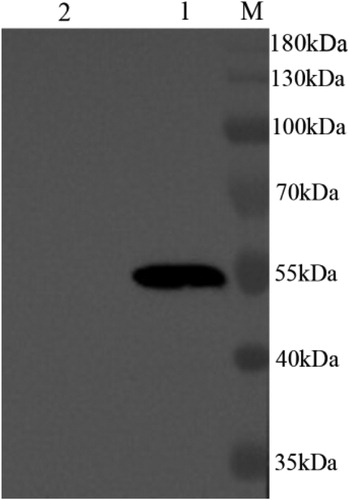
As shown in , the prepared cell wall-anchored fusion protein from DCpep-3-1E-expressing lactococci reacted with 3-1E polyclonal antibody (Ma et al., Citation2011), and a protein band of approximately 55 kDa was observed. The results showed that cell wall-anchored fusion protein DCpep-3-1E was expressed in the induced recombinant lactococci.
Figure 2. Westernblot detection of cell wall-anchored fusion protein DCpep-3-1E-CWA. Target fusion protein DCpep-3-1E-CWA separated by 12% SDS-PAGE was transferred to nitrocellulose membranes, probed with Rabbit anti-3-1E polyclonal antibodies (Ma et al., Citation2011). The target band corresponding to DCpep-3-1E-CWA (55 kDa) was observed. M. Protein Marker; Lane 1, DCpep-3-1E-CWA fusion protein from nisin-induced L. lactis NZ9000/pTX8048-SP-DCpep-3-1E-CWA; Lane 2, cell wall protein from nisin-induced L. lactis NZ9000/pTX8048 (negative control).
DCpep-3-1E induced humoral immune responses
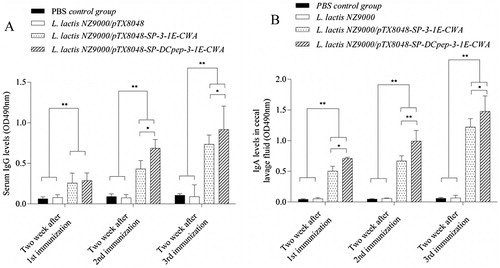
The antibody response induced by DCpep-3-1E protein delivered by L. lactis was determined by the indirect ELISA method. As shown in (A,B), at 2 weeks post each immunization, the induced sera IgG levels, secreted IgA (sIgA) levels in caecal lavage fluid from L. lactis NZ9000/pTX8048-SP-DCpep-3-1E-CWA immunized group were, respectively, significantly higher and higher as compared to PBS control group and L. lactis NZ9000/pTX8048 group (p < 0.01), and L. lactis NZ9000/pTX8048-SP-3-1E-CWA group (p < 0.05). The results indicated that DCpep significantly promoted antibody response in chickens.
Figure 3. The levels of IgG in serum (A) and secreted IgA (sIgA) in caecal lavage fluid (B) from immunized chickens in each group. Chickens in each group were orally immunized with L. lactis NZ9000/pTX8048-SP-DCpep-3-1E-CWA, L. lactis NZ9000/pTX8048-SP-3-1E-CWA, L. lactis NZ9000/pTX8048, and PBS (pH 7.2), respectively. In the stage of immunization, chickens in both unchallenged control group and challenged control group were sham-inoculated orally with PBS (pH 7.2), therefore the two groups were merged and called as PBS control group. Each with a 100 μl volume containing 1 × 1010 CFU live recombinant bacteria for three consecutive days at 2-week intervals for a total of three immunizations. The sera and caecal lavage fluid were prepared from five chickens in each group after each immunization. 3-1E specific serum IgG and secreted IgA (sIgA) levels in caecal lavage fluid were detected by indirect ELISA. The values represent mean ± SD (n = 5). *p < 0.05, **p < 0.01.
DCpep-3-1E induced cellular immune responses
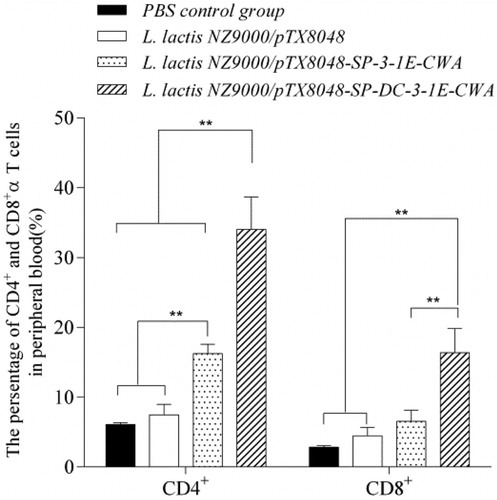
As shown in , at 2 weeks post-tertiary oral immunization, the proportions of CD4+ and CD8α+T cells in peripheral blood from chickens in the group immunized with L. lactis NZ9000/pTX8048-SP-DCpep-3-1E-CWA was significantly higher than those from the groups immunized with PBS, L. lactis NZ9000 and L. lactis NZ9000/pTX8048-SP-3-1E-CWA group (p < 0.01). The proportions of CD4+T cells in L. lactis NZ9000/pTX8048-SP-3-1E-CWA group were significantly higher than those from PBS and L. lactis NZ9000 group (p < 0.01). No significant difference was observed between PBS control group and L. lactis NZ9000 group (p > 0.05). The results indicated that DCpep effectively enhanced the T lymphocyte responses in chickens.
Figure 4. The proportion of CD4+ and CD8α+ T cells in peripheral blood from chickens in each group post-tertiary immunization. Chickens in each group were orally immunized with L. lactis NZ9000/pTX8048-SP-DCpep-3-1E-CWA, L. lactis NZ9000/pTX8048-SP-3-1E-CWA, L. lactis NZ9000/pTX8048, and PBS (pH 7.2), respectively. In the stage of immunization, chickens in both unchallenged control group and challenged control group were sham-inoculated orally with PBS (pH 7.2), therefore the two groups were merged and called as PBS control group. At 2 weeks post-tertiary oral immunization, lymphocytes suspension was, respectively, prepared from peripheral blood of five chickens in each group after three immunizations. The isolated lymphocytes were incubated with fluorescein-isothiocyanate (FITC)-conjugated mouse anti-chicken CD4+ antibody (0.5 mg/ml) and phycoerythrin (PE)-conjugated mouse anti-chicken CD8α+ antibody (0.5 mg/ml) (Southern Biotech). The proportion of T lymphocytes subtypes was measured by flow cytometry (Epics X MCL, Beckman Coulter). The values represent mean ± SD (n = 5). *p < 0.05, **p < 0.01.
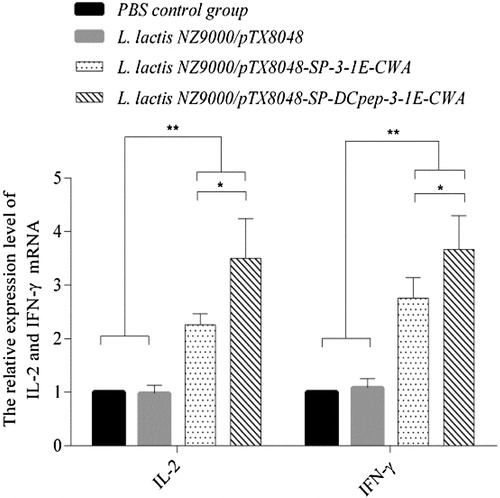
At 2 weeks post-tertiary oral immunization, the mRNA levels of ChIL-2 and ChIFN-γ from chickens in the groups immunized with 3-1E-expressing and DCpep-3-1E-expressing lactococci were highly significantly higher than those from PBS control group and L. lactis NZ9000 group (p < 0.01). The mRNA levels of ChIL-2 and ChIFN-γ in DCpep-3-1E-expressing lactococci group were higher as compared to that in the 3-1E-expressing lactococci group (p < 0.05) (). The results demonstrated that the introduction of DCpep in recombinant lactococci effectively promoted the production of cytokines in chickens.
Figure 5. The mRNA expression levels of ChIFN-γ and ChIL-2 in the spleen of chickens in each group post-tertiary immunization. Chickens in each group were orally immunized with L. lactis NZ9000/pTX8048-SP-DCpep-3-1E-CWA, L. lactis NZ9000/pTX8048-SP-3-1E-CWA, L. lactis NZ9000/pTX8048, and PBS (pH 7.2), respectively. In the stage of immunization, chickens in both unchallenged control group and challenged control group were sham-inoculated orally with PBS (pH 7.2), therefore the two groups were merged and called as PBS control group. At 2 weeks post-tertiary oral immunization, the mRNA expression levels of ChIFN-γ and ChIL-2 in the spleen of five chickens in each group was detected by real-time PCR. The mRNA levels of individual chicken in each group were divided by mRNA levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of the same chicken to normalize the relative mRNA levels of ChIL-2 and ChIFN-γ. Data are expressed as mean ± SD (n = 5). *p < 0.05, **p < 0.01.
Evaluation of immune protection
Oral immunization with DCpep-3-1E-expressing and 3-1E-expressing lactococci improved the body weight gain (BWG), alleviated lesion scores and decreased oocyst decrease ratio as compared to L. lactis NZ9000/pTX8048 group and PBS control group (p < 0.01) (, (A,B)). Although average lesion scores in caeca of chickens immunized with DCpep-3-1E-expressing lactococci were relatively slight compared with 3-1E-expressing lactococci, statistical differences in BWG and oocyst decrease ratio were not observed between the two groups.
Figure 6. Immune efficacies by oral immunization with live recombinant L. lactis NZ9000 expressing DCpep-3-1E fusion protein. Chickens in each group were orally inoculated with L. lactis NZ9000/pTX8048, L. lactis NZ9000/pTX8048-SP-3-1E-CWA and L. lactis NZ9000/pTX8048-SP-DCpep-3-1E-CWA, each with a 100 μl volume containing 1 × 1010 CFU recombinant bacteria for three consecutive days at 2-week intervals for a total of three immunizations. Chickens in unchallenged control group and challenged control group were both orally fed with 100 μl PBS (pH 7.2). At 54 days of age, all chickens except in the unchallenged control group were orally challenged with 2 × 104 E. tenella sporulated oocysts. Ten chickens from each group were selected to assess lesions in caeca on day 7 post infection (PI) according to the reported method (Johnson & Reid,Citation1970). (A). Faeces from ten chickens in each group were respectively collected between days 7 and 11 PI to count oocyst shedding per chicken. Calculation of oocyst decrease ratio was calculated as follows: (the number of oocysts from challenged control chickens − vaccinated chickens)/challenged control chickens ×100%. (B). Data are expressed as mean ± SD. *p < 0.05, **p < 0.01.
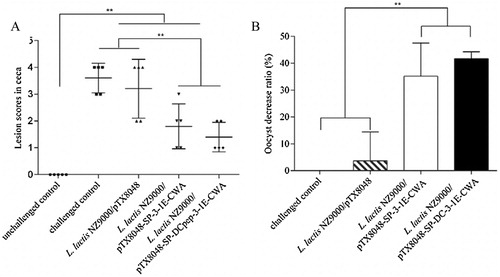
Table 3. Evaluation of protective effects against coccidiosis after immunization with recombinant live E. faecalis expressing target protein.
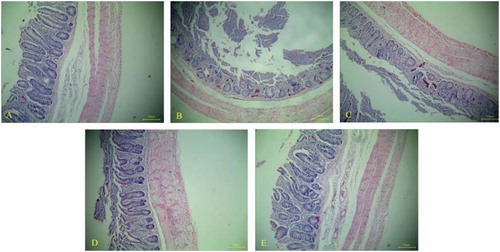
The sections of caeca from chickens in the unchallenged control group showed no obvious histopathological changes ((A)). For caeca sections on day 7 PI, the intestinal villi were severely compromised, and a large number of red cells and infiltrated inflammatory cells were observed in the challenged control group and L. lactis NZ9000 group ((B,C)). In contrast, although infiltrated inflammatory cells in the mucous layer and even in the muscular layer were observed, the histopathological changes in caeca sections from DCpep-3-1E-expressing and 3-1E-expressing lactococci group were both relatively moderate ((D,E)).
Figure 7. Histopathological lesions in caecum of chickens in each group on day 7 post challenge, stained with haematoxylin and eosin (HE). Caeca sections from chickens in unchallenged control group showing no obvious histopathological changes, and intestinal villi were regularly arranged (A, ×100). For caecum tissues in challenged control group (B, ×100) and vector control group (C, ×100), the structure of intestinal villi were broken, and a large number of red cells and infiltrated inflamed cells were observed. The histopathological changes in caecum of chickens orally treated with 3-1E-expressing (D, ×100) and DCpep-3-1E-expressing (E, ×100) lactococci were both relatively moderate.
Discussion
Avian coccidiosis, a kind of widespread intestinal infectious diseases caused by protozoan Eimeria leads to immense economic losses to the world poultry industry (Blake, Pastor-Fernandez, Nolan, & Tomley, Citation2017; Dalloul & Lillehoj, Citation2006). Currently, the dominated control methods including anticoccidial drugs and live vaccines gradually displayed drawbacks reflected by drug-resistant Eimeria species, drug residues in poultry products and the reversion to virulence for attenuated vaccines. The exploration of novel vaccines has become research hot point, and several delivery tools have been used to deliver target Eimeria proteins, including Escherichia coli (Yin et al., Citation2015), Bacillus subtilis (Lin et al., Citation2015), Mycobacterium bovis Bacillus Calmette-Guerin (Wang et al., Citation2014), attenuated Salmonella enterica serovar Typhimurium (Konjufca, Jenkins, Wang, Juarez-Rodriguez, & Curtiss, Citation2008), Pichia pastoris (Chen et al., Citation2015), fowlpox virus (Yang et al., Citation2008), tobacco (Sathish et al., Citation2011), nanoparticles (Jenkins et al., Citation2018; Zhang et al., Citation2012), Lactobacillus plantarum (Yang et al., Citation2017), Lactococcus lactis (Li et al., Citation2018; Ma et al., Citation2013; Ma et al., Citation2017), recombinant Eimeria (Tang et al., Citation2019). Based on our previous study using lactic acid bacteria as a delivery tool, we postulate that the immune responses and efficacies provided by oral immunization of recombinant lactococci could be improved by introducing immune enhancers into the live recombinant bacteria. To verify this hypothesis, we successfully constructed recombinant L. lactis NZ9000 co-expressing dendritic cell-targeting peptide (DCpep) and E. tenella 3-1E on the surface of bacteria in the present study.
The finding from the animal experiment showed that cell surface display of DCpep-3-1E in recombinant lactococci efficiently elicited more stronger 3-1E specific antibody response and cell-mediated immunity (CMI) compared to 3-1E-expressing lactococci. This enhanced immune responses could be explained by the fact that display of DCpep on the surface of recombinant live L. lactis NZ9000 effectively interact to its target receptor on DCs surface, and then activate the related signal pathway to secret important cytokines that modulated systemic immune responses (Curiel et al., Citation2004; Subramaniam et al., Citation2017).
Several related reports demonstrated that CMI, as well as humoral immunity, plays a key role in resisting coccidial infection (Lillehoj, Citation1998; Wallach, Citation2010). So we expected to observe that the enhanced immune responses in the group immunized with DCpep-3-1E-expressing lactococci accordingly afforded more protection against homologous Eimeria infection, as demonstrated by lower average lesion scores in caeca, increased body weight gain (BWG), decreased oocyst decrease ratio, compared to the group immunized with 3-1E-expressing lactococci. However, although chickens immunized with the two 3-1E-expressing lactococci displayed higher BWG, lower average lesion scores in caeca and higher oocyst decrease ratio compared to chickens in the challenged control group and L. lactis NZ9000 group, the statistical differences between the two 3-1E-expressing lactococci immunized groups were not observed. The explanation of these results could reside in the fact that the enhanced cellular and humoral immune responses elicited by the cell surface-displayed DCpep-3-1E were not as strong as that induced by other traditional vaccines enough to influence the general pathological change in intestine and BWG. This result is in accordance with our previous report (Li et al., Citation2018; Ma et al., Citation2017). In our subsequent study, more immunizing dose of live bacteria would be explored to acquire more obvious immune protections. The present study suggested that live lactococci co-expressing target antigen and other immune enhancers may be a promising approach to induce more significant immunity and immune efficacy against pathogen infection.
Oral vaccines using lactic acid bacteria as delivery tools are much safer, less expensive and more convenient, but the bottleneck of improving target protein expression levels in the live recombinant bacteria was not broke through till now, which seriously hindered its application. Therefore, the introduction of immune enhancer into live recombinant bacteria to increase the immune recognition between surface-displayed protein and immune cells, and further effectively activated immune cells to elicit stronger immune response and efficacy would be further explored in the subsequent research work.
Conclusion
Oral immunization to chickens with recombinant L. lactis NZ9000 co-expressing dendritic cell-targeting peptide (DCpep) and E. tenella 3-1E on the surface of bacteria elicited higher systemic immune responses. The present work demonstrated that co-expressing of target antigen and immune enhancers may be a promising approach to enhance immune responses and efficacy against pathogen infection.
Compliance with ethical standards
Animal experiments were performed according to the regulations of the Animal Experiment Ethics Committee of Northeast Agricultural University, Harbin, Heilongjiang Province, China.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Blake, D. P., Pastor-Fernandez, I., Nolan, M. J., & Tomley, F. M. (2017). Recombinant anticoccidial vaccines – a cup half full? Infection Genetics and Evolution, 55, 358–365. doi: 10.1016/j.meegid.2017.10.009
- Blake, D. P., & Tomley, F. M. (2014). Securing poultry production from the ever-present Eimeria challenge. Trends in Parasitology, 30, 12–19. doi: 10.1016/j.pt.2013.10.003
- Chen, P. P., Lv, J. F., Zhang, J., Sun, H., Chen, Z. T., Li, H. M., … Zhao, X. M. (2015). Evaluation of immune protective efficacies of Eimeria tenella EtMic1 polypeptides with different domain recombination displayed on yeast surface. Experimental Parasitology, 155, 1–7. doi: 10.1016/j.exppara.2015.04.020
- Chirdo, F. G., Millington, O. R., Beacock-Sharp, H., & Mowat, A. M. (2005). Immunomodulatory dendritic cells in intestinal lamina propria. European Journal of Immunology, 35, 1831–1840. doi: 10.1002/eji.200425882
- Curiel, T. J., Morris, C., Brumlik, M., Landry, S. J., Finstad, K., Nelson, A., … Mohamadzadeh, M. (2004). Peptides identified through phage display direct immunogenic antigen to dendritic cells. Journal of Immunology, 172, 7425–7431. doi: 10.4049/jimmunol.172.12.7425
- Dalloul, R. A., & Lillehoj, H. S. (2006). Poultry coccidiosis: Recent advancements in control measures and vaccine development. Expert Review of Vaccines, 5, 143–163. doi: 10.1586/14760584.5.1.143
- Douillard, F. P., Mahony, J., Campanacci, V., Cambillau, C., & van Sinderen, D. (2011). Construction of two Lactococcus lactis expression vectors combining the gateway and the nisin controlled expression systems. Plasmid, 66, 129–135. doi: 10.1016/j.plasmid.2011.07.001
- Jenkins, M. C., Stevens, L., O'Brien, C., Parker, C., Miska, K., & Konjufca, V. (2018). Incorporation of a recombinant Eimeria maxima IMP1 antigen into nanoparticles confers protective immunity against E. Maxima challenge infection. Vaccine, 36, 1126–1131. doi: 10.1016/j.vaccine.2017.11.014
- Jiang, L. L., Lin, J. J., Han, H. Y., Dong, H., Zhao, Q. P., Zhu, S. H., & Huang, B. (2012). Identification and characterization of Eimeria tenella apical membrane antigen-1 (AMA1). PLoS One, 7, 7. doi: 10.1371/journal.pone.0041115
- Johnson, J., & Reid, W. M. (1970). Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Experimental Parasitology, 28, 30–36. doi: 10.1016/0014-4894(70)90063-9
- Konjufca, V., Jenkins, M., Wang, S. F., Juarez-Rodriguez, M. D., & Curtiss, R. (2008). Immunogenicity of recombinant attenuated Salmonella enterica serovar typhimurium vaccine strains carrying a gene that encodes Eimeria tenella antigen SO7. Infection and Immunity, 76, 5745–5753. doi: 10.1128/iai.00897-08
- Li, J., Wang, F., Ma, C., Huang, Y., Wang, D., & Ma, D. (2018). Recombinant lactococcus lactis expressing Eimeria tenella AMA1 protein and its immunological effects against homologous challenge. Experimental Parasitology, 191, 1–8. doi: 10.1016/j.exppara.2018.05.003
- Lillehoj, H. S. (1998). Role of T lymphocytes and cytokines in coccidiosis. International Journal for Parasitology, 28, 1071–1081. doi: 10.1016/s0020-7519(98)00075-7
- Lillehoj, H. S., & Lillehoj, E. P. (2000). Avian coccidiosis. A review of acquired intestinal immunity and vaccination strategies. Avian Diseases, 44, 408–425. doi: 10.2307/1592556
- Lillehoj, H. S., & Trout, J. M. (1996). Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clinical Microbiology Reviews, 9, 349–360. doi: 10.1128/CMR.9.3.349
- Lin, Z. W., Shi, Y. Y., Deng, B., Mao, X. F., Yu, D. Y., & Li, W. F. (2015). Protective immunity against Eimeria tenella infection in chickens following oral immunization with Bacillus subtilis expressing Eimeria tenella 3-1E protein. Parasitology Research, 114, 3229–3236. doi: 10.1007/s00436-015-4539-3
- Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 25, 402–408. doi: 10.1006/meth.2001.1262
- Ma, D. M., Gao, M. Y., Dalloul, R. A., Ge, J. W., Ma, C. L., & Li, J. (2013). Protective effects of oral immunization with live Lactococcus lactis expressing Eimeria tenella 3-1E protein. Parasitology Research, 112, 4161–4167. doi: 10.1007/s00436-013-3607-9
- Ma, D. X., Ma, C. L., Pan, L., Li, G. X., Yang, J. H., Hong, J. H., … Ren, X. F. (2011). Vaccination of chickens with DNA vaccine encoding Eimeria acervulina 3-1E and chicken IL-15 offers protection against homologous challenge. Experimental Parasitology, 127, 208–214. doi: 10.1016/j.exppara.2010.07.015
- Ma, C. L., Zhang, L. L., Gao, M. Y., & Ma, D. X. (2017). Construction of Lactococcus lactis expressing secreted and anchored Eimeria tenella 3-1E protein and comparison of protective immunity against homologous challenge. Experimental Parasitology, 178, 14–20. doi: 10.1016/j.exppara.2017.05.001
- Mohamadzadeh, M., Duong, T., Sandwick, S. J., Hoover, T., & Klaenhammer, T. R. (2009). Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proceedings of the National Academy of Sciences of the United States of America, 106, 4331–4336. doi: 10.1073/pnas.0900029106
- Rose, M. E., & Hesketh, P. (1979). Immunity to coccidiosis: T-lymphocyte- or B-lymphocyte-deficient animals. Infection Immunity, 26, 630–637. doi: 10.1128/IAI.26.2.630-637.1979
- Sathish, K., Sriraman, R., Subramanian, B. M., Rao, N. H., Balaji, K., Narasu, M. L., & Srinivasan, V. A. (2011). Plant expressed EtMIC2 is an effective immunogen in conferring protection against chicken coccidiosis. Vaccine, 29, 9201–9208. doi: 10.1016/j.vaccine.2011.09.117
- Shirley, M. W., Smith, A. L., & Tomley, F. M. (2005). The biology of avian Eimeria with an emphasis on their control by vaccination. Advances in Parasitology, 60, 285–330. doi: 10.1016/S0065-308X(05)60005-X
- Subramaniam, S., Cao, D. J., Tian, D. B., Cao, Q. M., Overend, C., Yugo, D. M., … Meng, X. J. (2017). Efficient priming of CD4 T cells by Langerin-expressing dendritic cells targeted with porcine epidemic diarrhea virus spike protein domains in pigs. Virus Research, 227, 212–219. doi: 10.1016/j.virusres.2016.10.007
- Tang, X., Wang, C., Liang, L., Hu, D., Zhang, S., Duan, C., … Cui, S. (2019). Co-immunization with two recombinant Eimeria tenella lines expressing immunoprotective antigens of E. Maxima elicits enhanced protection against E. Maxima infection. Parasites & Vectors, 12, 347. doi: 10.1186/s13071-019-3605-6
- Wallach, M. (2010). Role of antibody in immunity and control of chicken coccidiosis. Trends in Parasitology, 26, 382–387. doi: 10.1016/j.pt.2010.04.004
- Wang, Q. Y., Chen, L. F., Li, J. H., Zheng, J., Cai, N., Gong, P. T., … Zhang, X. C. (2014). A novel recombinant BCG vaccine encoding Eimeria tenella rhomboid and chicken IL-2 induces protective immunity against coccidiosis. Korean Journal of Parasitology, 52, 251–256. doi: 10.3347/kjp.2014.52.3.251
- Wang, D., Zhang, Y., Ma, C. L., Ma, D. X., Zhao, Q., Wang, F., … Zhou, E. M. (2018). Live recombinant Lactococcus lactis expressing avian hepatitis virus ORF2 protein: Immunoprotection against homologous virus challenge in chickens. Vaccine, 36, 1108–1115. doi: 10.1016/j.vaccine.2018.01.003
- Yang, G., Li, J., Zhang, X., Zhao, Q., Liu, Q., & Gong, P. (2008). Eimeria tenella: Construction of a recombinant fowlpox virus expressing rhomboid gene and its protective efficacy against homologous infection. Experimental Parasitology, 119, 30–36. doi: 10.1016/j.exppara.2007.12.009
- Yang, G. L., Yao, J. Y., Yang, W. T., Jiang, Y. L., Du, J. F., Huang, H. B., … Wang, C. F. (2017). Construction and immunological evaluation of recombinant Lactobacillus plantarum expressing SO7 of Eimeria tenella fusion DC-targeting peptide. Veterinary Parasitology, 236, 7–13. doi: 10.1016/j.vetpar.2017.01.023
- Yin, G. W., Lin, Q., Qiu, J. H., Qin, M., Tang, X. M., Suo, X., … Liu, X. Y. (2015). Immunogenicity and protective efficacy of an Eimeria vaccine candidate based on Eimeria tenella immune mapped protein 1 and chicken CD40 ligand. Veterinary Parasitology, 210, 19–24. doi: 10.1016/j.vetpar.2015.03.012
- Zhang, D. F., Xu, H., Sun, B. B., Li, J. Q., Zhou, Q. J., Zhang, H. L., & Du, A. F. (2012). Adjuvant effect of ginsenoside-based nanoparticles (ginsomes) on the recombinant vaccine against Eimeria tenella in chickens. Parasitology Research, 110, 2445–2453. doi: 10.1007/s00436-011-2784-7
