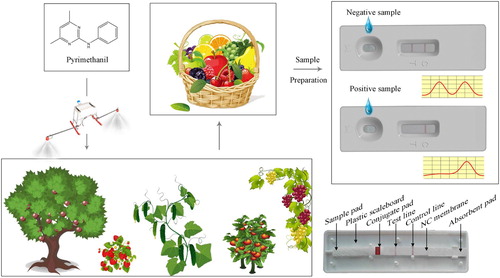
ABSTRACT
Pyrimethanil is one of anilinopyrimidine fungicides widely used in crop protection and frequently found in a variety of crops, including fruits, vegetables, and cereals. This paper describes the development of the first reported immunochromatography test strip (ICTS) based on colloidal gold-labeled monoclonal antibody for the detection of pyrimethanil. Under optimal conditions, the visual cut-off value of ICTS for pyrimethanil was 40 ng/mL. Using a portable strip reader, the 50% inhibition concentration and calculated LOD of pyrimethanil in the PBS samples were 4.8 and 0.9 ng/mL, respectively. When used to analyse fortified samples of fruit and vegetable, acceptable recovery rates of 83.3–105.7% with the coefficient of variation values below 11.7% were obtained. Analysis of blindly fortified samples by the proposed ICTS gave similar results to those of the ic-ELISA and HPLC-MS/MS methods. These results indicate the proposed ICTS is a rapid, simple analytical method for quantitative on-site monitoring of pyrimethanil in foodstuffs.
Introduction
Pyrimethanil () is a chemical fungicide belonging to the anilinopyrimidine family that is highly active against a broad-spectrum of plant pathogenic fungi (Mandrile, Giovannozzi, Durbiano, Martra, & Rossi, Citation2018). Pyrimethanil is employed for fruit, vegetable and ornamental crops against pathogen caused by Venturia inaequalis, Alternaria solani, and/or Botrytis cinerea (Esteve-Turrillas et al., Citation2015; Shim, Abd El-Aty, Choi, & Kang, Citation2007). Since long-term studies have shown certain toxicity and potential carcinogenicity in experimental animal (Fenik, Tankiewicz, & Biziuk, Citation2011; Mandrile et al., Citation2018; Park et al., Citation2013). The maximum residue limits (MRLs) of pyrimethanil for garden crops have been set to 0.2−10 mg/kg in different countries and regions (Liang et al., Citation2013; Mandrile et al., Citation2018). To ensure food safety to protect human health, the development of a simple, rapid, low cost, and high sensitive monitoring method has been required for the analysis of pyrimethanil in foodstuffs.
In order to avoid contaminated foodstuffs moving up the food chain, several methods have been reported for the detection of pyrimethanil in foodstuffs, including surface-enhanced raman scattering (Mandrile et al., Citation2018), liquid chromatography (Shim et al., Citation2007), gas chromatography (Amvrazi & Tsiropoulos, Citation2009), gas chromatography-mass spectrometry (Gonzalez-Rodriguez, Rial-Otero, Cancho-Grande, & Simal-Gandara, Citation2008; Raeppel et al., Citation2011; Rodriguez-Cabo, Rodriguez, Ramil, & Cela, Citation2011), high-performance liquid chromatography (Zhou et al., Citation2011), liquid chromatography technique coupled with tandem mass spectrometry (Ortelli, Edder, & Corvi, Citation2004; Park et al., Citation2013), and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) (Liang et al., Citation2013). These analytical methods are sensitive and accurate, whilst costly equipment, skilled personnel high cost, and unsuitable for on-site detection are their drawbacks. Consequently, rapid immunoassay methods such as ELISA indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) (Cao, Shi, Le, Tang, & Xie, Citation2019; Chen et al., Citation2019; Esteve-Turrillas, Mercader, Agullo, Abad-Somovilla, & Abad-Fuentes, Citation2015; Mercader, Esteve-Turrillas, Agullo, Abad-Somovilla, & Abad-Fuentes, Citation2012) and colloidal gold immunochromatography test strip (ICTS) (Chen et al., Citation2019) have been widely used for the rapid detection of pyrimethanil residue. These methods and their reported detection sensitivities are listed in . However, ic-ELISA still require costly equipment and significant time (3 h) and unsuitable for on-site use, compared with the ICST (10 min).
Table 1. A review of immunoassay methods for the determination of pyrimethanil in recent years.
The ICTS is a screening method used for on-site testing because of its several advantages, such as fast, ease of use, high sensitive, and low cost (Le et al., Citation2011; Le, Yan, Xu, & Hao, Citation2013). Colloidal gold particles are the most widely used as labels in immunoassays because of their unique characteristics, such as high specific surface area, colour, excellent electrical conductivity, and good biocompatibility (Le et al., Citation2013). Moreover, colloidal gold-based ICTS integrated with a portable strip reader could provide a simple, rapid, sensitive testing system for agricultural products monitoring. Although Chen and collaborators have also reported ICTS, which was only suitable for qualitative detection of pyrimethanil, with a cut-off value of 50 ng/mL in cucumber sample. To the best of our knowledge, development of an ICTS for simultaneous qualitative and quantitative detection of pyrimethanil has not been published previously.
In this study, highly specific and sensitive monoclonal antibodies (mAb) were conjugated with colloidal gold particles to create an integrated biorecognition element, colloidal gold-labeled mAb probes. A one-step ICTS with the use of colloidal gold-labeled probes was developed for the detection of pyrimethanil. A portable strip reader was used to record relative optical density (ROD) on the test line (ROD T) and control line (ROD C) for possible on-site quantitative detection of pyrimethanil in food samples within 10 min. The accuracy and reproductivity of the ICTS were verified by comparing this method with the ic-ELISA and HPLC-MS/MS methods.
Materials and methods
Materials and reagents
Pyrimethanil and the employed fungicide standards were purchased from Dr. Ehrenstorfer Corporation (Augsburg, Germany). Fungicide stock solutions were prepared in N,N-dimethylformamide and kept at –20°C in amber glass vials. Chloroauric acid (HAuCl4·3H2O), sodium citrate (C6H5Na3O7·2H2O), and bovine serum albumin (BSA) were purchased from Sigma Corporation (St. Louis, MO). Goat anti-mouse IgG was obtained from Sino-American Biotechnology Corporation (Luoyang, China). Hapten 6-(3-(4,6-dimethylpyrimidin-2-ylamino)phenyl)hexanoic acid (pMm), anti-pyrimethanil monoclonal antibodie (anti-pyrimethanil mAbs), and pMm–BSA were produced in our laboratory (Cao et al., Citation2019). All other chemicals and organic solvents were of analytical grade or higher. Sample pad, glass fibre, absorbent pad, and nitrocellulose (NC) membrane were purchased from Millipore Corporation (Billerica, MA). The water was filtered using a Millipore Milli-Q purification system.
Equipment
The BioDot (Irvine, CA, USA) XYZ Platform combining motion control with a BioJet Quanti3000TM dispenser, an AirJet Quanti3000TM dispenser, and the programmable strip cutter CM4000 were provided by Gene Corporation Limited (ShangHai Branch, China). Colour sensor-based portable strip reader was purchased from Shanghai Jiening Biotechnology Co., Ltd (ShangHai, China). An Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, CA, USA) coupled to a Quattro Premier XE tandem quadrupole mass spectrometer was obtained from Waters Corporation (Manchester, UK).
Preparation of colloidal golds and colloidal gold-labeled mAb probes
The colloidal gold nanoparticles were synthesized via citrate reduction of HAuCl4·3H2O according to a reported method with slight modifications (Le et al., Citation2012). Briefly, 100 mL of 0.01% (w/v) HAuCl4·3H2O solution was brought to a boil and then added a solution of 1% sodium citrate (2 mL). The reaction is determined to reach completion when the solution colour changes from clear to a brilliant wine red. After cooling at room temperature, the colloidal gold solution was then purified by centrifugation at 6000 rpm for 15 min and then redispersed in ultrapure water. The core nanoparticle diameters were characterized by transmission electron microscope (TEM) with FEI Tecnai G20 (Hillsboro, OR, USA). Finally, the obtained colloidal golds were supplemented with 0.05% (m/v) of sodium azide and stored at 4°C for further use.
The colloidal gold-labeled mAb probes were prepared by conjugating colloidal gold with anti-pyrimethanil mAbs as previously described with slight modifications (Le et al., Citation2013). Briefly, the anti-pyrimethanil mAb was purified by the caprylic acid-ammonium sulphate method and then by the protein G immunoaffinity column (Le et al., Citation2011). Anti-pyrimethanil mAb (2 μg/mL, 1 mL) was added dropwise to 50 mL of colloidal gold solution (pH 7.5). The pH of the colloidal gold solution was adjusted with 0.1M K2CO3. The mixture was stirred vigorously for 30 min. Then, 5 mL 10% (w/v) BSA aqueous solution was added dropwise into the mixture, and then magnetic stirred at room temperature for 30 min. The resulting colloidal gold-labeled mAb probes were centrifuged twice at 12,000 rpm for 30 min at 4°C each. Subsequently, the probes were resuspended with blocking buffer (0.01 M PBS (pH 7.4) containing 2% BSA, 2.5% sucrose, 3% PVP K30, 2% BSA, 0.5%Triton-X and 0.02% NaN3). The colloidal gold solution and colloidal gold-labeled probes were characterized with UV-vis spectra between 400 and 650 nm.
Preparation of the ICTS
The pyrimethanil competitive ICTS consisted of a sample pad, conjugate pad, NC membrane containing the test and control lines, and absorbent pad. The sample pad was previously treated with blocking buffer (0.01 M PBS (pH 7.4) containing 2% BSA, 2.5% sucrose, 3% PVP K30, 2% BSA, 0.5%Triton-X and 0.02% NaN3) and then dried at 37°C before use. Subsequently, the colloidal gold-labeled mAb probes were dispensed onto the conjugate pad at a speed of 6 μL/cm using an AirJet Quanti3000TM, and then dried for 2 h at 37°C. With an AirJet Quanti 3000 dispenser, the test line and control line of the NC membrane were dispensed with 0.25 mg/mL of pMm–BSA conjugate at 0.6 μL/cm and 0.5 mg/mL of goat anti-mouse IgG at 0.4 μL/cm, respectively, and then dried at 37°C for 2 h. A distance of 4 mm was chosen between the test line and control line. Subsequently, the sample pad, conjugate pad, blotted NC membrane, and absorbent pad were laminated and pasted to a plastic scaleboard. Then, the whole assembled scaleboard was cut into 3 mm-wide strips using a programmable strip cutter CM4000. Finally, these assembled strips were mounted in plastic cassettes and stored with desiccator at room temperature until use.
Principle of the test
The principle of the test was illustrated in . One hundred microliters of pyrimethanil standard solution or diluted samples solution were applied onto the sample pad. The solution was migrating along the conjugate pad into the NC membrane through capillary action. The specific colloidal gold-labeled probes, which were solubilized from the conjugate pad by redissolving in the sample solution, reacted with pyrimethanil (if it was present in the sample). When a detectable level of pyrimethanil was present in the sample, the free pyrimethanil bound to the gold-labeled mAb probes, preventing the probes binding to the pyrimethanil-BSA in the test line. Therefore, the more the levels of pyrimethanil present in the sample, the more shallow the test line coloured. If no pyrimethanil is present, the limited amounts of colloidal-gold-labeled mAb probes would be trapped by the immobilized pMm–BSA conjugate, and then a visible red test line would appear. If no control line was present, the test was considered to be invalid. After 10 min, the results could be directly observed with the naked eyes. The relative optical densities (RODs) on the test line (RODT) and control line (RODC) were recorded using a portable strip reader.
Sample preparation
The grape, strawberry, peach, tomato, apple, and cucumber crops were purchased from local markets (Chongqing, China), and were confirmed by HPLC-MS/MS analysis to be free of pyrimethanil. The modified QuEChERS method was employed for the extraction and purification of fungicide residues from food samples (Cao et al., Citation2019; Esteve-Turrillas et al., Citation2015; Lehotay, Citation2007). The sample pre-treatment and subsequent ic-ELISA and HPLC-MS/MS analysis were carried out, as reported previoudly (Cao et al., Citation2019; Esteve-Turrillas et al., Citation2015).
Evaluation of the ICTS performance
For qualitative evaluation, the visual cut-off level of ICTS was defined as the minimum concentration of analyte when the colour on the test line has disappeared (Kong et al., Citation2017; Xie, Zhang, & Le, Citation2017).
The quantitative analysis was performed by measuring the RODs of the test line and control line with the use of strip reader. The standard solutions were prepared by spiking pyrimethanil stock solutions to PBS (pH 7.4). The final concentrations of pyrimethanil in the standard solution were 0, 0.625, 1.25, 2.5, 5, 10, 20, 40, and 80 ng/mL. RODT and RODC, as well as the RODT/RODC ratio, were measured by the portable strip reader, and then calculated by software. The RODT/RODC ratio was used to offset the background noise. The inhibition curves were constructed by plotting B/B0 × 100% against the logarithm of the pyrimethanil concentration, where B and B0 designated the RODT/RODC values of the pyrimethanil positive sample and the negative control, respectively.
The 50% inhibition concentration (IC50) was calculated (Xie et al., Citation2017). The determination of calculated LOD (cLOD) was based on the pyrimethanil concentration that generated a competitive inhibition rate of 10% (IC10).
The specificity of the ICTS was investigated by testing two other anilinopyrimidine fungicides (cyprodinil and mepanipyrim) and six widely used fungicides in fruit and vegetables (kresoxim-methyl, trifloxystrobin, pyraclostrobin, azoxystrobin, dimoxystrobin, and fluoxastrobin). The standard solutions of the pyrimethanil and other fungicides were prepared at various concentrations in the range 40–500 ng/mL, which were used to evaluate the specificity of the ICTS. The specificity was assessed based on the colour on the test line of every strip.
Accuracy and reproductivity of the ICTS
The accuracy and reproductivity of the ICTS were evaluated by analysing the recovery and coefficient of variation (CV) of the intra- and inter-assay. The samples were fortified with pyrimethanil at a concentration of 5, 10, and 50 μg/kg. After pre-treatment of the fortified samples, the extraction solutions were detected by ICTS. The recovery (%) was calculated by the following equation: (conc. measured/conc. fortified) × 100%. The intra-assay was performed on the same day with five replicates at each fortified level, whereas the inter-assay was performed on three consecutive days with five replicates for each fortified level.
Comparison of the ICTS with ic-ELISA and HPLC-MS/MS
To demonstrate the applicability of the ICTS, the blindly fortified samples were analysed for pyrimethanil residues using the ICTS, ic-ELISA, and HPLC-MS/MS, and the measured results were compared. The thirty fruit and vegetable samples, including five grape samples, five strawberry samples, five peach samples, five tomato samples, five apple samples, and five cucumber samples were purchased from local markets. These samples were blindly fortified with pyrimethanil at different concentration levels ranging from 5 μg/kg to 50 μg/kg. A side-by-side comparison between the ICTS, ic-ELISA and HPLC-MS/MS was conducted using the same fortified sample.
Results and discussion
Construction of the colloidal gold-labeled mAb probe
Colloidal gold particle provides suitable optical, magnetic, electronic, and bonding properties for the development of biosensor assay. In this study, colloidal gold particles were prepared by a chemical method using the reduction of HAuCl4·3H2O. The gold ions were directly reduced to gold atoms by the sodium citrate, and many of the gold atoms immediately accumulated into colloidal gold solution. As shown in Supplementary Figure 1S(a), the TEM image analysis revealed that the colloidal gold particles were homogeneous in size distribution ranging 22.6–26.3 nm with a mean diameter of about 24.2 ± 2.6 nm. No agglomeration of colloidal gold particles was detected, indicating that the colloidal gold particles were stable in solution. The UV–vis spectra were used to characterize the properties of the absorption spectra of free colloidal gold particle and colloidal gold-labeled mAb probes (Supplementary Figure 1 (b)). The colloidal gold particles exhibit a UV absorption at 519 nm. For colloidal golds-labeled mAb probes, the absorb peak shifted from 519 to 527 nm. The surface plasmon band of the colloidal gold-labeled mAb probe was distinctly broadened and red shifted by about 8 nm compared to colloidal gold particle. Along with the antibody conjugated on the colloidal gold surface, its size increased and the absorb peak shifted. These results confirmed that the anti-pyrimethanil mAbs were successfully coupled on the colloidal gold surface.
To have strong adsorption between the gold and antibody conjugates, a preliminary titration for conjugation between colloidal gold and antibody was performed. Optimal conditions of pH and mAb concentration for the probes could be determined by comparing the absorption in the wavelength range of 520 to 580 nm (A520–A580). Gold colloid solutions adjusted to pH range of 5.5–9.0 were pipetted into a series of tubes. Anti-pyrimethanil mAb solution (0.25–2 μg/mL, 1 mL) was added to each colloidal gold solution diluted in a series of concentrations. Each tube received 1 mL of 10% NaCl and was shaken for 5 min. Absorption of each tube at 520 and 580 nm was determined 10 min later. The optimum pH of the gold-labeled antibody solution was determined to be 7.5, as shown in Supplementary Figure 2S(a). At this pH, 1.25 μg/mL of anti-pyrimethanil mAb was confirmed to be the minimal concentration for stabilizing colloidal gold solution (Supplementary Figure 2S (b)). To ensure a sufficiently high mAb concentration stability and conjugation with colloidal gold particle, 1.5 μg/mL of anti-pyrimethanil mAb (increasing 20% of mAb based on 1.25 μg/mL) was used for the probes.
Optimization of the ICTS
In general, the analytical performance of the ICTS is mainly affected by many parameters, such as the antibody sensitivity, amounts of probes, coating antigen, NC membrane, concentration of secondary antibody (goat anti-mouse IgG), blocking buffer, and immunoreagent amount. This paper focuses on evaluating the effect of the blocking buffer, coating antigen, and immunoreagent amount.
Blocking buffers for the sample pads and conjugate pads were evaluated to study its effect on mAbs and analytes. In this study, the effects of blocking solution on the release speed of probe, the reaction time of the test strip, and the background colour of the NC membrane were investigated. The optimum blacking buffer was one that produced a rapid release speed, a short reaction time and a light-pink background colour. According to the specificity, sensitivity, reaction speed, and background colour of the NC membrane after the reaction, 0.01 M PBS (pH 7.4) containing 2.5% sucrose, 2% BSA, 0.5%Triton-X, 3% PVP K30, and 0.02% NaN3 was chosen as the optimal blocking buffer for the sample and conjugate pads (Supplementary Table 1S).
In order to improve the sensitivity of the ICTS, the effects of immobilization concentration and incorporation rate of coating antigen (pMm–BSA) were optimized using the checkerboard test. The strips were optimized with negative (0 ng/mL) and positive samples (40 ng/mL of pyrimethanil) using different experimental conditions. As deduced from , when the incorporation rate of coating antigen (pMm–BSA) for test line was 16:1, the negative and positive colour contrasts were more obvious. Meanwhile, the linear shape of test line was more uniform. We also discovered that the colour intensity of negative and positive contrast of the test line was the most obvious, the colour intensity on test line 3 (T3) was strongest when the coating concentration was 0.25 mg/mL. Therefore, the optimal concentration and incorporation rate of pMm–BSA on the test line were 0.5 mg/mL and 16:1, respectively. The immunoreagent concentrations of ICTS were evaluated as the above procedure. Finally, the optimum combination of 6 μL/cm of colloidal gold-labeled mAb probe, 0.6 μL/cm of pMm–BSA (0.25 mg/mL), and 0.4 μL/cm of anti-mouse IgG (0.5 mg/mL) were dispensed on the test strip. Under these optimized conditions, the ICTS showed a distinctive test line and good sensitivity.
Figure 2. The effect of immobilization concentration and incorporation rates of coating antigen. Concentration of coating antigen (pMm–BSA) on the T1, T2, T3 and T4 were 1, 0.5, 0.25 and 0.125 mg/mL, respectively. Incorporation rates of pMm–BSA were 8:1, 16:1 and 32:1. The standard concentration: negative (0 ng/mL); positive samples (40 ng/mL).

Properties of the ICTS
The properties of the ICTS were determined by analysing pyrimethanil standards. The pyrimethanil standards were diluted in 0.1 M PBS (pH 7.4) at concentrations of 0.625–80 ng/mL. As shown in , when the concentration of pyrimethanil was 10 ng/mL, the colour intensity of the test line was similar or weaker than that of the test line for the negative sample. When the concentration of pyrimethanil was higher than 40 ng/mL, the red line disappeared and only the control line showed red band. Therefore, the visual cut-off value of the ICTS for pyrimethanil was 40 ng/mL.
Figure 3. Series of pyrimethanil standard samples tested by developed ICTS and visual results examined after 10 min. The standard solutions of pyrimethanil at each final concentration of 0, 0.625, 1.25, 2.5, 5, 10, 20, 40, and 80 ng/mL in sample (0.1 M PBS, pH 7.4).

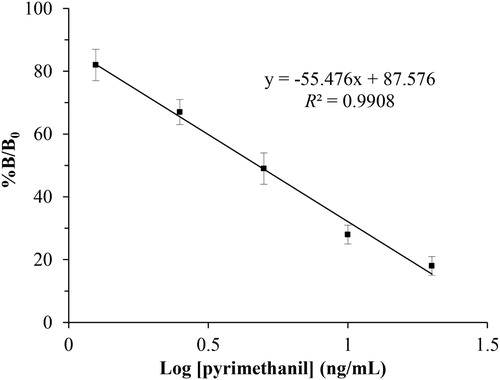
To establish ICTS method for the quantitative determination of pyrimethanil, the ROD of the test and control lines were scanned by a portable strip reader. Under optimal experimental conditions, the standard curve for the quantitative detection of pyrimethanil was constructed by plotting B/B0 × 100% against the concentration (log C). As shown in , the standard curve exhibited a good linear range from 1.25 ng/mL to 20 ng/mL, with an acceptable correlation coefficient (R2 = 0.9908). The IC50 for pyrimethanil in PBS sample was 4.8 ng/mL and the cLOD was 0.9 ng/mL. In addition, the matrix effects are one of the most common challenges in performing immunoassays on complex samples. In this study, to evaluate the influence of the matrix on the proposed method, a variety of fruits and vegetables were extracted and purificated by modified QuEChERS method. The matrix effects of fruits and vegetables were preliminarily investigated. The results demonstrated that the matrix has no significant effect on the sensitivity of the ICTS followed by the extraction and purification method using a modified QuEChERS method.
Figure 4. Standard curve of the developed ICTS for pyrimethanil quantitation in PBS (n = 5). Competitive inhibition rate was defined as B/B0 × 100%, where B0 and B represent the RODT/RODC values of the sample with/without pyrimethanil, respectively. Typical calibration curves of the ICTS by the portable strip reader with increasing pyrimethanil concentrations, from top to bottom: 0, 0.625, 1.25, 2.5, 5, 10, 20, 40, and 80 ng/mL, respectively. Good linearity of the calibration curve was achieved for pyrimethanil in the range of 1.25–20 ng/mL.
The specificity of the ICTS was investigated by testing two other anilinopyrimidine fungicides and six widely used fungicides in fruit and vegetable. As shown in Supplementary Figure 3S, 500 ng/mL of above-mentioned each fungicide standared solution was selected for the specific asssay, and the pink bands of test line were obvious. For the sample of pyrimethanil standared solution (40 ng/mL), the pink band of test line was not observed (Supplementary Figure 3S). The developed ICTS for pyrimethanil detection exhibited negligible cross-reactivity with the eight fungicides; this finding was also consistent with our previous results using ic-ELISA method (Cao et al., Citation2019).
Accuracy and reproductivity of the ICTS
To further evaluate the accuracy and reproductivity of the proposed ICTS for sample analysis, six collected samples (grape, strawberry, peach, tomato, apple, and cucumber) were fortified with pyrimethanil at each final concentration of 5, 10, and 50 μg/kg. Samples were investigated using the developed ICTS. The recoveries and reproductivity were calculated and summarized in . Satisfactory recoveries were obtained in the range from 83.3% to 105.7%, and CVs (intra-assay and inter-assay) ranged from 6.3% to 11.7%. These results indicated that the proposed method could be satisfactorily applied to the determination of pyrimethanil residue in fruits and vegetables.
Table 2. Accuracy and reproductivity of the ICTS in pyrimethanil-fortified samples.
Comparison of ICTS with ic-ELISA and HPLC-MS/MS
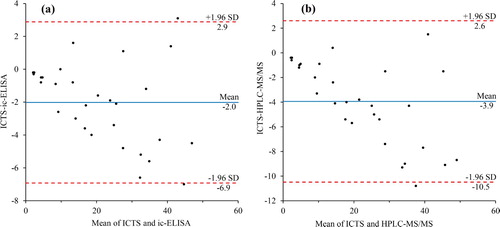
Thirty food samples blindly fortified with pyrimethanil were analysed by the ICTS, ic-ELISA, and HPLC-MS/MS. Bland-Altman plot for pyrimethanil measured by ICTS and ic-ELISA was shown in (a). The results showed that the mean bias was – 2.0 μg/kg, and the 95% limits of agreement defined as the mean ± 1.96 SD were between – 6.9 and 2.9 μg/kg. Similarly, Bland-Altman plot for the ICTS and HPLC-MS/MS appears in (b), the mean bias ± 1.96 SD were between – 10.5 and 2.6 μg/kg. It is evident that results from the ICTS and ic-ELISA and results from the ICTS and HPLC-MS/MS were not significantly different (significant level α = 0.05). As shown by a side-by-side comparison, there was an excellent correlation between ICTS and ic-ELISA (y = 1.0791x + 0.5858, R² = 0.9807) and ICTS and HPLC-MS/MS (y = 1.1339x + 1.2299, R² = 0.9677). Thus, there were no major differences in the quantitative analyses comparison among the three methods, which indicated that the developed ICTS was reliable for the detection of pyrimethanil in fruits and vegetables.
Conclusions
In this study, the colloidal gold-labeled mAb probes were prepared, and an ICTS was developed for quantitative or qualitative analysis pyrimethanil in fruits and vegetables. The whole procedure was simplified, and the assay time was shorten comparing with the ic-ELISA we developed before (Cao et al., Citation2019). For qualitative evaluation, the cut-off values of ICTS for pyrimethanil was 40 ng/mL The cut-off value of the proposed strip was lower than that of strip (cut-off value of 50 ng/mL) in the previous literature (Chen et al., Citation2019). By scanning the ROD of the test line, this ICTS could be quantitatively analysed by the strip reader. Recovery rates of 83.3–105.7% and CVs of 6.3–11.7% demonstrated high accuracy and reproductivity of the ICTS. Moreover, the proposed ICTS showed a good agreement with confirmatory ic-ELISA and HPLC-MS/MS method. In conclusion, the established ICTS method is simple, convenient and suitable for rapid, on-site monitoring of pyrimethanil residues in foodstuffs.
Supplemental Material
Download MS Word (2.5 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Amvrazi, E. G., & Tsiropoulos, N. G. (2009). Application of single-drop microextraction coupled with gas chromatography for the determination of multiclass pesticides in vegetables with nitrogen phosphorus and electron capture detection. Journal of Chromatography A, 1216(14), 2789–2797. doi: 10.1016/j.chroma.2008.07.070
- Cao, Y., Shi, H. X., Le, T., Tang, R., & Xie, Y. (2019). Development a monoclonal antibody based enzyme linked immunosorbent assay for screening pyrimethanil in fruits and vegetables. Food and Agricultural Immunology, 30(1), 548–563. doi: 10.1080/09540105.2019.1608160
- Chen, Z., Wu, X., Xu, L., Liu, L., Kuang, H., & Cui, G. (2019). Development of immunocolloidal strip for rapid detection of pyrimethanil. Food and Agricultural Immunology, 30(1), 1239–1252. doi: 10.1080/09540105.2019.1677566
- Esteve-Turrillas, F. A., Abad-Somovilla, A., Quinones-Reyes, G., Agullo, C., Mercader, J. V., & Abad-Fuentes, A. (2015). Monoclonal antibody-based immunoassays for cyprodinil residue analysis in QuEChERS-based fruit extracts. Food Chemistry, 187, 530–536. doi: 10.1016/j.foodchem.2015.04.119
- Esteve-Turrillas, F. A., Mercader, J. V., Agullo, C., Abad-Somovilla, A., & Abad-Fuentes, A. (2015). Site-heterologous haptens and competitive monoclonal antibody-based immunoassays for pyrimethanil residue analysis in foodstuffs. LWT – Food Science and Technology, 63(1), 604–611. doi: 10.1016/j.lwt.2015.03.074
- Fenik, J., Tankiewicz, M., & Biziuk, M. (2011). Properties and determination of pesticides in fruits and vegetables. TrAC Trends in Analytical Chemistry, 30, 814–826. doi: 10.1016/j.trac.2011.02.008
- Gonzalez-Rodriguez, R. M., Rial-Otero, R., Cancho-Grande, B., & Simal-Gandara, J. (2008). Determination of 23 pesticide residues in leafy vegetables using gas chromatography-ion trap mass spectrometry and analyte protectants. Journal of Chromatography A, 1196-1197, 100–109. doi: 10.1016/j.chroma.2008.02.087
- Kong, D., Xie, Z., Liu, L., Song, S., Kuang, H., & Xu, C. (2017). Development of ic-ELISA and lateral-flow immunochromatographic assay strip for the detection of vancomycin in raw milk and animal feed. Food and Agricultural Immunology, 28(3), 414–426. doi: 10.1080/09540105.2017.1293014
- Le, T., Xu, J., Jia, Y. Y., He, H. Q., Niu, X. D., & Chen, Y. (2012). Development and validation of an immunochromatographic assay for the rapid detection of quinoxaline-2-carboxylic acid, the major metabolite of carbadox in the edible tissues of pigs. Food Additives & Contaminants: Part A, 29(6), 925–934. doi: 10.1080/19440049.2012.662703
- Le, T., Yan, P., Xu, J., & Hao, Y. (2013). A novel colloidal gold-based lateral flow immunoassay for rapid simultaneous detection of cyromazine and melamine in foods of animal origin. Food Chemistry, 138(2-3), 1610–1615. doi: 10.1016/j.foodchem.2012.11.077
- Le, T., Yu, H., Wang, X., Ngom, B., Guo, Y., & Bi, D. (2011). Development and validation of an immunochromatographic test strip for rapid detection of doxycycline residues in swine muscle and liver. Food and Agricultural Immunology, 22(3), 235–246. doi: 10.1080/09540105.2011.556713
- Lehotay, S. J. (2007). Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: Collaborative study. Journal of Aoac International, 90(2), 485–520. doi: 10.1093/jaoac/90.2.485
- Liang, X., Liu, X., Dong, F., Xu, J., Qin, D., Li, Y., … Zheng, Y. (2013). Simultaneous determination of pyrimethanil, cyprodinil, mepanipyrim and its metabolite in fresh and home-processed fruit and vegetables by a QuEChERS method coupled with UPLC-MS/MS. Food Additives & Contaminants: Part A, 30(4), 713–721. doi: 10.1080/19440049.2013.768777
- Mandrile, L., Giovannozzi, A. M., Durbiano, F., Martra, G., & Rossi, A. M. (2018). Rapid and sensitive detection of pyrimethanil residues on pome fruits by surface enhanced Raman scattering. Food Chemistry, 244, 16–24. doi: 10.1016/j.foodchem.2017.10.003
- Mercader, J. V., Esteve-Turrillas, F. A., Agullo, C., Abad-Somovilla, A., & Abad-Fuentes, A. (2012). Antibody generation and immunoassay development in diverse formats for pyrimethanil specific and sensitive analysis. The Analyst, 137(23), 5672–5679. doi: 10.1039/c2an35801h
- Ortelli, D., Edder, P., & Corvi, C. (2004). Multiresidue analysis of 74 pesticides in fruits and vegetables by liquid chromatography-electrospray-tandem mass spectrometry. Analytica Chimica Acta, 520(1–2), 33–45. doi: 10.1016/j.aca.2004.03.037
- Park, J. H., Park, J. S., Abd El-Aty, A. M., Rahman, M. M., Na, T. W., & Shim, J. H. (2013). Analysis of imidacloprid and pyrimethanil in shallot (Allium ascalonicum) grown under greenhouse conditions using tandem mass spectrometry: Establishment of pre-harvest residue limits. Biomedical Chromatography, 27(4), 451–457. doi: 10.1002/bmc.2812
- Raeppel, C., Nief, M., Fabritius, M., Racault, L., Appenzeller, B. M., & Millet, M. (2011). Simultaneous analysis of pesticides from different chemical classes by using a derivatisation step and gas chromatography-mass spectrometry. Journal of Chromatography A, 1218(44), 8123–8129. doi: 10.1016/j.chroma.2011.08.098
- Rodriguez-Cabo, T., Rodriguez, I., Ramil, M., & Cela, R. (2011). Dispersive liquid-liquid microextraction using non-chlorinated, lighter than water solvents for gas chromatography mass spectrometry determination of fungicides in wine. Journal of Chromatography A, 1218(38), 6603–6611. doi: 10.1016/j.chroma.2011.07.054
- Shim, J. H., Abd El-Aty, A. M., Choi, J. H., & Kang, C. A. (2007). Determination of field-incurred pyrimethanil residues in persimmon (Diospyros kaki Linn) by liquid chromatography. Biomedical Chromatography, 21(12), 1279–1283. doi: 10.1002/bmc.884
- Xie, Y., Zhang, L., & Le, T. (2017). An immunochromatography test strip for rapid, quantitative and sensitive detection of furazolidone metabolite, 3-amino-2-oxazolidinone, in animal tissues. Food and Agricultural Immunology, 28(3), 403–413. doi: 10.1080/09540105.2017.1293013
- Zhou, Y. W., Han, L. T., Cheng, J., Guo, F., Zhi, X. R., Hu, H. L., & Chen, G. (2011). Dispersive liquid-liquid microextraction based on the solidification of a floating organic droplet for simultaneous analysis of diethofencarb and pyrimethanil in apple pulp and peel. Analytical and Bioanalytical Chemistry, 399(5), 1901–1906. doi: 10.1007/s00216-010-4567-x