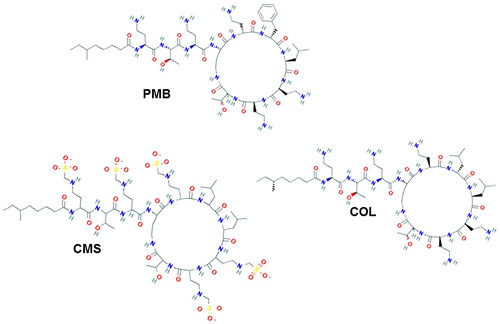
ABSTRACT
Polyclonal antibodies were obtained by immunizing rabbits with polymyxin B (PMB) conjugated to bovine serum albumin. Based on the heterologous coating conjugate of PMB with periodate-oxidized gelatin and the resulting antibodies, an indirect competitive enzyme-linked immunosorbent assay (ELISA) was developed to detect residual colistin (COL), a structural analogue of PMB in livestock products. The developed analysis was characterized by close specificity towards PMB (100%) and COL (88%), sensitivity (IC50 = 5.7 ng/mL), dynamic range (IC20-IC80 = 1.0–80 ng/mL) and the detection limit of COL (IC10 = 0.4 ng/mL). It was possible to quantify the antibiotic in milk and eggs without special and labour-intensive sample pretreatment using a simple 10- and 50-fold dilution of the corresponding samples. The developed analysis system made it possible to record the maximum residue limit of COL in dairy products (50 μg/kg) and eggs (300 μg/kg) with 89–104% recovery of analyte.
KEYWORDS:
Introduction
Polymyxins are decapeptide antibiotics produced by the spore-forming bacteria from Bacillus genus – B. polymyxa, B. circulans, B. colistinus. Structurally, these compounds are cyclic heptapeptides with lipophilic tail. Practically significant antibiotics, polymyxin B (PMB) and colistin (COL) are structurally similar, differ only in the sixth amino acid (D-phenylalanine or D-leucine, respectively), and therefore have a similar antimicrobial effect and cross-resistance mechanism (Velkov, Roberts, Nation, Thompson, & Li, Citation2013). According to the mechanism of antibacterial activity, they belong to membrane cationic surface-active compounds that cause a violation of the permeability of the cell wall and the death of the pathogen. Their lipophilic substituent ensures the penetration of the antibiotic molecule through the outer membrane of the microorganism, and thanks to the five free amino groups, PMs firmly bind to lipid A moiety of the lipopolysaccharides from gram-negative microorganisms (Brown & Dawson, Citation2015; Ryder, Wu, McKelvey, McGuire, & Schilke, Citation2014).
Despite the fact that these antimicrobial agents were introduced into medical practice as early as the 1950s, they had only limited use due to pronounced nephro- and neurotoxicity. However, due to the growth and spread of resistance to most modern antibiotics among gram-negative microorganisms, especially Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae, WHO has included COL in the list of critically important reserve antimicrobials (WHO, Citation2011). Along with medical practice, COL was also actively used to treat mastitis in cows, intestinal infections of farm animals and birds, and as a food supplement to stimulate livestock growth (EMEA, Citation2002). However, the large-scale use of PM in medicine, veterinary medicine and agriculture has provoked the formation of pathogens that are resistant to these drugs, which are currently recorded in more than 30 countries (Baron, Hadjadj, Rolain, & Olaitan, Citation2016).
As measures against the development and spread of antibiotic-resistant pathogenic microflora, the use of COL as a growth stimulant was banned (Walsh & Wu, Citation2016) and its maximum residue limits (MRL) in animal products were established. Thus, in the Russian Federation and EU countries, the residual COL content in meat should not exceed the level of 150 μg/kg, in milk – 50 and 300 μg/kg in eggs (Hygienic Requirements in Respect of the Safety and Nutritional Value of Foodstuffs, Citation2019).
To detect antibacterial compounds in animal products, there are methods including determination of biological activity, i.e. growth inhibition of sensitive test microorganism (Barnard, Citation1984), physicochemical methods based on isolating an analyte from a sample and identifying it by characteristic properties or molecular weight (Boison, Lee, & Matus, Citation2015; Kaufman & Widmer, Citation2013), as well as methods for the specific recognition of the target compound by receptor or receptor-like structures, for example, by antibodies (Oliveri Conti et al., Citation2015).
Microbiological tests are quite lengthy (up to 20 h), unsuitable for analysis a combination of several antibiotics in samples or other natural substances that have antimicrobial effects (organic acids, enzymes, immunoglobulins), and do not always satisfy in sensitivity, not higher than 100–150 μg/kg (Leroy, Decolin, Nicolas, Archimbault, & Nicolas, Citation1989).
Chromatographic methods are capable to identify COL with high sensitivity, but additional derivatization with fluorophores or the use of mass spectrometry is required for detection (Fu et al., Citation2018; Morovjan, Csokan, & Nemeth-Konda, Citation1998). The complexity and high costs of the analysis do not allow using this procedure routinely for large-scale screening.
A limited number of studies have been devoted to the creation of immunoassay of polymyxins (Kitagawa, Ohtani, Maeno, Fujiwara, & Kimura, Citation1985; Suhren & Knappstein, Citation2005; Xu, Burkin, Eremin, Dias, & Zhang, Citation2019), and the methods for determining the antibiotic in meat products, milk, and fish presented in them were characterized by moderate sensitivity with a detection limit of 9–30 ng/mL.
The present work is aimed at developing a sensitive competitive enzyme-linked immunosorbent assay (ELISA) to satisfy the quantitation of COL in dairy products for which the MRL value is the lowest (50 μg/kg), as well as to adapt the assay for measuring the residual COL in eggs, the object for which immunoassay has not yet been described.
Material and methods
Chemicals
The sulphates of PMB and COL (polymyxin E) were obtained from AppliChem (Darmstadt, Germany), goat anti-rabbit IgG labelled with peroxidase was purchased from IMTEK (Moscow, Russia), colistin methanesulphonate (CMS), bovine serum albumin (BSA), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) were the products of Sigma (St Louis, USA), 2,4,6-trinitrobenzyl sulphonic acid (TNBS), ϵ-aminocaproic acid (ACA), and NaBH4 were from Serva (Heidelberg, Germany). Gelatin (Gel) was from Bio-Rad (USA), and skim milk powder (SM) was from Fluka (Switzerland). A two-component substrate solution with 3,3′,5,5′-tetramethylbenzidine (TMB) was provided by Bioservice (Moscow, Russia). The samples of milk, cream and eggs were produced in Russia and purchased from local retail.
Synthesis of conjugated antigens
BSA-PMB×50(edc)
To activate the carboxyl groups of the protein, 25 mg of crystalline EDC (130 μmol) was added to a solution of BSA (4 mg, 60 nmol) in 1.0 mL Н2О and stirred for 40 min on a magnetic stirrer at room temperature. Then, the activated BSA was added to PMB (4.16 mg, 3 μmol) in 0.5 mL of carbonate–bicarbonate buffer pH 9.6 (CBB) and stirred for 2 h at room temperature. Unreacted ingredients were removed during overnight dialysis against 6 L distilled water using dialysis membrane tubes with cutoff 12400 Da Sigma (St. Louis, MO, USA).
Gel-PMB×10-30(edc), Gel-COL×10-50(edc)
The same conjugation procedure was conducted for preparation of Gel-conjugates. Crystalline EDC (80 mg, 416 μmol) was added to Gel solution (16 mg, 4 × 25 nmol) in 2.0 mL Н2О and activated under stirring for 40 min. The solution was divided into four equal portions, which were added to PMB or COL solutions in CBB containing 10- and 50-fold molar excesses of hapten over Gel. The reaction mixtures were stirred at room temperature for 2 h, and then dialyzed against 6 L of distilled water for two days.
Gel(pi)-PMB×10-30, Gel(pi)-COL×10-50
A solution containing Gel (16 mg, 4 × 25 nmol) in 1.4 mL of distilled water was supplemented with sodium periodate (4.3 mg, 20 μmol) up to 2 mL of the final volume and stirred on a magnetic stirrer for 15 min. The oxidized protein was dialyzed overnight against water at 4°C. The volume of dialysate obtained was divided into four portions, to which 10 mg/mL solution of antibiotics in CBB were added. The amounts of PMB was taken as 10- and 30-fold molar excess (350 μg and 1.05 mg), and 10- and 50-fold molar excess of COL (341 μg and 1.71 mg) over the protein carrier was taken, respectively. After 2 h-incubation under stirring on a magnetic stirrer, 100 μL of sodium borohydride solution (2 mg/mL) was added to each reaction mixture and stirred for another 2 h. The resulting conjugates were dialyzed against 6 L of water for 2 days at 4°C.
Evaluation of prepared conjugates
To confirm the formation of a conjugated immunogen, a comparative quantification of the free amino groups in BSA and in BSA conjugated to PMB was performed based on the method described previously (Habeeb, Citation1966). The studied proteins (100 μL) were added to the plate wells in 0.1 M NaHCO3 (pH 8.5), supplemented with 0.1 mM TNBS (50 μL) in the same buffer and incubated for 2h in a thermoshaker ELMI (Latvia) at 37°C. After 2 h, 50 μL of 10% SDS and 25 μL of 1M HCl were added to the wells, and UV spectra were recorded using Shimatzu UV-1800 spectrophotometer (Japan). Under the same conditions, the solutions of the amino group calibrant, ACA (8–500 μM), were incubated and examined. Using the absorption peak values (340 nm), a curve was plotted versus the concentration of ACA, and the linear calibration was used to calculate the number of free amino groups in protein samples.
Immunization and antibody preparation
The BSA-PMB×50(edc) immunogen emulsified in Freund's complete adjuvant was injected subcutaneously into rabbits (2.5–3 kg) at 8–10 points in the back at a dose of 100 μg. Repeated injections of the immunogen were carried out monthly at the same dose of immunogen in incomplete Freund's adjuvant. A week after each booster injection, a blood portion was taken from the marginal vein of the ear of rabbits, the serum was separated and stored equilibrated with an equal volume of glycerol at −15°C.
Immune response control and optimization of ELISA
The maturing of the immune response in animals was analysed by the interaction of antiserum samples in indirect ELISA with conjugated antigens adsorbed on 96-well Costar plates. The wells were filled with 0.1 mL of conjugate solution in a concentration range of 0.01–1 μg/mL in CBB and incubated for 16 h at 4°C. Then, the plates were washed 3–5 times with 0.15 M phosphate-buffered saline pH 7.5 containing tween 20 (PBS-t), and 0.1 mL of PBS-t and antiserum in serial dilutions in the same buffer with 1% BSA were combined in the wells. After 1 h-incubation at 25°C, the plate was washed again; 0.1 mL of goat anti-rabbit IgG antibody conjugated with horseradish peroxidase was filled into the wells and incubated 1 h at 37°C. After washing, the enzymatic reaction was allowed to develop for 30 min with a substrate solution containing TMB (0.1 ml per well) and then was terminated by adding 100 μL of 0.5 M sulphuric acid. The absorbance was registered at 450 nm using a StatFax 2100 plate reader (Awareness Technologies, USA).
Immunoreagents in concentrations that provided a reaction absorbance of 0.8–1.2 were used in a competitive assay. For this, the COL standard solutions were prepared in PBS-t (10000-0.1 and 0 ng/mL) and added to the wells together with the antibody working solution. The antibody binding at zero concentration of the antibiotic was taken as 100% (B0), and for each antibiotic concentration the binding level was calculated as a percentage (B/B0). The dependence of these indicators was presented in the form of standard curves. Graphical processing and calculations were carried out using Origin 8.0 software (Originlab Corporation, USA). Of the conjugates with different hapten loads, one was selected that provided a better sensitivity of analyte determination. The sensitivity value was taken as half-inhibition concentration (IC50), the measuring range was between IC20 and IC80 values. The detection limit of assay was defined as (IC10) (Xu et al., Citation2019). The cross-reactivity of antibodies was calculated as the ratio of IC50 PBM/ IC50 ANALOGUE expressed as a percentage.
Examination of matrix effect
Milk is a multi-component product containing various proteins, saturated and unsaturated fats, carbohydrates, vitamins and minerals. The matrix of milk can greatly affect the interaction of antibodies with antigen. The composition of the chicken egg is no less complicated. A number of proteins and enzymes, various carbohydrates and lipids, cholesterol, natural and fortified micronutrients can also affect immune binding.
To reproduce such interference, skim milk powder (SM) was used. A solution of this reagent was used to prepare calibration curves. The matrix effect of diluted SM reagent corresponded to the exploration of real samples of cow's milk.
Sample pretreatment and recovery rate estimation
The matrix samples found to be COL-free by preliminary testing were used for recovery test. Blank milk samples with a fat content of 1.5–10% were fortified with COL to obtain final concentrations of 100, 50, 25 ng/mL, and concentrations of 600, 300 and 150 ng/mL were created in egg homogenates. For degreasing, a portion of milk cooled at 4°C was centrifuged for 20 min at 3000 × g, and then the layer of fat was removed from the surface. The delipidized milk samples were thoroughly mixed, diluted with PSB-t 10 times and tested in ELISA. The homogenized eggs were analysed by developed test after 50-fold dilution with PSB-t. The recovery rate was determined as a ratio of the average concentration found for various samples to the fortified concentration.
Results and discussion
Characterization of conjugated antigen
Polymyxins are low molecular weight compounds (900–1300 Da) that are not able to elicit an immune response. To produce specific antibodies, it was necessary to conjugate polymyxin with a protein macromolecule ().
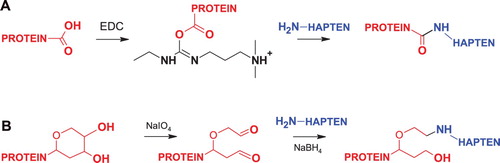
Both PMB and COL bear primary amino groups that can serve as functional groups for conjugation with protein carriers. Nevertheless, their significant number (n = 5) complicates the formation of a chemical bond at particular position. For this reason, it was not practical to use for conjugation homobifunctional cross-linking agents such as glutaraldehyde, bis-N-hydroxysuccinimide ester derivatives or bis-imidoesters. In order to minimize possible cross-linking between the hapten molecules, we activated the carboxyl groups of the protein, thereby ensuring their direct binding to the sterically available amino groups of PMB ((A)). Guided by a similar principle, we performed an alternative conjugation of PMB and COL with periodate-oxidized glycoprotein, Gel ((B)). Thus, the resultant conjugates were formed using a zero-length spacer arm between protein and hapten as shown in the scheme.
Figure 2. Scheme of synthesis conjugated antigens in carbodiimide condensation method (A) and by reductive amination of periodate-oxidazed glycoprotein (B).
Polymyxins are oligopeptide molecules and have no specific spectral characteristics in the UV-Vis region that distinguish them from proteins. In this regard, to evaluate the prepared immunogen conjugate, a comparative analysis of the free amino groups in BSA and BSA-PMB×50(edc) was performed using TMBS ().
Figure 3. Spectra of BSA and BSA-PMB×50(edc) solutions (0.1 mg/mL) treated with TNBS (A). Calibration line for the determination of free amino groups (B).
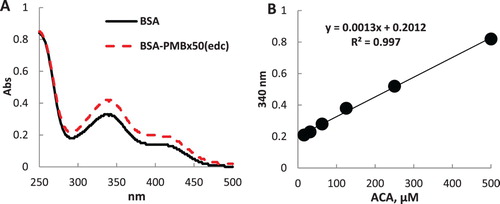
As can be seen from the spectrogram in (A), the conjugate BSA-PMB×50(edc) (dashed line) enriched by hapten NH2-groups were able to bind a larger number of TMBS molecules as compared to BSA (solid line), as evidenced by a more pronounced peak at 340 nm. Using ACA as a reference substance with single primary amine ((B)), the growth of NH2-groups in the immunogen conjugate was calculated as 46.5 mol/mol. If we assume that, under conjugation of 50-fold molar excess of PMB to BSA, only one most sterically accessible NH2-group was involved in linking, then four remaining amines were labelled with TNBS. In this case, the hapten load was 11.6 mol/mol.
The formation of Gel-based conjugates was confirmed by their interaction as coating antigens with anti-PBM antibody in ELISA (see the section below).
Antibody production and ELISA optimization
Control of the immune response in dynamics showed that after the 6th immunization, serum preparations had a working titer (1/6000) and could provide the best level of assay sensitivity (IC50 = 5 ng/mL). The study of anti-BSA-PMB×50(edc) # 6 interaction with various coating antigens revealed that intensive binding to the conjugates homologous in synthesis method, Gel-PMB(edc) and Gel-COL(edc) was poorly inhibited by free hapten, so they were no considered in following experiments. The conjugates prepared by heterologous synthesis method when adsorbed on polystyrene provided a more sensitive determination of the analytes. From each group of PMB- and COL-conjugates the representatives with certain hapten load were selected, that became the basis of more sensitive ELISA variants. They are presented in .
Table 1. The specificity and sensitivity of ELISAs based on heterologous antigens.
The data from the table indicate similar recognition of polymyxins, which could vary slightly depending on the coated hapten. So, in comparison with the coating PMB-based antigen, the use of the COL-based coating antigen contributed to a better recognition of COL (183% vs. 88%). CMS was not detected by the obtained antibodies, since all its primary amino groups are substituted by methyl sulphonic residues (). CMS is a prodrug that converts to recognizable COL after hydrolysis in vivo or in vitro (Bergen, Li, Rayner, & Nation, Citation2006).
ELISA based on coating Gel(pi)-PMB×10 was selected for the quantitative determination of COL, it was characterized by almost the same recognition of COL and PBM, better parameters of sensitivity (IC50 = 5.7 ng/mL), a wide measurement range (IC20-IC80 = 1.0–80 ng/mL) and had a significantly lower detection limit (LOD) (IC10 = 0.4 ng/mL) compared with immunosystems (LOD = 9–30 ng/mL) in previously published reports (Kitagawa et al., Citation1985; Suhren & Knappstein, Citation2005; Xu et al., Citation2019).
Examination of matrix effect
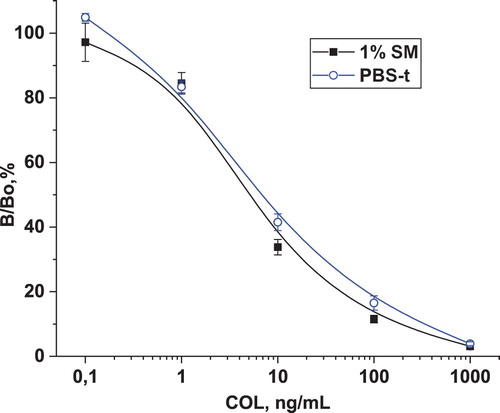
In order to adapt the ELISA system to the measurement of COL in foodstuff, dairy products and chicken eggs, it was necessary to evaluate the effect of sample matrix on the immunochemical interaction “antibody–antigen” and develop a procedure of sample preparation to eliminate possible interferences. As we have repeatedly seen, the matrix effect of milk consists in some suppression of the binding of antibodies to antigen (Burkin, Nuriev, Wang, & Galvidis, Citation2018; Galvidis & Burkin, Citation2010). It can be seen from that when diluting, the milk matrix effect weakened, and the optical signal of reaction was restored. At a 1:50 dilution, it almost reached the level of antibody binding in PSB-t, however, this degree of sample dilution significantly increased the assay LOD. In this regard, to simulate the effect of the milk matrix, a buffer containing 1% SM was used, the effect of which was equivalent to milk diluted 10 times ().
Figure 4. Interaction of anti-BSA-PMB×50(edc) with Gel(pi)-PMB×10 in various matrices. Each column represents the average value (n = 4), the error value corresponds to the standard deviation.
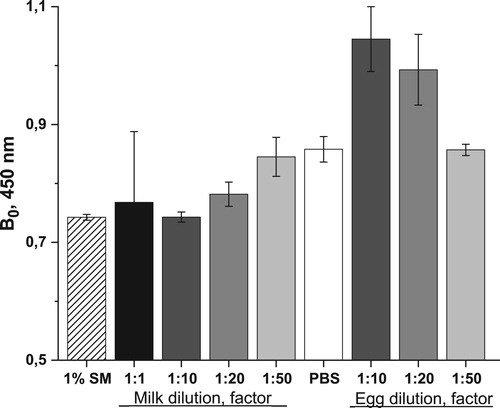
Egg homogenate had quite opposite effect on immune binding, increasing the resultant optical signal (). Immunoassays of various analytes in eggs are often preceded by a rather laborious sample preparation. So, for example, for analysis of tetracyclines (Wang, Xia, Liu, Wang, & Liu, Citation2019), six coccidiostatics (Bienenmann-Ploum et al., Citation2012) and antibiotics from amphenicol group (Lei et al., Citation2018), the sample preparation included extraction with acetonitrile, removing of the precipitated ingredients by centrifugation, separation of the organic supernatant and its complete evaporation, re-dissolution of the precipitate in a buffer with hexane or methanol, additional centrifugation to obtain a supernatant for testing in immunoassay.
In this work, the dilution of the samples by a factor of 50 turned out to be effective, while the drawbacks were completely avoided, reaching the level of interaction in the PSB-t. Such a significant dilution was not critical for the sensitivity of the analysis, since the limitations set for residual COL in eggs (300 μg/mL) are less stringent than for milk (50 μg/mL).
Thus, the interference from the matrix of the studied objects was identified and eliminated by simulating the matrix effect and dilution. Sample preparation was as simple as possible and meant diluting samples with assay buffer. Typical calibration curves for measuring COL contamination of milk and eggs are shown in .
Recovery rate examination
The adequacy of the selected sample preparation and the accuracy of the measurements made using the developed assay were tested in experiments with the artificial fortification of samples with COL at concentrations corresponding to 0.5, 1, and 2 MRL. The analyte recovery rate was found to be high 89–104% (), and reproducibility was satisfactory (1.9–16%) for detecting threshold concentrations of COL in livestock production.
Table 2. Recovery rate of COL from milk and egg samples fortified with antibiotic at threshold content.
Conclusion
Thus, in the result of immunization rabbits with the conjugate of the oligopeptide antibiotic PMB with BSA the polyclonal antibodies were produced that were capable of closely recognizing PMB (100%) and its structural analogue COL (88%). Based on the obtained antibodies, a competitive ELISA was developed to determine the residual COL content in livestock products, cow's milk and chicken eggs. The developed test was characterized by a higher sensitivity compared to previously published reports (Kitagawa et al., Citation1985; Suhren & Knappstein, Citation2005; Wang et al., Citation2019; Xu et al., Citation2019). A significant improvement in the limit of determination (up to 0.4 ng/mL) made it possible to detect the MRL concentrations of COL in dairy products and eggs without laborious sample pretreatment using simple dilution of the samples.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Barnard, J. H. (1984). Potency of polymyxin B1 and B2 fractions by turbidimetric assays and agar plate diffusion assay. Analytical Proceedings, 21, 238–240. View Record in Scopus.
- Baron, S., Hadjadj, L., Rolain, J.-M., & Olaitan, A. O. (2016). Molecular mechanisms of polymyxin resistance: Knowns and unknowns. International Journal of Antimicrobial Agents, 48(6), 583–591. doi:10.1016/j.ijantimicag.2016.06.023
- Bergen, P. J., Li, J., Rayner, C. R., & Nation, R. L. (2006). Colistin methanesulfonate is an inactive prodrug of colistin against pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy, 50(6), 1953–1958. doi:10.1128/AAC.00035-06
- Bienenmann-Ploum, M. E., Huet, A. C., Campbell, K., Fodey, T. L., Vincent, U., Haasnoot, … Nielen, M. W. F. (2012). Development of a five-plex flow cytometric immunoassay for the simultaneous detection of six coccidiostats in feed and eggs. Analytical and Bioanalytical Chemistry, 404(5), 1361–1373. doi:10.1007/s00216-012-6214-1
- Boison, J. O., Lee, S., & Matus, J. (2015). A multi-residue method for the determination of seven polypeptide drug residues in chicken muscle tissues by LC-MS/MS. Analytical and Bioanalytical Chemistry, 407(14), 4065–4078. doi:10.1007/s00216-015-8644-z
- Brown, P., & Dawson, M. J. (2015). Chapter Three – A perspective on the next generation of antibacterial agents derived by manipulation of natural products. Progress in Medicinal Chemistry, 54, 135–184. doi:10.1016/bs.pmch.2014.10.001
- Burkin, M. A., Nuriev, R. I., Wang, Z., & Galvidis, I. A. (2018). Development of sandwich double-competitive ELISA for sulfonamides. Comparative analytical characteristics and matrix effect resistance. Food Analytical Methods, 11(3), 663–674. doi:10.1007/s12161-017-1036-6
- The European Agency for The Evaluation of Medicinal Products. (2002). Colistin. Summary report 2002. EMEA/MRL/815/02-FINAL. Google Scholar.
- Fu, Q., Li, X., Zheng, K., Ke, Y., Wang, Y., Wang, L., … Xia, X. (2018). Determination of colistin in animal tissues, egg, milk, and feed by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chemistry, 248, 166–172. doi:10.1016/j.foodchem.2017.12.029
- Galvidis, I. A., & Burkin, M. A. (2010). Monoclonal antibody-based enzymelinked immunosorbent assay for the aminoglycoside antibiotic kanamycin in foodstuffs. Russian Journal of Bioorganic Chemistry, 36(6), 722–729. doi:10.1134/S1068162010060087
- Habeeb, A. F. S. A. (1966). Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Analytical Biochemistry, 14(3), 328–336. doi:10.1016/0003-2697(66)90275-2
- Hygienic Requirements in Respect of the Safety and Nutritional Value of Foodstuffs, SanPiN 2.3.2.1078-01, as Amended. (2019). 2. Chapter 1. Requirements for the safety and nutrition of foods of the unified sanitary-epidemiological and hygiene requirements of the commission of the customs union of Russia, Belarus and Kazakhstan. http://docs.cntd.ru/document/902249109.
- Kaufman, A., & Widmer, M. (2013). Quantitative analysis of polypeptide antibiotic residues in a variety of food matrices by liquid chromatography coupled to tandem mass spectrometry. Analytica Chimica Acta, 797, 81–88. doi:0.1016/j.aca.2013.08.032 doi: 10.1016/j.aca.2013.08.032
- Kitagawa, T., Ohtani, W., Maeno, Y., Fujiwara, K., & Kimura, Y. (1985). Sensitive enzyme immunoassay of colistin and its application to detect residual colistin in rainbow trout tissue. Journal of the Association of Official Analytical Chemists, 68(4), 661–664. https://www.scopus.com/inward/record.url?eid=2-s2.0-0022403664&partnerID=10&rel=R3.0.0.
- Lei, X., Xu, L., Song, S., Liu, L., & Kuang, H. (2018). Development of an ultrasensitive ic-ELISA and immunochromatographic strip assay for the simultaneous detection of florfenicol and thiamphenicol in eggs. Food and Agricultural Immunology, 29(1), 254–266. doi:10.1080/09540105.2017.1371114
- Leroy, P., Decolin, D., Nicolas, S., Archimbault, P., & Nicolas, A. (1989). Residue determination of two co-administered antibacterial agents — cephalexin and colistin — in calf tissues using high-performance liquid chromatography and microbiological methods. Journal of Pharmaceutical and Biomedical Analysis, 7(12), 1837–1846. doi:10.1016/0731-7085(89)80201-8
- Morovjan, G., Csokán, P. P., & Németh-Konda, L. (1998). HPLC determination of colistin and aminoglycoside antibiotics in feeds by post-column derivatization and fluorescence detection. Chromatographia, 48(1–2), 32–36. doi:10.1007/BF02467512
- Oliveri Conti, G., Copat, C., Wang, Z., D’Agati, P., Cristaldi, A., & Ferrante, M. (2015). Determination of illegal antimicrobials in aquaculture feed and fish: An ELISA study. Food Control, 50, 937–941. doi:10.1016/j.foodcont.2014.10.050
- Ryder, M. P., Wu, X., McKelvey, G. R., McGuire, J., & Schilke, K. F. (2014). Binding interactions of bacterial lipopolysaccharide and the cationic amphiphilic peptides polymyxin B and WLBU2. Colloids and Surfaces B: Biointerfaces, 120, 81–87. doi:10.1016/j.colsurfb.2014.05.004
- Suhren, G., & Knappstein, K. (2005). Detection of colistin in spiked and incurred milk samples by LC- and ELISA-technique. Analytica Chimica Acta, 529(1–2), 97–101. doi:10.1016/j.aca.2004.09.030
- Velkov, T., Roberts, K. D., Nation, R. L., Thompson, P. E., & Li, J. (2013). Pharmacology of polymyxins: New insights into an ‘old’ class of antibiotics. Future Microbiology, 8(6), 711–724. doi:10.2217/fmb.13.39
- Walsh, T. R., & Wu, Y. (2016). China bans colistin as a feed additive for animals. The Lancet Infectious Diseases, 16(10), 1102–1103. doi:10.1016/S1473-3099(16)30329-2
- Wang, G., Xia, W. Q., Liu, J. X., Wang, J. P., & Liu, J. (2019). Directional evolution of TetR protein and development of a fluoroimmunoassay for screening of tetracyclines in egg. Microchemical Journal, 150, 104184. doi:10.1016/j.microc.2019.104184
- Wang, J., Zhou, J., Chen, Y., Zhang, X., Jin, Y., Cui, X., … He, L. (2019). Rapid one-step enzyme immunoassay and lateral flow immunochromatographic assay for colistin in animal feed and food. Journal of Animal Science and Biotechnology, 10(1), 82. doi:10.1186/s40104-019-0389-7
- WHO. (2011). Critically important antimicrobials for human medicine. Retrieved from http://apps.who.int/iris/bitstream/10665/77376/1/9789241504485_eng.pdf.
- Xu, L., Burkin, M., Eremin, S., Dias, A. C. P., & Zhang, X. (2019). Development of competitive ELISA and CLEIA for quantitative analysis of polymyxin B. Food Analytical Methods, 12(6), 1412–1419. doi:10.1007/s12161-019-01477-9