ABSTRACT
In this study, a monoclonal antibody with selectivity for clopidol was produced based on a new hapten. The 50% inhibition concentration (IC50) for the developed ic-ELISA was 0.275 ng/mL. Cross-reactivity tests showed that the monoclonal antibody had high specificity for clopidol. Using this antibody, a lateral-flow colloidal gold immunoassay (LCGA) was created for the rapid screening of clopidol in raw chicken samples. With the naked eye, the cut-off value of the strip assay was 5 μg/kg for clopidol in chicken samples. Therefore, this LCGA provides a sensitive and rapid method for on-site detection of clopidol in chicken samples.
1. Introduction
Clopidol (CLOP) is a pyridine compound, with the chemical name 3,5-dichloro-2,6-dimethyl-4-hydroxypyridine, which is an anticoccidial agent. It is widely used in the livestock and poultry breeding industry. However continuous or excessive use will cause it to accumulate in the human body through the food chain. Studies have shown that CLOP has teratogenicity and is therefore very harmful to human health (Pietruk, Olejnik, Jedziniak, & Szprengier-Juszkiewicz, Citation2015). At present, countries including the European Union, Japan, Canada, and the United States have established a maximum limit of chlorhexidine residues in various animal tissues, and China has also set a maximum allowable limit of chlorhexidine in chicken meat at 50 μg/kg.
Various methods have been reported for detecting residues of CLOP and other anticoccidial drugs in different matrices. Such methods include high performance liquid chromatography-mass spectrometry (HPLC-MS) (Germany, Citation2019; Kim, Ryu, Chung, & Kim, Citation2018; Matus & Boison, Citation2016) and liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) (Barreto, Ribeiro, Hoff, & Dalla Costa, Citation2017). Although these methods have the advantages of high sensitivity and specificity, these methods are complicated and time-consuming to operate, and also require expensive equipment and professional operators, thus limiting their broad application. In addition, it is difficult to screen a large number of samples in a short period of time using these methods. Compared with the above methods, immunochromatographic test strips have great advantages in conventional screening. Immunochromatographic test strips are suitable for high-throughput applications and allow fast analysis.
At present, there are few studies using ic-ELISA to detect CLOP, and the detection line is high. In this study, we produced sensitive and specific antibodies to CLOP, and developed a gold nanoparticle-based lateral flow strip assay to detect CLOP residues in chicken.
2. Materials and methods
2.1. Chemicals and apparatus
Standards including diclazuril, CLOP, robenidine, dinitolmide and sulfaquinoxaline were purchased from J & K Scientific Ltd. (Beijing, China). Bovine serum albumin (BSA), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), N-hydroxysuccinimide (NHS), gold chloride trihydrate, Freund’s incomplete adjuvant, Tween-20, gelatin, Freund’s complete adjuvant and enzyme immunoassay-grade horseradish peroxidase-labeled goat anti-mouse immunoglobulin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Hypoxanthine-thymidine (HT) supplement, RPMI-1640 cell culture medium, polyethylene glycol 1500 and calf serum were acquired from Gibco BRL (Paisley, UK). All other reagents used were of chemical grade.
Absorbance pad, polyvinyl chloride pads, nitrocellulose (NC) membrane, and sample pad (glass-fiber membrane) were acquired from Jie Yi Biotechnology Co., Ltd. (Shanghai, China). The CM4000 Guillotine Cutting Module was obtained from Kinbio Tech Co., Ltd. (Shanghai, China).
The antigen and hapten were characterized using a UV/VIS scanner (Bokin instruments, Tsushima, Japan). A Waters Maldi Synapt QToF mass spectrometer (Shanghai, China) was used to characterize the hapten. A Milli-Q synthesis system (Millipore Co., Bedford, MA, USA) was used to produce ultrapure water. In addition a multi-scan MKS microplate reader (Thermo Labsystems Company, Beijing, China), vortex (Shanghai Huxi Analysis Instrument Factory Co., Ltd., Shanghai, China) and water bath (Shanghai Instrument Group Co., Ltd., Supply & Sales Co., Shanghai, China) were used in this study.
2.2. Preparation of haptens and antigens
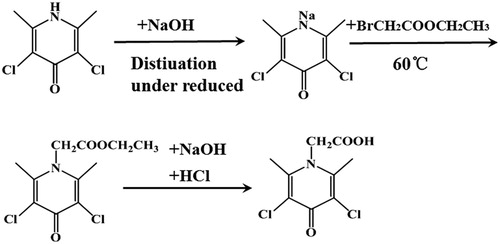
A scheme for the production of CLOP hapten containing a carboxyl group is shown in . In short, 10 mmol of CLOP was completely dissolved in 25 mL of 0.5 mol/L NaOH, the water was then removed by vacuum distillation. After washing with water and drying to room temperature, the primary product was dissolved in 20 mL of N,N-dimethylformamide (DMF), then 0.6 mL of ethyl bromoacetate was added dropwise. The chemical reaction time extended to 4 h at 60°C. After cooling to room temperature, 200 mL of distilled water was added, and the mixture was cooled in a 4°C refrigerator overnight. After three steps of vacuum filtration, water separation and filtration, the CLOP derivative containing an ester bond was obtained. This product was added to 20 mL of distilled water, then heated to 85°C. Next, 0.5 mol/L NaOH solution was added dropwise until all the solid was completely dissolved. After cooling to room temperature, the pH was adjusted to 4 by adding 1 mol/L HCl solution dropwise. The reaction mixture was stored at 4°C overnight. Finally, the three steps of filtration, recrystallization, and drying were performed to obtain the CLOP hapten.
The CLOP-hapten was coupled to BSA as the immunogen and coupled to OVA as the coating antigen (Su, Chen, He, & Yang, Citation2017; Wang et al., Citation2019). Briefly, to conjugate the CLOP-hapten with BSA (immunogen) and OVA (coating antigen), 3.24 mg of CLOP-hapten, 5.8 mg of N-hydroxysuccinimide, and 9.7 mg of EDC were dissolved in 0.6 mL of DMF and stirred for 6 h at room temperature. The solution was added to the BSA solution (7.5 mg of BSA dissolved in 2 mL of 0.1 M carbonate–bicarbonate buffer; pH 9.5). After stirring overnight, the solution was dialyzed to obtain pure immunogen and stored at −20°C. The coating antigen was prepared in the same way.
2.3. Monoclonal antibody (mAb) production and characterization
All animal experiments were in strict accordance with Chinese laws and guidelines which were approved by the Animal Ethics Committee of Jiangnan University. Twenty BALB/c mice (6 weeks of age) were divided into two groups with 10 mice per group. Each group was injected with CLOP-hapten-BSA following a protocol described previously (Wang et al., Citation2017; Lei, Xu, Song, Liu, & Kuang, Citation2018; Peng et al., Citation2017). The immunogen CLOP-hapten-BSA was used to immunize each mouse. In the first immunization, the immunogen (100 μg) was emulsified with Freund's complete adjuvant and injected into the neck. After three weeks, booster immunizations (50 μg) were performed. Mouse serum was collected and then the titer and specificity to CLOP were evaluated by an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) following each immunization. The mouse with the highest titer and specificity to CLOP was chosen as the spleen donor for cell fusion.
The detailed procedure for cell fusion was as described previously (Xiaoxin, Liqiang, Gang, Xiaoling, & Hua, Citation2019; Xinyi et al., Citation2019; Yuxi et al., Citation2019). After three subclones, a preferably selected stable antibody-producing cell strain was expanded. Finally, one part of each stable antibody-producing clone was stored in liquid nitrogen, and the other was injected into mice to produce ascites. Seven to eight days after the injection, ascites was collected from the abdominal cavity of the mice and purified by caprylic acid–ammonium sulfate precipitation. After dialysis at 4°C against PBS for 2–3 days, the purified mAb was stored at −20°C until use (Lingling et al., Citation2019; Zhongxing, Xiaoling, Liguang, Hua, & Chuanlai, Citation2019).
Sensitivity and specificity of the anti-CLOP mAb were analyzed using an ic-ELISA. The appropriate concentration of coating antigen and mAb were determined by a checkerboard assay and this method was performed as formerly reported (Lu et al., Citation2018). A standard mAb curve (0, 0.1, 0.25, 0.5, 1, 2, and 5 ng/mL) was created and OD450 values were plotted against CLOP concentration. The antibody sensitivity was characterized based on the IC50 and the limit of detection (LOD). The specificity of the anti-CLOP mAb was investigated by evaluating the cross-reactivity (CR) of the mAb, including diclazuril, sulfaquinoxaline, robenidine and dinitolmide. CR of the mAb was tested using the following formula:
CR (%) = (IC50 of clopidol/IC50 of the analogue) × 100%.
2.4. Gold nanoparticle (GNP) synthesis
GNPs were synthesized using a sodium citrate reduction method described previously (Chen et al., Citation2015; Guo, Wu, Liu, Kuang, & Xu, Citation2018; Zeng, Jiang, Liu, Song, & Kuang, Citation2018). In the first step, 100 mL of the chloroauric acid mixture (HAuC4, 0.01%, w/v) was heated in a flask to full boiling. In the second step, 4 mL of freshly-prepared trisodium citrate solution was added to the above mixed solution (1%, w/v). As the solution continued to boil, the colour of the solution gradually changed to wine red and was then stirred for 15–20 min. The final product was cooled and stored at 4°C.
2.5. Preparation of GNP-labeled mAb
The GNP-labeled anti-CLOP mAb was prepared using methods described previously (Shanshan et al., Citation2019). In short, 4 µg antibody (0.2 mg/mL) diluted in PBS was added to 2 mL of GNP solution (adjusted the pH of GNP solution to 8 by 0.1 mol/L K2CO3) with gentle shaking, and then the liquid was stirred for 50 min at room temperature. After blocking all free GNPs with 2 mL of 10% BSA aqueous solution, the solution was allowed to stand at room temperature for 1 h. GNP-labeled anti-CLOP mAb was washed 4–5 times with resuspension buffer (0.02 mol/L Tris [pH 8.2], 0.15% Tween, 10 mmol/L PBS containing 2% BSA, 0.2% PEG, and 3% sucrose) by centrifugation (20,000 × g, 30 min) at 4°C. The final gold-labeled anti-CLOP mAb was resuspended in borate buffer and stored at 4°C.
2.6. Assembly of the immunochromatographic strip
The immunochromatographic strips consisted of four sections: nitrocellulose (NC) membrane, sample pad, absorption pad and polyvinylchloride (PVC) backing card. The strip assembly step was performed on the basis of methods described previously (Isanga et al., Citation2017; Kong et al., Citation2016; Niu et al., Citation2018). The goat anti-mouse IgG antibody (1 mg/mL) and the coating antigen (1 mg/mL) at optimal concentrations as determined above, were each sprayed onto the NC membrane with a dispenser, forming the test line (T-line) and the control line (C-line). The NC membrane was dried at 37°C for 2 h, and then sealed and stored. The sample pad was saturated with 0.03 mol/mL PBS containing 3% BSA, 0.1% Tween 20, and 2% sucrose and dried at room temperature for 3 h. The NC membrane was assembled on the centre of the PVC backing card and then the absorbent pad and the sample pad were assembled on the NC film near the ends of the C-line and the T-line.
2.7. Sample pretreatment
A raw chicken breast sample was purchased from a local market and confirmed to be CLOP-free by LC-MS. The sample pretreatment was performed as described previously (Li, Xiya, et al., Citation2018; Wang et al., Citation2019). First, 10 mL of methanol was added to a 3 g sample of minced chicken. The mixture was shaken for 5 min and centrifuged at 10,000 × g for 10 min. The resulting supernatant was placed in a clean 15–20 mL centrifuge tube. Secondly, N-hexane was added to the mixture, and then the supernatant liquid was discarded after centrifugation at 4,000 × g for 10 min; this step was repeated several times. Thirdly, 5 mL of the lower liquid was evaporated to dryness under a stream of nitrogen. The residue was resuspended in 2 mL of a methanol phosphate buffer solution and tested for the presence of CLOP.
2.8 Test procedure and principle of the strip assay
The testing process was rapid and low-cost using methods described previously (Jiajia, Liqiang, Shanshan, Gang, & Hua, Citation2018; Li, Liu, Song, Kuang, & Xu, Citation2018). In short, 60 μL of the GNP-labeled mAb solution and 110 μL of the sample extract were mixed until combined in a microwell plate and allowed to sit at room temperature for 10 min. After this, 100 μL of the mixed solution was added to the sample pad and moved towards the absorbent pad. The results were acquired after 15 min.
By means of capillary action, the mixture moved across the NC membrane and combined with the goat anti-mouse IgG and coating antigen attached to the NC membrane. When CLOP was not present in the sample extract, the GNP-labeled mAb moved freely to the absorbent pad and was captured by the coating antigen of the T line, which then changed colour, producing a red line. Excess GNP-labeled mAb continued moving and was captured by the goat anti-mouse IgG, also producing a red control line. When CLOP was contained in the sample extract, the small molecules first reacted to the limited amount of GNP-labeled mAb and moved to the test area. The more CLOP contained in the sample extract, the less free GNP-labeled mAb remained to be caught by the coating antigen attached to the T-line. Therefore, the colour density of the T-line was inversely proportional to the concentration of CLOP in the sample, and the control line must be visible for the test to be valid, regardless of the presence or absence of CLOP in the sample.
3. Results and discussion
3.1. Hapten and antigen characterization
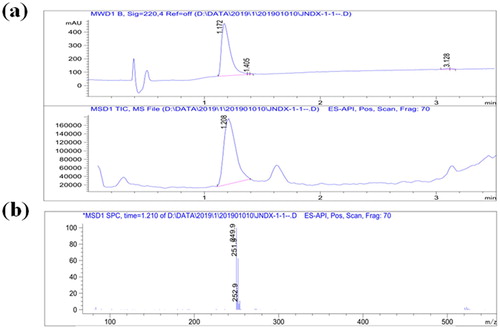
CLOP has a low molecular weight and cannot induce a specific immune response. Therefore, CLOP must first be coupled to a carrier protein to make it immunogenic. Since the protein did not contain a reactive group, a carboxyl group was introduced into CLOP (). The derivative CLOP-hapten was analyzed by LC-MS. As shown in (a), the main product visible on the chromatogram had a retention time of 1.2 min indicating a very high yield and the main UV peak appeared at 1.2 min. Meanwhile, substantial data obtained from MS (shown in (b)), revealed a fragment with a molecular weight of 250 Da, which was consistent with the molecular size of CLOP derivatives. Therefore derivation of CLOP-hapten was very successful.
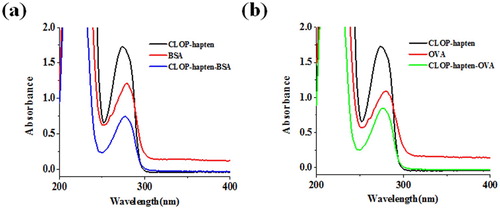
The CLOP-hapten was linked to a carrier protein using the carbodiimide method and the antigen was characterized by ultraviolet spectroscopy. (a) shows that the CLOP-hapten had a peak at 275 nm and BSA had a peak at 280 nm. CLOP-hapten-BSA exhibited one peak at 278 nm. These confirmed that the CLOP-hapten was linked successfully with BSA. (b) shows that the maximum absorbance of CLOP-hapten-OVA was between the maximum absorbance of CLOP-hapten and the maximum absorbance of OVA, indicating that the coated antigen was successfully synthesized.
3.2. Characterization of a mAb against CLOP
The antibody is the core of any immunoassay. In this study, a CLOP antibody was successfully obtained by the process of mouse immunization and cell fusion. Four cell lines (2B1, 3H3, 2D3, and 4F5.) were acqiured in our experiment. We selected 3H3 mAb, because its IC50 value (0.275 ng/mL) was the lowest. The concentrations selected of coating antigen and 3H3 mAb were 0.03 and 0.3 μg/mL, respectively. The sensitivity and specificity of the anti-CLOP mAb were determined by ic-ELISA. The sensitivity of the the mAb against CLOP was evaluated using a standard curve. The standard curve () of CLOP detection was created and the equation was y = 0.0868 + 1.71367/(1 + [x/0.45959]0.99679), with a correlation coefficient (R2) of 0.999, an LOD value of 0.018 ng/mL, an IC50 value of 0.275 ng/mL, and a linear range for CLOP (IC20–IC80) of 0.032–0.896 ng/mL. The specificity of the the mAb against CLOP was evaluated the using CR of anti-CLOP mAb to CLOP-related chemicals. As shown in , the mAb is highly specific for CLOP, where the CR of anti-CLOP mAb to CLOP-related chemicals was less than 0.4%. Due to its high affinity and specificity, the anti-CLOP mAb was selected for further experiments.
Figure 4. Characterization of the mAb against CLOP. Standard curve for CLOP detection with the mAb against CLOP in the ic-ELISA. Each point is the mean of ± SD of three replicates. IC50 of the the mAb against CLOP was 0.275 ng/mL, LOD was 0.018 ng/mL, and the linear range of detection was from 0.032 ng/mL to 0.896 ng/mL.
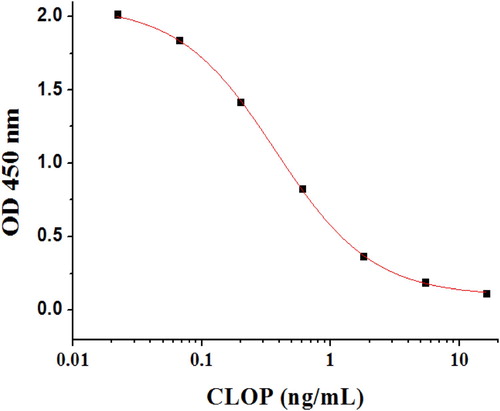
Table 1. Cross-reactivity of analogues and CLOP to the mAb against CLOP in the ic-ELSIA.
3.3. Optimization of a GNP-based lateral-flow colloidal gold immunoassay (LCGA)
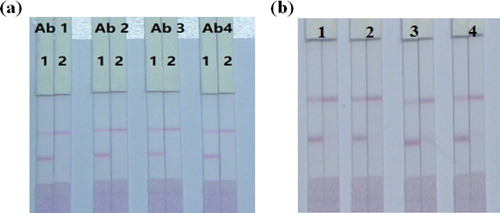
The GNP-labeled mAb is an identification element, where CLOP competes with the coating antigen bound on the test line for GNP-labeled mAb. A couple of previous studies (Wang et al., Citation2020; Wang et al., Citation2015) have proven that the antibodies and pH are essential to the function of GNP-labeled anti-CLOP mAb conjugates. Thus, in this study, GNP solutions were tested at different pH values (4.5, 5.5, 6.5, 7.5, and 8.5). The pH of the solution was changed using 0.1 M K2CO3, and the outcomes were evaluated using an ic-ELISA (Wang et al., Citation2020). The optimal pH was found to be 9.0. For screening the antibodies, four kinds of antibodies (2B1, 3H3, 2D3, and 4F5) were optimized and a dilution series of mAb concentrations (6, 8, 10, and 12 µg/mL) were adopted for the chemical synthesis of the GNP-mAb at pH 9.0. As shown in (a), the results showed that the optimum mAb was 3H3. As shown in (b), the outcomes showed that the strip assay using 8 µg mAb in 1 mL of GNP solution was appropriate.
Figure 5. Optimization of different antibodies; (a) Images of the detection of CLOP in different kinds antibodies. Ab1, Ab2, Ab3, and Ab4 were 2B1, 3H3, 2D3, and 4F5. (b) Images of the detection of CLOP in different concentrations of antibodies. 1 = 6 µg/mL, 2 = 8 µg/mL, 3 = 10 µg/mL, 4 = 12 µg/mL.
Another factor affecting the test strip performance was related to the conditions under which goat anti-mouse IgG and coating antigen were immobilized on the NC membrane. It was found that changing the pH affected the adsorption of protein onto the NC membrane. In this study, PBS, phosphate buffer (pH 6.5), and carbonate buffer (pH 9.6) were chosen to optimize coating conditions. The results showed that, in terms of signal and sensitivity, carbonate buffer provided the best results, a finding which was similar to other studies (Kong et al., Citation2017).
Generally, suspension buffers are composed of stabilizers, surfactants, and protein protectants, which also affect GNP-labeled mAbs. In this work, different concentrations of the stabilizer (2%, 4%, and 6% sucrose), protein protection reagent (0.2%, 0.4%, and 0.6% BSA), and surfactant (0.2%, 0.4%, and 0.6% Tween 20) were optimized iteratively. We found the optimal sensitivity and stability of the GNP-based lateral-flow assay were obtained with 0.3% Tween 20, 0.3% BSA, and 3% sucrose.
3.4. Sensitivity of the strip assay
To characterize the test property of the GNP-based lateral-flow assay for CLOP, different concentrations of CLOP (0, 0.25, 0.5, 1, 2.5, 5, 10, and 25 μg/kg) were added to blank chicken samples. After 15 min the results were observed by the naked eye. As shown in (a), the cut-off value was 5 μg/kg for the GNP-based lateral-flow assay. If the CLOP concentration was < 1 μg/kg, no difference was discernible between the T line and the control and at that point the sample was deemed to be negative. If the concentration of CLOP in the sample was between 1 and 5 μg/kg, the T-line colour was lighter than the control, and the sample was considered to be weakly positive. If the concentration of CLOP was ≥ 5 μg/kg, the T-line was invisible and at that point the sample was deemed to be positive.
Figure 6. Sensitivity test of strip. Representative photo of detection a set of chicken samples spiked CLOP by the LCGA strip assay. 1 = 0 μg/kg, 2 = 0.25 μg/kg, 3 = 0.5 μg/kg, 4 = 1 μg/kg, 5 = 2.5 μg/kg, 6 = 5 μg/kg, 7 = 10 μg/kg and 6 = 25 μg/kg. Cut-off value was 5 μg/kg.
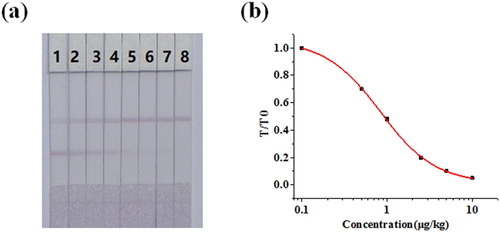
The LCGA was a quantitative measurement, and the colour intensity was determined by a strip scanning reader. T/T0 was defined as the ratio of the colour intensity of the measured sample to a negative sample on the T-line, and it could be used to quantify the signal. LOD was rated as the concentration of CLOP when the suppression ratio of the signal of the negative sample was 10%. (b) shows the calibration curve generated and the equation was y = 0.01135 + 1.0457/(1 + [x/0.83341]1.33469), with the R2 of 0.999. According to this calibration curve, the LOD was 0.14 μg/kg.
Compared with other approaches (Barreto et al., Citation2017; Germany, Citation2019), LCGA could detect CLOP faster and more conveniently. In the previous LC-MS / MS study (Suk et al., Citation2017), the LOD value was 3.3 μg/kg that was higher than the LOD of the LCGA. When compared with previous the immunoassay for the detection of CLOP, this strip assay could detect CLOP with a lower cut-off and LOD value, and a higher sensitively. Moreover, one of the main developmental directions in the field of food safety and quality control are development of rapid, real-time inspection methods for harmful substances in foods or raw materials. Therefore, the LCGA with faster detection and lower LOD could be applied as a high efficiency tool for screening hazardous substances in food or raw materials.
3.5. Analysis of CLOP in chicken samples
A chicken sample which was determined to be negative for CLOP by LC-MS was spiked with different concentrations of CLOP (0, 0.25, 0.5, 1, 2.5, 5, 10, and 25 μg/kg) and tested by ic-ELISA and LCGA. Each sample was analyzed five times by both methods. The concentration of CLOP was calculated from the calibration curve.
shows that the recovery of CLOP using the ic-ELISA method was between 87.95% and 99.5%, while the recovery using the strip assay was between 86.8% and 108.3%. The findings showed that there was no significant difference between the results of the strip assay and the ic-ELISA, and there was no noticeable difference between the two. The outcomes of the coefficient of variation (CV) indicated that the LCGA had good stability and was satisfactory compared with the test values on both sides of the detection range; the values in the middle of the detection range were more stable.
Table 2. Analysis of CLOP in chicken samples by ic-ELISA and the strip assay (n = 5).
4. Conclusion
In summary, we successfully produced the anti-CLOP mAb using a new CLOP-hapten with an IC50 value of 0.275 ng/mL under optimum conditions. In addition, the LCGA was developed for the rapid, stable and sensitive detection of CLOP in chicken, then reaction conditions for the method were optimized. For qualitative or semi-quantitative detection, the cutoff value of CLOP detected was 5 μg/kg. For quantitative detection, a calibration curve was established, for which the LOD was 0.14 μg/kg CLOP in chicken. Recovery rates for LCGA were 95.7–98.4% for CLOP in chicken, which was very close to the results using ic-ELISA. Thus, the LCGA is suitable for use in large-scale screening of CLOP residues in real chicken samples.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Barreto, F., Ribeiro, C., Hoff, R. B., & Dalla Costa, T. (2017). A simple and high-throughput method for determination and confirmation of 14 coccidiostats in poultry muscle and eggs using liquid chromatography - quadrupole linear ion trap - tandem mass spectrometry (HPLC-QqLIT-MS/MS): Validation according to European Union 2002/657/EC. Talanta, 168, 43–51.
- Chen, Y. N., Wang, Y. W., Liu, L. Q., Wu, X. L., Xu, L. G., Kuang, H., … Xu, C. L. (2015). A gold immunochromatographic assay for the rapid and simultaneous detection of fifteen beta-lactams. Nanoscale, 7(39), 16381–16388.
- Germany, D. I. f. N. e. (2019). Animal feeding stuffs: Methods of sampling and analysis - Screening and determination of authorized coccidiostats at additive and 1 % and 3 % cross-contamination level, and of non-registered coccidiostats and of one antibiotic at sub-additive levels, in compound feed with high performance liquid chromatography - tandem mass spectrometry detection (LC-MS/MS). German Standard, 17299, 56.
- Guo, L. L., Wu, X. L., Liu, L. Q., Kuang, H., & Xu, C. L. (2018). Gold nanoparticle-based paper sensor for simultaneous detection of 11 Benzimidazoles by one monoclonal antibody. Small, 14(6), 8.
- Isanga, J., Tochi, B. N., Mukunzi, D., Chen, Y. N., Liu, L. Q., Kuang, H., & Xu, C. L. (2017). Development of a specific monoclonal antibody assay and a rapid testing strip for the detection of apramycin residues in food samples. Food and Agricultural Immunology, 28(1), 49–66.
- Jiajia, S., Liqiang, L., Shanshan, S., Gang, C., & Hua, K. (2018). Development of an immunochromatographic strip assay for three major capsaicinoids based on an ultrasensitive monoclonal antibody. Food and Agricultural Immunology, 29(1), 930–940.
- Kim, C., Ryu, H. D., Chung, E. G., & Kim, Y. (2018). Determination of 18 veterinary antibiotics in environmental water using high-performance liquid chromatography-q-orbitrap combined with on-line solid-phase extraction. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 1084, 158–165.
- Kong, D. Z., Liu, L. Q., Song, S. S., Suryoprabowo, S., Li, A. K., Kuang, H., … Xu, C. L. (2016). A gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins. Nanoscale, 8(9), 5245–5253.
- Kong, D. Z., Xie, Z. J., Liu, L. Q., Song, S. S., Kuang, H., Cui, G., & Xu, C. L. (2017). Development of indirect competitive ELISA and lateral-flow immunochromatographic assay strip for the detection of sterigmatocystin in cereal products. Food and Agricultural Immunology, 28(2), 260–273.
- Lei, X. L., Xu, L. G., Song, S. S., Liu, L. Q., & Kuang, H. (2018). Development of an ultrasensitive ic-ELISA and immunochromatographic strip assay for the simultaneous detection of florfenicol and thiamphenicol in eggs. Food and Agricultural Immunology, 29(1), 254–266.
- Li, H. F., Xiya, S. Q., Zhang, X. Y., Li, C. L., Dong, B. L., Mujtaba, M. G., … Wang, Z. H. (2018). Generic hapten synthesis, broad-specificity monoclonal antibodies Preparation, and Ultrasensitive ELISA for five Antibacterial Synergists in chicken and Milk. Journal of Agricultural and Food Chemistry, 66(42), 11170–11179.
- Li, Y., Liu, L. Q., Song, S. S., Kuang, H., & Xu, C. L. (2018). A rapid and semi-quantitative gold nanoparticles based strip Sensor for Polymyxin B sulfate residues. Nanomaterials, 8(3), 12.
- Lingling, G., Liqiang, L., Gang, C., Shufeng, M., Xiaoling, W., & Hua, K. (2019). Gold immunochromatographic assay for kitasamycin and josamycin residues screening in milk and egg samples. Food and Agricultural Immunology, 30(1), 1189–1201.
- Lu, L., Wei, J., Liguang, X., Liqiang, L., Shanshan, S., & Hua, K. (2018). Development of IC-ELISA and immunochromatographic strip assay for the detection of flunixin meglumine in milk. Food and Agricultural Immunology, 29(1), 193–203.
- Matus, J. L., & Boison, J. O. (2016). A multi-residue method for 17 anticoccidial drugs and ractopamine in animal tissues by liquid chromatography-tandem mass spectrometry and time-of-flight mass spectrometry. Drug Testing and Analysis, 8(5-6), 465–476.
- Niu, Y. B., Wang, D. F., Cui, L. Y., Wang, B. X., Pang, X. J., & Yu, P. X. (2018). Monoclonal antibody-based colloid gold immunochromatographic strip for the rapid detection of Tomato zonate spot tospovirus. Virology Journal, 15, 9.
- Peng, J., Liu, L. Q., Xu, L. G., Song, S. S., Kuang, H., Cui, G., & Xu, C. L. (2017). Gold nanoparticle-based paper sensor for ultrasensitive and multiple detection of 32 (fluoro)quinolones by one monoclonal antibody. Nano Research, 10(1), 108–120.
- Pietruk, K., Olejnik, M., Jedziniak, P., & Szprengier-Juszkiewicz, T. (2015). Determination of fifteen coccidiostats in feed at carry-over levels using liquid chromatography-mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 112, 50–59.
- Shanshan, S., Steven, S., Liqiang, L., Qiankun, Z., Xiaoling, W., & Hua, K. (2019). Development of an immunochromatographic strip test for rapid detection of sodium nifurstyrenate in fish. Food and Agricultural Immunology, 30(1), 236–247.
- Su, P., Chen, X. N., He, Z. J., & Yang, Y. (2017). Preparation of Polyclonal antibody and development of a Biotin-streptavidin-based ELISA method for detecting Kanamycin in Milk and Honey. Chemical Research in Chinese Universities, 33(6), 876–881.
- Suk, C. H., Young-Jun, L., Rahman, M. M., Kabir, M. H., Park, B., Eok, K. J., & Shim, J. H. (2017). Development of simultaneous analytical method of veterinary antibiotics in manure using liquid chromatography coupled with tandem mass spectrometry. Korean Journal of Environmental Agriculture, 36(3), 201–210.
- Wang, J., Wang, Y. L., Pan, Y. H., Chen, D. M., Liu, Z. L., Feng, L., … Yuan, Z. H. (2017). Preparation of a generic monoclonal antibody and development of a highly sensitive indirect competitive ELISA for the detection of phenothiazines in animal feed. Food Chemistry, 221, 1004–1013.
- Wang, Y., Deng, R. G., Zhang, G. P., Li, Q. M., Yang, J. F., Sun, Y. N., … Hu, X. F. (2015). Rapid and sensitive detection of the food Allergen Glycinin in powdered Milk using a lateral flow colloidal gold immunoassay strip test. Journal of Agricultural and Food Chemistry, 63(8), 2172–2178.
- Wang, Z., Wu, X., Liu, L., Xu, L., Kuang, H., & Xu, C. (2020). Rapid and sensitive detection of diclazuril in chicken samples using a gold nanoparticle-based lateral-flow strip. Food Chemistry, 312, 126116.
- Wang, Z. X., Zhang, J., Liu, L. Q., Wu, X. L., Kuang, H., Xu, C. L., & Xu, L. G. (2019). A colorimetric paper-based sensor for toltrazuril and its metabolites in feed, chicken, and egg samples. Food Chemistry, 276, 707–713.
- Xiaoxin, X., Liqiang, L., Gang, C., Xiaoling, W., & Hua, K. (2019). Development of an immunochromatography assay for salinomycin and methyl salinomycin in honey. Food and Agricultural Immunology, 30(1), 995–1006.
- Xinyi, S., Liqiang, L., Liguang, X., Wei, M., Xiaoling, W., Gang, C., & Hua, K. (2019). Rapid detection of praziquantel using monoclonal antibody-based ic-ELISA and immunochromatographic strips. Food and Agricultural Immunology, 30(1), 913–923.
- Yuxi, C., Liqiang, L., Gang, C., Xiaoling, W., Hua, K., & Chuanlai, X. (2019). Development of an immunochromatographic strip for the detection of rosiglitazone in functional foods based on monoclonal antibodies. Analytical Methods, 11(38), 4910–4916.
- Zeng, L., Jiang, W., Liu, L. Q., Song, S. S., & Kuang, H. (2018). Development of ic-ELISA and lateral-flow immunochromatographic strip for detection of vitamin B-2 in an energy drink and vitamin tablets. Food and Agricultural Immunology, 29(1), 121–132.
- Zhongxing, W., Xiaoling, W., Liguang, X., Hua, K., & Chuanlai, X. (2019). Detection of triclabendazole and three metabolites in bovine muscle samples with a gold nanoparticle-based lateral flow immunoassay. Analytical Methods, 11(42), 5478–5486.