?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Carprofen (CAR) is a non-steroidal anti-inflammatory drug commonly used to treat various musculoskeletal diseases and infections in food animals. While CAR residues in food animal tissues caused by veterinary medicine abuse are a potential danger to human health. In this study, a high-affinity monoclonal antibody (mAb) against CAR was prepared, and then an immunochromatographic strip for rapidly screening CAR in bovine muscle was developed to help enforce regulatory compliance. The visual detection limit (cut-off value) by the naked eye of the fabricated test strip was 12.5 ng g−1. Scanning by the strip reader, the IC50 of the test strip was calculated as 1.743 ng g−1, and the limit of detection (LOD, IC10) was 0.283 ng g−1. The proposed method provided an efficient tool for monitoring CAR in bovine muscle. Meantime, our work suggested great potential for the analysis of CAR residues in other food matrixes.
Introduction
Carprofen (CAR), one of the non-steroidal anti-inflammatory drugs (NSAIDs), is frequently employed for anti-inflammatory, antipyretic and analgesic in humans (Starek & Krzek, Citation2009). NSAIDs exert an anti-inflammatory effect by reversibly binding to cyclooxygenase (COX) to inhibit the conversion of arachidonic acid to prostaglandins (Barreto et al., Citation2019). In veterinary medicine, CAR is applied in the treatment of colic and musculoskeletal disorders, infectious diseases, mastitis metritis agalactia syndrome, mastitis, heat shock caused by an increase of environment temperature and reducing stress during transportation in livestock and poultry (Bobkov & Zbinden, Citation2018; Caplen et al., Citation2013, Citation2013; Jedziniak, Szprengierjuszkiewicz, & Olejnik, Citation2009). However, as CAR has been increasingly used by farmers in raising domestic animals, improper use and disrespect for the required withdrawal period become emerged in recent years (Sundlof, Citation2014). These events may result in CAR residues in animal tissues or products and constitute potential health risks for consumers. It is well known that veterinary drug residues can directly induced allergic reactions and hormonal imbalances (Pugajeva, Ikkere, Judjallo, & Bartkevics, Citation2019). It has been experimentally confirmed that NSAIDs have various toxic effects on human including aplastic anemia/agranulocytosis,(Jiang, Fuller, Hsieh, & Rao, Citation2018) gastrointestinal ulcers (Lefkowith, Citation1999; Misurac et al., Citation2013), renal dysfunction (Griffin, Yared, & Ray, Citation2000; Whelton, Citation1999), hepatic failure (Carrillo-Jimenez & Nurnberger, Citation2000; Lee, Citation2004) and cardiovascular toxicity (Gislason, Citation2006; Naidoo et al., Citation2018; Smolinske, Hall, Vandenberg, Spoerke, & McBride, Citation1990). In response to these food safety concerns, European Union (EU) has recommended the maximum residue limits (MRLs) for CAR (Gentili et al., Citation2012) in bovine muscle as 500 µg kg−1, bovine liver as 1000 µg kg−1 and bovine kidney as 1000 µg kg−1. Moreover, CAR was a target in the 2017 EU monitoring plan (Bobkov & Zbinden, Citation2018). To meet these regulatory requirements, it is imperative to establish practical and effective approaches for the detection of CAR residues in food animal tissues.
Multiple physicochemical analysis techniques have been developed for either screening or quantitatively testing CAR in numerous matrixes. For instance, high performance liquid chromatography-ultraviolet detection (HPLC-UV) (Barreto et al., Citation2019; Kamaruzaman, Sanagi, Endud, Wan Ibrahim, & Yahaya, Citation2013), liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Britzi & Schwartsburd, Citation2019; Desmarchelier et al., Citation2018; Igualada, Moragues, & Pitarch, Citation2007; Wang, Jia, et al., Citation2019), ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) (Liu et al., Citation2019) and so on. Even though these instrumental detection tools pose excellent sensitivity, stability and reproducibility, they are high-priced, time-consuming and require trained personnel, which make them unsuitable for on-site screening of the high-throughput samples (Chen et al., Citation2019). Whereas, immunological detection methods are simple and reliable. Especially, due to the benefits of visual interpretation and quickness the immunochromatographic strip (ICGIS) has been considerably employed for monitoring many veterinary drugs, such as nitroxinil (Na et al., Citation2019), halofuginone (Song et al., Citation2019), sildenafil (Wang et al., Citation2019), cimaterol (Liu, Liu, Cui, Wu, & Kuang, Citation2019), melamine (Guo et al., Citation2019), spiramycin (Guo et al., Citation2019) and so on. Up to now, few researches on the immunoassay strip for detecting CAR have been reported. To compliance with the regulatory limits of CAR, we intended to develop a lateral flow immunochromatographic strip for the rapid screening of CAR. Herein, we designed an immunogen by conjugating CAR with bovine serum albumin (BSA) and obtained a target monoclonal antibody (mAb). Afterwards, an immunoassay strip for qualitatively and quantitatively monitoring CAR in bovine muscle was successfully fabricated.
Materials and methods
Reagents and apparatus
Carprofen (CAR), flunixin (FLU), ketoprofen (KET), tolfenamic acid (TFA) and diclofenac sodium (DLO) standards were bought from Aladdin Chemistry Co. Ltd. (Shanghai, China). Horseradish peroxidase (HRP)-labelled goat anti-mouse immunoglobulin (IgG) antibody were obtained from Sino American Biotechnology Co. (Luoyang, China). Aspirin (ASP), BSA, ovalbumin (OVA), 1-ethyl-carbodiimidehydrochloride (EDC), N-hydroxysuccinimide (NHS), Freund’s complete adjuvant (FCA), Freund’s incomplete adjuvant (FIA), hypoxanthine aminopterin thymidine (HAT), hypoxanthine thymidine (HT) and mouse monoclonal antibody isotyping kit were purchased from Sigma (St. Louis, USA). RPMI-1640 medium was from Solarbio (Beijing, China) and PEG1500 was from Roche (Mannheim, Germany). Ninety-six-well reaction plates were purchased from Nunc (Roskilde, Denmark). Polystyrene hard board, nitrocellulose (NC) membrane, glass fibre and absorbent pad were purchased from Millipore (Bedford, USA). The Microplate Reader 550 was obtained from Bio-Rad (Richmond, USA). The XYZ Biostrip Dispenser, CM 4000 Cutter and TSR3000 Membrane Strip Reader were all bought from Bio-Dot (Richmond, USA).
Eight-week-old female BALB/c mice were provided by the Laboratory Animal Center, Zhengzhou University, China and kept in Key Laboratory of Animal Immunology (KLAI), Henan Academy of Agricultural Sciences. The animal experiments were approved by the Animal Ethics Committee and preformed according by the guidelines of KLAI.
Synthesis of immunogen and coating antigen
Since CAR possesses a free carboxyl group, the active ester method was conducted to conjugate CAR with the carrier proteins BSA (CAR-BSA) and OVA (CAR-OVA) as the immunogen and coating antigen, respectively. First, 10 mg of CAR was dissolved in 1 mL of methanol and the pH of the solution was adjusted to 5.0 using 0.1 mmol L−1 HCl. Then 10.5 mg EDC and 6.3 mg NHS were added and this mixture solution was reacted under stirring for 15 min at room temperature. Second, the reaction mixture was added dropwise into 1 mL of CBS solution containing 12.1 mg of BSA. Then the mixture was reacted under stirring for 4 h at room temperature. Third, the reaction solution was dialyzed for 3 d in PBS buffer. The ultimate reactants were frozen at −20°C. The CAR-OVA was synthesized by the same method as above.
Preparation of mAb
The preparation of mAb against CAR was according to the previous article (Na et al., Citation2019). Three female eight-week-old BALB/c mice received four subcutaneous injections of immunogen CAR-BSA with the dose of 50 µg each mouse. In the first immunization, 100 µL of PBS solution containing CAR-BSA was fully emulsified with an equal volume of FCA. In the other three immunizations, FCA was superseded by FIA as the emulsifier. Next, mouse sera were collected on the seventh day after the fourth immunization and determined by the indirect competitive ELISA (ic-ELISA). The mouse secreted the optimal antibodies was chosen as a spleen donor. Then the superior mouse acquired an enhanced abdominal immunity. Three days later, the splenocytes were fused with mouse myeloma cells. After screening the supernatant of the hybridoma cells, the positive wells were continuously subcloned until the target monoclonal cell line was picked out. The cultured monoclonal cells were intraperitoneally injected into paraffin-treated mice to produce ascites. Finally, the ascites fluid was purified by the octanoic acid-ammonium sulfate method (CA-SA) and stored at −80°C.
Ic-ELISA
The properties of the anti-CAR mAb were identified by ic-ELISA. The procedure of ic-ELISA was referred to the reported literature (Na et al., Citation2019). The optimum concentration of coating antigen was determined as 0.375 µg mL−1 by ic-ELISA. First, 100 µL of coating antigen was added into each well of the polyethylene microplate, and the plate was incubated at 37°C for 2 h. After washing the plate four times by PBST, each well was blocked with 250 µL of 5% skimmed milk and incubated at 37°C for 1 h. Then the plate was washed four times and stored at 4°C for later use. CAR was dissolved in methanol to prepare 1 mg mL−1 of CAR standard solution. The standard solution was diluted with PBS (pH 7.4) to seven concentrations of 0.625, 0.313, 0.156, 0.078, 0.039, 0.020 and 0 ng mL−1. The ic-ELISA comprised the following steps. First, 100 µL of the diluted CAR standard solution and 100 µL of anti-CAR mAb were successively added into the well, then the plate was incubated at 37°C for 15 min. Following a wash step, each reaction well was added 100 µL of HRP-conjugated goat anti-mouse IgG and incubated at 37°C for 30 min. The plate was washed again and then loaded with 100 µL of TMB substrate per well at 37°C for 15 min. The colour reaction was terminated by 50 µL of 2 M sulfuric acid per well. Ultimately, the reaction plate was measured by the microplate reader at 450 nm and the optical density values (ODs) were obtained. The standard linear curve was constructed by plotting the ratio of the positive and negative ODs against the logarithm of a range concentrations of CAR (0.020–0.625 ng mL−1). The half inhibition concentration (IC50) could be calculated from the curve. The each cross-reactivity rate (CR) of the mAb with other analogues was computed according to the following formula:
Preparation of gold nanoparticles and gold-labelled mAb
The gold nanoparticles (AuNPs) were generated by the traditional reduction of gold chloride with trisodium citrate (Frens, Citation1973; Sun et al., Citation2018). First of all, 100 mL of 0.01% (w/v) gold chloride tetrahydrate (HAuCl4) solution was added to a conical flask and heated to boiling. Next, 1.6 mL of 1% trisodium citrate solution was rapidly added to the boiling solution under stirring. The mixture solution gradually changed from grey to wine red and continued to boil for 5 min, then cooled to room temperature. The resulting solution was stored at 4°C until use.
In order to determine the appropriate concentration of the mAb labelled with AuNPs, 2 µL of the mAb was added to 58 µL of ultrapure water (UPW) in reaction microplate and 2-fold serially diluted in UPW. 125 µL of colloidal gold solution with pH adjusted to 8.2 by 0.1 M K2CO3 was added in the reaction well. After the solution was reacted at room temperature for 10 min, 125 µL of 9% NaCl solution was added. With the decrease of the mAb, the colour of the liquid changed from bright red to blue. The optimal concentration of mAb was the minimum concentration that did not cause the colour change. Afterwards, 10 µL of mAb solution was dissolved in 200 µL of UPW, and then 10 mL of gold solution was added. The mixture was reacted at room temperature for 30 min under stirring and 2 mL of 10% BSA solution was added to block the unbound sites of the AuNPs. 30 min later, the reaction solution was centrifuged at 12500 × g at 4°C for 30 min. Then the supernatant was carefully discarded and the remaining substances were resuspended in HB buffer [20 mmol L−1 borate buffer (pH 9.0) containing 1% BSA, 3% sucrose and 0.03% sodium azide]. The labelled mAb solution was kept at 4°C for later use.
Characterization of immunochromatographic strip
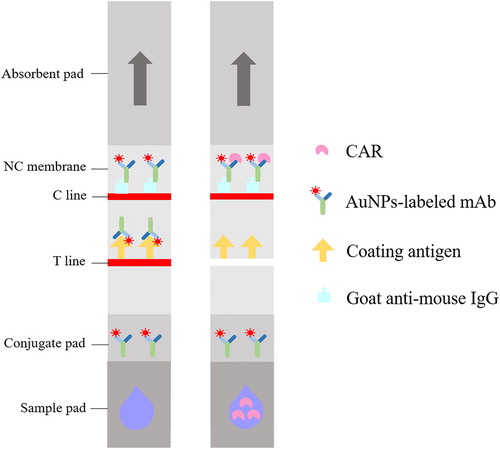
The assembly diagram of the immunochromatographic strip with the ic-ELISA format was shown in . The test strip was fabricated with a semirigid polyethylene sheet (backing card), a sample pad, a conjugate pad, a nitrocellulose (NC) membrane and an absorbent pad. The NC membrane was sprayed with one test line (T line, CAR-OVA) and one control line (C tine, goat anti-mouse IgG) by an XYZ Biostrip Dispenser. After drying at 42°C for 1 h, the membrane was blocked with 2% (w/v) BSA and attached to the centre of the backing card. The conjugate pad was coated with 7 µL cm−1 of AuNPs-labelled mAb solution and dried at 37°C for 40 min. The sample pad was immersed in 100 mM PB buffer (1% BSA, 5% sucrose, 0.3% Tween 20 and 0.05% sodium azide) and dried at 42°C for 4 h. An absorbent pad maintaining capillary force was attached to the other end of the backing sheet to promote liquid flow. Finally, the assembled card was cut into strips 3 mm wide and stored in a vacuum sealed pouch.
The principle of immunochromatographic assay
The detection principle of the strip test was similar to ic-ELSIA and depicted in . After the strip was inserted into the sample extract, the fluid flowed towards the absorbent pad under capillary force. In the absence of CAR, as the extract flowed through the conjugate pad, the AuNPs-labelled mAb was dissolved, released and then captured by CAR-OVA immobilized on the T line. Hence, the test zone appeared a bright red band. If CAR existed in the extract, CAR would compete against the capture antigen on the T line to specifically bind to the limited mAb. As the concentration of CAR in the sample increased, the colour of the test line became lighter and even disappeared. However, in either case the AuNPs-labelled mAb would eventually be intercepted by the goat anti-mouse IgG on the C line. Therefore, the control zone would always show one red band, otherwise it proved that the strip was invalid.
Preparation of spiked bovine muscle samples
The bovine muscle samples were bought from the local supermarket. 4 g of the muscle was transferred to a ceramic mortar and ground thoroughly. Then 40 mL of methanol was added and the mixture was moved to a 50 mL centrifuge tube. The mixed solution was vortexed for 2 min and then ultrasonically extracted for 10 min. Next, the extracting solution was centrifuged at 10000 × g for 10 min and then the supernatant was mindfully collected. After the clarified extract was dried in a stream of nitrogen, the remainder was re-dissolved in 4 mL of PBS buffer containing 10% methanol. 1 mg mL−1 of CAR standard was diluted with the final extract to a series of concentrations for the identification of the strip sensitivity. In the recovery test, the muscle samples spiked with three concentrations of CAR (1, 3 and 5 ng g−1) were extracted as described above. Then each sample was determined by the same batch of the strips in triplicate for intra-assay test and three different batches for inter-assay test.
Results and discussion
Characterization of immunoreagents for CAR
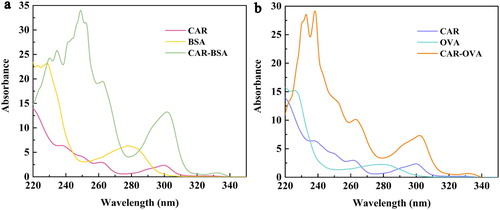
The characterizations of the immunogen CAR-BSA and the coating antigen CAR-OVA were performed by scanning UV spectrum. The results were shown in . Actually, CAR had an extremely distinct characteristic absorption peak (CAP) at 300 nm and the CAP of BSA or OVA was at 280 nm. While, CAR-BSA or CAR-OVA also had a CAP at 300 nm and the peak shape of CAR-BSA or CAR-OVA was similar to that of CAR. It could be preliminarily judged that the coupling of CAR and BSA/OVA was successful.
Identification of mAb properties
Through the cell screening, a monoclonal cell line 1F5-C8 with the highest sensitivity to CAR was obtained. As shown as in (b), the equation of the competitive inhibition linear standard curve for CAR of 1F5-C8 was y = −0.3579x + 0.1505. The coefficient of determination (R2) of the standard equation was 0.997, which indicated the curve fitted the sample data well. Calculated from the equation, the IC50 value was 0.106 ng g−1. The titre of the mAb against CAR was 1.02 × 106. The mAb was identified as IgG1 and the result was described as (a). To assess the CR, several substances with similar structures to CAR such as aspirin (ASP), flunicin (FLU), diclofenac sodium (DIC), ketotifen (KET) and tolfenamic acid (TOL) were analysed by the ic-ELISA. The result was shown in , 1F5-C8 was considered not to recognize other analogues, the CRs were all less than 1.0 × 106. Generally, 1F5-C8 with high sensitivity and desirable specificity could be used for further experiments on the strip.
Figure 3. The subtype of 1F5-C8 (a) and the standard curve of CAR detection by the ic-ELISA in PBS (b).
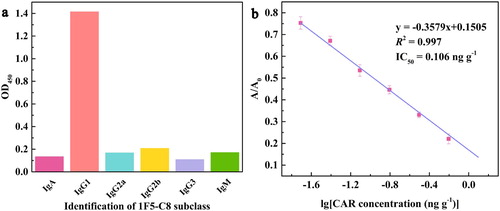
Table 1. The cross-reaction of CAR with other compounds by ic-ELISA in bovine muscle samples.
Analytical performance of the immunochromatographic assay
During the preparation of the test strip, the colour depth was used to judge the optimal parameters of the strip. summarized the optimized parameters of the test strip. After a series of colour comparisons, the optimal concentration of the coating antigen on T line was identified as 0.3 mg mL−1 (55 ng cm−1) and the optimal concentration of the mAb labelled with gold nanoparticles was 12.5 µg mL−1 (75 ng cm−1). The optimized reaction buffer was PBS containing 10% methanol with the pH of 7.4.
Table 2. The optimized experimental parameters of the test strip.
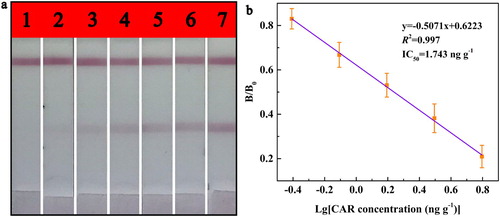
The CAR standard was diluted with the extract solution to a range of concentrations (12.50, 6.25, 3.13, 1.57, 0.79, 0.40 and 0 ng g−1) for determining the sensitivity of the strip in bovine muscle sample. The visual detection limit (cut-off value) by the naked eye was specified as the minimum CAR concentration that caused the T line colourless. As shown as in (a), the colour of the T line disappeared completely when CAR concentration was 12.5 ng g−1. Therefore, the cut-off value was considered as 12.5 ng g−1. The strip could be used to quantitatively detect CAR by scanning with the TSR3000 Membrane Strip Reader. The result was shown in (b), the equation of the standard linear curve was y = −0.5071x + 0.6223 with R2 of 0.998. Then, the IC50 value was calculated as 1.743 ng g−1 and the LOD was 0.283 ng g−1, the linearity region (IC20-IC80) for measuring CAR ranged from 0.446 ng g−1–6.804 ng g−1.
Recovery test of CAR with the immunochromatographic assay
A recovery experiment was conducted to evaluate the accuracy and precision of the strip. The bovine samples spiked with 1, 3 and 5 ng g−1 of CAR were respectively tested by the same or three different batches of strips. The results of the recovery test were shown in . In the intra-assay, the recovery rates ranged from 82.51% to 90.55% and the highest CV was 7.29%. In the inter-assay, the recovery rates were from 79.53% to 87.58% and the highest CV was 8.81%. Hence, the developed test strip posed satisfactory accuracy and precision.
Table 3. Recoveries and CVs of the test strips for CAR spiked into bovine muscle samples.
Conclusions
In this study, an effective immunogen (CAR-BSA) was prepared and a highly sensitive and specific mAb against CAR was obtained. On this basis, we presented an immunochromatographic strip based on indirect competitive format for real-timely monitoring CAR in bovine muscle. The entire test process took only 10 min and the result could be judged by the naked eye. In general, the strip exhibited excellent sensitivity with the cutoff value of 12.5 ng g−1 and a low LOD of 0.283 ng g−1. Meanwhile, the accuracy and precision of the strip were also allowable. The proposed strip could be used not only for screening CAR on field, but also offered the potential to detect CAR in other food matrixes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Barreto, F., Jank, L., Castilhos, T., Rau, R. B., Andrade Tomaszewski, C., Ribeiro, C., & Hillesheim, D. R. (2019). Chapter 13 - chemical residues and Mycotoxins in raw milk. In L. A. Nero & A. F. De Carvalho (Eds.), Raw milk (pp. 273–293). London: Academic Press.
- Bobkov, M., & Zbinden, P. (2018). Occurrence of veterinary drug residues in poultry and products thereof. a review. CHIMIA International Journal for Chemistry, 72(10), 707–712. doi: 10.2533/chimia.2018.707
- Britzi, M., & Schwartsburd, F. (2019). Development and validation of a high-throughput method for the determination of eight non-steroidal anti-inflammatory drugs and Chloramphenicol in milk, using liquid chromatography-tandem mass Spectroscopy. International Journal Analyt Bioanalyt Methods, 1(005), 1–12.
- Caplen, G., Baker, L., Hothersall, B., McKeegan, D. E. F., Sandilands, V., Sparks, N. H. C., … Murrell, J. C. (2013). Thermal nociception as a measure of non-steroidal anti-inflammatory drug effectiveness in broiler chickens with articular pain. The Veterinary Journal, 198(3), 616–619. doi: 10.1016/j.tvjl.2013.09.013
- Caplen, G., Colborne, G. R., Hothersall, B., Nicol, C. J., Waterman-Pearson, A. E., Weeks, C. A., & Murrell, J. C. (2013). Lame broiler chickens respond to non-steroidal anti-inflammatory drugs with objective changes in gait function: A controlled clinical trial. The Veterinary Journal, 196(3), 477–482. doi: 10.1016/j.tvjl.2012.12.007
- Carrillo-Jimenez, R., & Nurnberger, M. (2000). Celecoxib-induced acute pancreatitis and hepatitis: A case report. Archives of Internal Medicine, 160(4), 553–554. doi: 10.1001/archinte.160.4.553
- Chen, Z., Wu, X., Xu, L., Liu, L., Kuang, H., & Cui, G. (2019). Development of immunocolloidal strip for rapid detection of pyrimethanil. Food and Agricultural Immunology, 30(1), 1239–1252. doi: 10.1080/09540105.2019.1677566
- Desmarchelier, A., Fan, K., Minh Tien, M., Savoy, M. C., Tarres, A., Fuger, D., … Mottier, P. (2018). Determination of 105 antibiotic, anti-inflammatory, antiparasitic agents and tranquilizers by LC-MS/MS based on an acidic QuEChERS-like extraction. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 35(4), 646–660.
- Frens, G. (1973). Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature Physical Science, 241(105), 20–22. doi: 10.1038/physci241020a0
- Gentili, A., Caretti, F., Bellante, S., Rocca, L. M., Curini, R., & Venditti, A. (2012). Development and validation of two multiresidue liquid chromatography tandem mass spectrometry methods based on a versatile extraction procedure for isolating non-steroidal anti-inflammatory drugs from bovine milk and muscle tissue. Analytical and Bioanalytical Chemistry, 404(5), 1375–1388. doi: 10.1007/s00216-012-6231-0
- Gislason, G. H. (2006). Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective nonsteroidal antiinflammatory drugs after acute myocardial infarction. Circulation, 113(25), 2906–2913. doi: 10.1161/CIRCULATIONAHA.106.616219
- Griffin, M. R., Yared, A., & Ray, W. A. (2000). Nonsteroidal antiinflammatory drugs and acute renal failure in elderly persons. American Journal of Epidemiology, 151(5), 488–496. doi: 10.1093/oxfordjournals.aje.a010234
- Guo, L., Liu, L., Xu, L., Kuang, H., Cui, G., & Xu, C. (2019). Gold immunochromatography assay for the rapid detection of spiramycin in milk and beef samples based on a monoclonal antibody. Biotechnology Journal, 15(1), 1900224. doi: 10.1002/biot.201900224
- Guo, M., Wu, X., Song, S., Zheng, Q., Luo, P., Kuang, H., … Ye, L. (2019). Ultrasensitive anti-melamine monoclonal antibody and its use in the development of an immunochromatographic strip. Food and Agricultural Immunology, 30(1), 462–474. doi: 10.1080/09540105.2019.1590318
- Igualada, C., Moragues, F., & Pitarch, J. (2007). Rapid method for the determination of non-steroidal anti-inflammatory drugs in animal tissue by liquid chromatography–mass spectrometry with ion-trap detector. Analytica Chimica Acta, 586(1-2), 432–439. doi: 10.1016/j.aca.2006.10.040
- Jedziniak, P., Szprengierjuszkiewicz, T., & Olejnik, M. (2009). Multi-residue screening method for the determination of non-steroidal anti-inflammatory drug residues in cow's milk with HPLC-UV and its application to meloxicam residue depletion study. Bulletin of the Veterinary Institute in Puławy, 53(4), 731–739.
- Jiang, X., Fuller, D., Hsieh, Y. P., & Rao, Q. (2018). Monoclonal antibody-based ELISA for the quantification of porcine hemoglobin in meat products. Food Chemistry, 250, 170–179. doi: 10.1016/j.foodchem.2018.01.032
- Kamaruzaman, S., Sanagi, M. M., Endud, S., Wan Ibrahim, W. A., & Yahaya, N. (2013). MCM-41 solid phase membrane tip extraction combined with liquid chromatography for the determination of non-steroidal anti-inflammatory drugs in human urine. Journal of Chromatography B, 940, 59–65. doi: 10.1016/j.jchromb.2013.09.017
- Lee, W. M. (2004). Acetaminophen and the U.S. Acute liver failure study group: Lowering the risks of hepatic failure. Hepatology, 40(1), 6–9. doi: 10.1002/hep.20293
- Lefkowith, J. B. (1999). Cyclooxygenase-2 specificity and its clinical implications. The American Journal of Medicine, 106(5B), 43S–50S. doi: 10.1016/S0002-9343(99)00116-3
- Liu, S., Li, S., Yang, W., Gu, F., Xu, H., Wang, T., … Hou, X. (2019). Magnetic nanoparticle of metal-organic framework with core-shell structure as an adsorbent for magnetic solid phase extraction of non-steroidal anti-inflammatory drugs. Talanta, 194, 514–521. doi: 10.1016/j.talanta.2018.10.037
- Liu, Z., Liu, L., Cui, G., Wu, X., & Kuang, H. (2019). Development of an immunochromatographic strip assay based on a monoclonal antibody for detection of cimaterol. Food and Agricultural Immunology, 30(1), 1162–1173. doi: 10.1080/09540105.2019.1674787
- Misurac, J. M., Knoderer, C. A., Leiser, J. D., Nailescu, C., Wilson, A. C., & Andreoli, S. P. (2013). Nonsteroidal anti-inflammatory drugs are an important cause of acute kidney injury in children. The Journal of Pediatrics, 162(6), 1153–1159. doi: 10.1016/j.jpeds.2012.11.069
- Na, G., Hu, X., Yang, J., Sun, Y., Kwee, S., Tang, L., … Zhang, G. (2019). A rapid colloidal gold-based immunochromatographic strip assay for monitoring Nitroxynil in milk. Journal of the Science of Food and Agriculture, 100(5), 1860–1866. doi: 10.1002/jsfa.10074
- Naidoo, V., Taggart, M. A., Duncan, N., Wolter, K., Chipangura, J., Green, R. E., & Galligan, T. H. (2018). The use of toxicokinetics and exposure studies to show that carprofen in cattle tissue could lead to secondary toxicity and death in wild vultures. Chemosphere, 190, 80–89. doi: 10.1016/j.chemosphere.2017.08.167
- Pugajeva, I., Ikkere, L. E., Judjallo, E., & Bartkevics, V. (2019). Determination of residues and metabolites of more than 140 pharmacologically active substances in meat by liquid chromatography coupled to high resolution Orbitrap mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 166, 252–263. doi: 10.1016/j.jpba.2019.01.024
- Smolinske, S. C., Hall, A. H., Vandenberg, S. A., Spoerke, D. G., & McBride, P. V. (1990). Toxic effects of nonsteroidal anti-inflammatory drugs in overdose. Drug Safety, 5(4), 252–274. doi: 10.2165/00002018-199005040-00003
- Song, S., Suryoprabowo, S., Liu, L., Kuang, H., Xu, L., Ma, W., & Wu, X. (2019). Development of monoclonal antibody-based colloidal gold immunochromatographic assay for analysis of halofuginone in milk. Food and Agricultural Immunology, 30(1), 112–122. doi: 10.1080/09540105.2018.1550058
- Starek, M., & Krzek, J. (2009). A review of analytical techniques for determination of oxicams, nimesulide and nabumetone. Talanta, 77(3), 925–942. doi: 10.1016/j.talanta.2008.09.022
- Sun, Y., Yang, J., Yang, S., Sang, Q., Teng, M., Li, Q., … Zhang, G. (2018). Development of an immunochromatographic lateral flow strip for the simultaneous detection of aminoglycoside residues in milk. RSC Advances, 8(17), 9580–9586. doi: 10.1039/C8RA01116H
- Sundlof, S. F. (2014). Veterinary drugs residues: Veterinary drugs – general. Encyclopedia of Food Safety, 3, 35–38. doi: 10.1016/B978-0-12-378612-8.00248-1
- Wang, Y., Jia, M., Wu, X., Wang, T., Wang, J., & Hou, X. (2019). PEG modified column MIL-101(Cr)/PVA cryogel as a sorbent in stir bar solid phase extraction for determination of non-steroidal anti-inflammatory drugs in water samples. Microchemical Journal, 146, 214–219. doi: 10.1016/j.microc.2018.12.045
- Wang, Z., Wu, X., Liu, L., Xu, L., Kuang, H., & Xu, C. (2019). An immunochromatographic strip sensor for sildenafil and its analogues. Journal of Materials Chemistry B, 7(41), 6383–6389. doi: 10.1039/C9TB00280D
- Whelton, A. (1999). Nephrotoxicity of nonsteroidal anti-inflammatory drugs: Physiologic foundations and clinical implications. The American Journal of Medicine, 106(5B), 13S–24S. doi: 10.1016/S0002-9343(99)00113-8