ABSTRACT
Maximum permissible levels of mycotoxins in baby food may be 1% of those in ordinary food. Therefore, highly sensitive methods of mycotoxin control are in demand. To detect such low amounts, expensive instrumental methods are commonly used. Advantages of immunochromatographic analyses are their low cost and simple sample preparation; however, their sensitivity needs to be increased to contend with instrumental methods. A scheme for competitive immunochromatography with indirect labelling was implemented and developed for the detection of mycotoxin zearalenone (ZEA). Two separate reagents were used for the assay, namely free specific antibodies and antispecies antibodies conjugated with gold nanoparticles. This made it possible to simultaneously increase the sensitivity of the assay and the reliability of measurements. The instrumental detection limit of ZEA in baby food was 5 pg/mL (100 pg/g). Thus, the sensitivity attained is comparable with liquid chromatography characteristics. The duration of the analysis was 17 min.
1. Introduction
Detection of mycotoxins in children’s nutrition is extremely important because mycotoxins have teratogenic, mutagenic, and oncogenic effects; reduce immunity; and weaken the child’s overall health (Barug, Citation2006; Erkekoğlu et al., Citation2008; Peraica et al., Citation1999; Pestka, Citation1994; Pestka et al., Citation2004; Schatzmayr & Streit, Citation2013; Van Egmond et al., Citation2007). Owing to the high risk of disease caused by mycotoxin contamination (Erkekoğlu et al., Citation2008), the legislative regulations specify extremely low limits for mycotoxins in baby food. For example, according to the Recommendations of the European Commission 2007/1126/EU, the maximum permissible levels for zearalenone (ZEA) and aflatoxin B1 in baby food are 20 ng/g and 100 pg/g, respectively. These restrictions require detection of mycotoxins at picogram levels. Until now, such extremely low amounts of analyte could be determined only by complex instrumental methods of analysis, such as high-performance liquid chromatography (HPLC) (Kafouris et al., Citation2017; McNamee et al., Citation2017).
The simple and rapid detection of compounds under nonlaboratory conditions has been highly requested in recent years (Alldrick, Citation2014; Von Holst & Stroka, Citation2014). Immunochromatographic analysis (ICA) is one of the most efficient means of solving this problem and is actively used for the rapid detection of pathogens, disease markers, hormones, food toxicants, and so on (Mak et al., Citation2016; Raeisossadati et al., Citation2016; Urusov et al., Citation2010; Yang et al., Citation2015). ICA is a simple and time-saving method. However, picogram detection limits are unattainable with traditional immunochromatographic methods, and novel solutions are needed for mycotoxins’ control in baby food. Developing ICA for low-molecular-weight compounds is associated with additional difficulties because the common approaches to reducing the detection limit cause a loss of reliability.
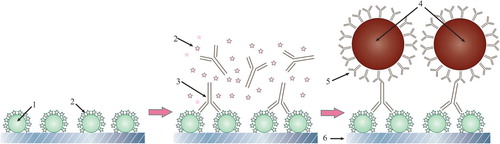
A competitive scheme in ICA is based on the interaction of labelled specific antibodies with the sample’s antigen and the antigen immobilized in the test strip’s analytical zone (Wong & Tse, Citation2009). The lower the content of the test compound in the sample, the more antibodies (and the marker) will bind in the analytical zone, causing a more intense colouration. Reducing the concentration of specific antibodies increases the test’s sensitivity, and in addition, in this case, lower antigen concentration can prevent the binding of antibodies in the analytical zone. However, in traditional immunochromatography, this decrease is accompanied by a decrease in the marker’s concentration and the intensity of the colouration, thereby leading to less reliable results. In the standard assay scheme (), an excess of antibodies is used in a conjugation, which provides more efficient binding. However, for a competitive scheme, the presence of several reactive antibodies on the surface of one particle may lead to an increase in the detection limit. This is caused by two factors. First, with a high surface density of immobilized antibodies, the probability of a polyvalent interaction between the conjugate and such a modified surface increases. Furthermore, competitive inhibition of this high-affinity binding requires high antigen concentrations. Second, as a result of the numerous antibodies immobilized on a particle, the interaction of a part of them with the antigen molecules in a sample does not interfere with the interaction of the conjugate with the immobilized antigen.
Figure 1. Scheme of traditional immunochromatographic analysis showing that a large amount of antigen in the sample is required to block all antigen-binding sites.
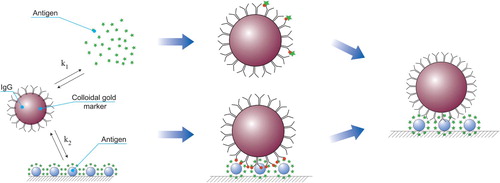
The antibody–marker conjugate is polyvalent and contains near 102 of antibodies on the surface of one particle (Sotnikov et al., Citation2015). Therefore, the conjugate binds to the antigen in the analytical zone (also polyvalent) with much higher affinity than with the free analyte of the sample. As a result, immunochromatography has a significantly lower sensitivity, as compared to enzyme-linked immunosorbent assay (ELISA) with the same immunoreagents (Huang et al., Citation2014).
Various methods of increasing sensitivity in immunochromatography have been suggested, but they all have critical shortcomings. Thus, the treatment of bound gold nanoparticles (GNPs) by silver salts with the formation of larger and brighter particles is widely used (Chiao et al., Citation2004; Linares et al., Citation2012; Panferov et al., Citation2016). However, silver salt solutions are insufficiently stable and so should be prepared directly before the assay. In addition, the use of this reagent enhances the background colouration. Alternate approaches are to use additional aggregating reagents, alternative markers, or detection methods (Dzantiev et al., Citation2014). The signal is increased in this way, but the problems of polyvalent conjugates and inefficient competition are not solved.
Previously, we proposed an alternative ICA scheme that avoids these limitations (Urusov et al., Citation2016, Citation2017). Conjugates of specific antibodies and GNPs were replaced with a combination of native specific antibodies and secondary (antispecies) antibodies conjugated to GNPs. Separating the stages of interaction of specific antibodies with the antigen to be analysed and the binding of the formed immune complexes to a detectable marker allows a significant increase in the ratio (marker: specific antibodies). The growth of this ratio leads to a higher sensitivity of detection. At the same time, the high signal (the intensity of the detected colouration in the analytical zone) and, accordingly, the reliability of the visual evaluation of the analysis results and the accuracy of the quantitative determination are maintained.
In this paper, the indirect labelling approach was applied to create a highly sensitive assay for zearalenone (ZEA). This mycotoxin is found in food and feed products, posing a serious threat to human health and livestock (Heidtmann-Bemvenuti, Citation2011; Hussein & Brasel, Citation2001). ZEA changes the reproductive tract of animals and is associated with hyperplastic and neoplastic endometrial and human cervical cancer (Zinedine et al., Citation2007). Although ZEA has a relatively low acute toxicity, the European Union has established the lowest possible levels of Fusarium mycotoxins in food products. The maximum permissible levels of ZEA in baby food, according to the Commission Recommendation 2013/165/EU, are 20–100 μg/kg. Controlling the production of baby food is an important social and economic task. Our method can detect such small amounts of toxins in baby food through simple, time-saving, and inexpensive testing.
2. Materials and methods
2.1. Chemicals
The mouse monoclonal antibodies against ZEA were acquired from Jiangnan University (Wuxi, Jiangsu, China) (Kong et al., Citation2016). Dr. S. F. Biketov (Russian State Research Center for Applied Microbiology and Biotechnology, Obolensk, Moscow region, Russia; obolensk.org) provided the ZEA–bovine serum albumin (BSA) conjugate. The goat antimouse polyclonal antibodies were acquired from Arista Biologicals (Allentown, PA, USA; aristabiologicals.com). Antimouse immunoglobulins labelled with peroxidase were obtained from the N. F. Gamaleya Institute of Epidemiology and Microbiology (Moscow, Russia; www.gamaleya.ru). The BSA was acquired from MP Biomedicals (Santa Ana, CA, USA; mpbio.com). The sodium azide, 3,3´,5,5´-tetramethyl benzidine (TMB), Tween 20, and chloroauric acid were acquired from Sigma-Aldrich (St. Louis, MO, USA; sial.com). The Triton X-100 was obtained from Panreac Química SLU (Barcelona, Spain; itwreagents.com). The ZEA was acquired from Chromresurs (Moscow, Russia; chromresurs.ru). All other reagents were of analytical-grade purity or greater. Deionized water (18 MΩ·cm at 25°C; Simplicity Millipore, Billerica, MA, USA; www.millipore.com) was used for the preparation of all solutions.
The immunochromatographic test strips were fabricated from Hi-Flow membranes (Millipore, Billerica, MA, USA; merckmillipore.com). The reagents were applied with an IsoFlow dispenser (Imagene Technology, Hanover, NH, USA; imagenetechnology.com), and an Index Cutter-1 (A-Point Technologies, Gibbstown, NJ, USA) was used to cut the multimembrane composites into strips.
ELISA was implemented with Costar 9018 microplates (Corning, New York, NY, USA; www.corning.com). When conducting ELISA, absorbance of the reaction product at 450 nm was measured with a Zenyth 3100 microplate reader (Anthos Labtec Instruments, Salzburg, Austria; www.anthos-labtec.com).
2.2. Antibodies testing by ELISA
ZEA-BSA was incubated in microplate (Costar 96–Well Flat–Bottom EIA Plate, USA) wells overnight at 4°C at a concentration of 1 μg/mL in 100 µL of 50 mM phosphate buffer (PBS containing 100 mM NaCl, pH 7.4). After three washing steps with PBS containing 0.05% Triton X-100 (PBST), 100 µL of a solution of specific antibodies at a concentration of 200 ng/mL and antigen-containing samples at a concentration of 0.005–100 ng/mL for ZEA (in PBST) were added into the wells. The mixture was incubated at 37°C for 60 min. The formed immune complexes were detected by a peroxidase reaction. After the incubation and washing procedure, a solution of diluted peroxidase-labelled antimouse IgG (1:3000 in PBST) was added at 100 µL per well and incubated for 60 min at 37°C. After the incubation, the microplate was washed with PBST three times. To measure the peroxidase activity, the substrate solution (0.42 mM TMB and 1.8 mM H2O2 in 0.1 M citrate buffer, pH 4.0; 100 µL per well) was added. After incubation for 15 min at room temperature, 50 µL of 1 M H2SO4 was added to terminate the reaction. The absorbance of the reaction product was read at 450 nm with a microplate reader.
2.3. Immobilization of antibodies on gold nanoparticles
GNPs (50 µg/mL concentration, 30 nm diameter) were obtained according to the Frens method with modifications (Frens, Citation1973; Urusov et al., Citation2016, Citation2017). The pH of the GNP solution was adjusted to between 8.5 and 9.0 with potassium carbonate, followed by the addition of either specific or antispecies antibodies (10 µg/mL of GNP solution) diluted in 10 mM Tris buffer, pH 8.5 (TB). The resulting mixture was incubated for 45 min at room temperature. Next, a 10% aqueous solution of BSA (VGNP:VBSA = 40:1) was added, and the mixture was stirred vigorously for 15 min. The GNPs were pelleted by centrifugation at 15000 × g for 15 min at 4°C. The precipitate was resuspended in TB containing 1% BSA, 1% sucrose (TBSU), and 0.05% sodium azide. The obtained solution was stored at 4 °C.
2.4. Preparation of the immunochromatographic test strips
The ZEA–BSA conjugate and antispecies antibodies were dissolved in PBS to a concentration of 1.6 and 1.0 mg/mL respectively and then applied on nitrocellulose membrane (Millipore HF 135) fixed on a plastic support at a loading of 0.1 µL per 1 mm, using a dispenser. The nitrocellulose membrane was fastened with the top absorbing membrane.
For the traditional scheme, the same composite with the applied reagents and the absorbing membrane was used. In addition, antibodies to ZEA conjugated with GNPs were dissolved in TBSU containing 0.05% Tween 20 with D520 = 1.0 and applied to the glass–fiber conjugate pad (3.2 µL per 1 mm).
The membrane composites were cut into 3.5-mm wide test strips using a cutter and stored at room temperature in a sealed package containing silica gel.
2.5. Preparation and validation of tested samples
Baby foods (corn gruel) were purchased from local supermarkets. The samples were mixed with an extraction solution (70% methanol, 30% water) in a 1:5 [v/v] proportion and incubated with gentle mixing at room temperature for 30 min (Urusov et al., Citation2015; Yang et al., Citation2017). To separate the solids, the extract was filtered through the paper filters and kept at 4 °C for not more than 2 weeks. The extracts were analysed by HPLC as per Barbas et al. (Citation2005), and no ZEA were detected. ZEA solutions were introduced into the obtained extracts prior to performing the immunoassays.
2.6. Traditional ICA of ZEA
The test strips were vertically immersed in a series of samples with ZEA ranging from 0.05–9,000 ng/mL in baby food extracts mixed with PBST (1:2.5 [v/v]). After 17 min of incubation, the strips were scanned in a flatbed scanner (Canon Lide 90, Canon, Tokyo, Japan; canon.com) with a resolution of 600 dpi, without contrast or colour correction. The intensities of colouration were quantified using the Total Lab software (TotalLab, Newcastle upon Tyne, UK; totallab.com).
2.7. ICA of ZEA with indirect labelling
The test strips were vertically immersed in a mix of 30 µL food samples with ZEA (0.05–9,000 ng/mL in baby food extracts) and 70 µL specific antibody in PBST (50 ng/mL). Each test strip was incubated for 5 min. Next, the test strips were washed by immersing in PBST for 5 min and then incubated for 5 min in a solution of secondary antibodies conjugated with GNPs (D580 = 1). After 3 min of drying, the test strip was scanned. The total analysis time was 17 min. For statistical processing, all measurements were performed in triplicate.
2.8. Processing of ICA data
The dependence of the colour intensity on ZEA concentration was approximated by the four-parameter sigmoidal function y = (A − D)/(1 + [x/C]B ) + D, and the operating range for the quantitative detection (IC20–IC80) was calculated using the Origin 7.5 software (Origin Lab, Northampton, USA; originlab.com). The visual detection limit corresponded to the integrated colour intensity in the test zone equal to 25 arbitrary units. The instrumental detection limit corresponded to IC10.
3. Results and Discussion
3.1. Characteristics of specific antibodies
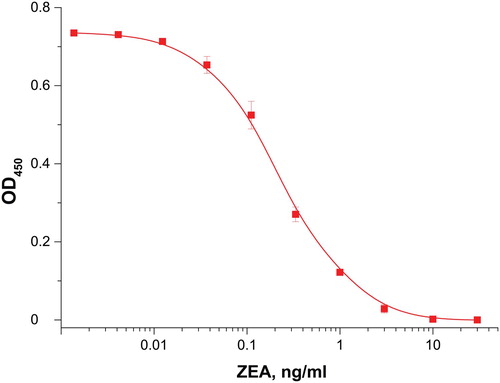
The reagents used for producing the immunochromatographic test strips were preliminarily characterized with the ELISA technique. The obtained concentration dependence for ZEA is shown in . The reached limit of ZEA detection is 0.05 ng/mL. The antibodies’ high affinity demonstrates them to be suitable reagents for the ICA systems under development.
3.2. ZEA measurements using a traditional ICA
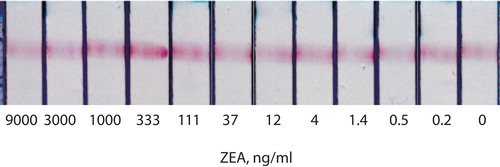
The test strips were made according to the optimal conditions (Urusov et al., Citation2011). For ZEA, competitive binding with the free antigen was not observed, even up to 9 μg/mL (), despite the affinity of the binding and the intense colouring of the analytical zone.
3.3. Principle of the proposed ICA with indirect labelling
The analysis scheme with the separation of the stages of competitive interaction and the introduction of a label is shown in . In the analytical zone, the conjugate of the antigen with the carrier protein is immobilized. The sample’s antigen binds to the free specific antibodies. If the concentration is above the threshold, then all valencies of the antibodies become occupied, and they do not bind in the analytical zone. Otherwise, the antibodies form an antigen-specific antibody complex in the analytical zone. To detect this, a conjugate of antispecies antibodies–GNP was passed along the test strip. Binding to specific antibodies provides the colouring of the analytical zone.
3.4. Choosing optimal parameters for the test system with indirect labelling
As previously shown (Urusov et al., Citation2014, Citation2016), some ICA parameters are universal and can be transferred to new analytes:
the chosen working membrane is a Hi-Flow HF135 membrane (Millipore), and it takes an average position in terms of flow rate and binding capacity and demonstrates high quality (uniform flow movement even for long incubations and several successive stages);
the conjugate of antispecies antibodies with colloidal label is taken with an excess (10 μg/mL) to exclude its influence on the assay parameters;
the absorbing membrane should ensure absorption of the entire volume of liquid flowing in several stages, and a bigger size than in the standard test is recommended.
Based on the properties of the ICA with indirect labelling (Urusov et al., Citation2016, Citation2017), the only characteristic requiring optimization for specific reactants is the concentration of free specific antibodies. This concentration is critical for ensuring the sensitivity of the analysis and the signal intensity because both a high signal and a reasonable detection limit should be provided (Urusov et al., Citation2017).
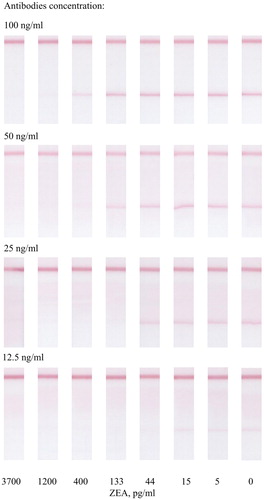
At a high concentration of antibodies (100 ng/mL), the sensitivity of the assay decreased (see ). At low antibody concentrations (25 and 12.5 ng/mL) the colouration was not sufficient and reproducible enough to reliably detect the analysis results. The 50 ng/mL concentration of antibodies was chosen as optimum. At such a concentration, the colouration of the zone is quite bright, and the ICA has the necessary sensitivity.
3.5. Characterizing ICA with indirect labelling
The test strips for ZEA ICA with indirect labelling were produced as described in the Materials and Methods section. They were tested for ZEA analysis in baby food (the added-found protocol), where the required detection limits are 20 μg/kg per the recommendations of the European Commission 2007/1126/EU. shows the results of the studies.
Figure 6. ICA of ZEA by indirect labelling. A: appearance of the strips’ analytical zones after testing extracts with different ZEA concentrations; B: calibration curve; dependence of the registered colouration from the ZEA concentration.
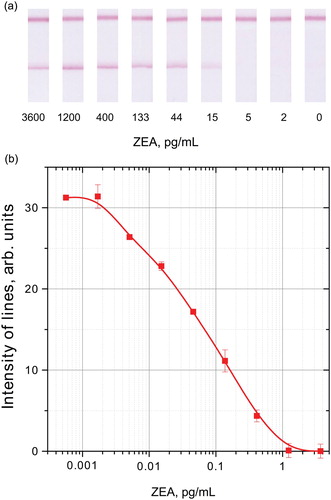
The combination of these factors led to a reduction in the detection limit while maintaining high colouration intensity. As a result, the instrumental registration was characterized by a detection limit of 5 pg/mL, and the disappearance of staining in the analytical zone was visually detected from 400 pg/mL. The standard deviation did not exceed 10%. The obtained results confirm the effectiveness of the indirect labelling principle in accordance with our previous studies of such assays (Urusov et al., Citation2014). As demonstrated earlier, in the traditional ICA with the same antibodies, the colouration of the analytical zone did not change, even at a ZEA concentration of 9 μg/mL, instead of high sensitivity of ELISA.
Thus, the developed ICA meets the practical requirements, and seems promising, for measuring the contamination of baby food and its raw materials. The added-found experiments showed high recovery of ZEA in corn gruel, from 96% to 109% (see ).
Table 1. Detection of ZEA by ICA with indirect labelling in corn gruel extracts.
The proposed separation of the competitive interaction stage and the detection of immune complexes allowed us to significantly reduce the number of specific antibodies and increase the content of the coloured marker. Thus, for the antibody–marker ratio equal to 300:1, the completion of one traditional test needs 8 × 1011 molecules of specific antibodies, whereas in the case of indirect labelling, the quantity decreased to 1010 molecules. Therefore, the consumption of antibodies is reduced 80 times, and it becomes possible to increase the amount of the marker without impairing the assay sensitivity.
These values are suitable for the detection of ZEA in baby food, the analysis of which had previously involved only physical methods (Romagnoli et al., Citation2010; Rubert et al., Citation2012). The instrumental detection limit of 5 pg/mL (100 pg/g) reached in our article is the lowest value for known ICA of ZEA. In published studies, the lowest detection limit was at 2.5 ng/mL in multiplex ICA (Chen et al., Citation2016), which is worse compared to both visual and instrumental detection limits of our study. Other developments demonstrated even higher values: 5 ng/mL (Shim et al., Citation2009), 6 ng/mL (Wang et al., Citation2013), 1–50 ng/mL (Liu et al., Citation2012), 50 ng/ml (Hao et al., Citation2018), and 80 ng/mL (Foubert et al., Citation2017).
Previously, we have used the separation of specific antibodies and a marker for the ICA of ZEA (Urusov et al., Citation2016); the reactants were combined and dried on one test strip, and the reached detection limit was not that low, measuring 350 pg/mL. In a new variant, the duration of the specific antibodies’ interaction with the sample’s antigen increased by the preliminary incubation of these reagents. This change makes the immunochemical reaction close to the equilibrium and thus helps reduce the detection limit. A preliminary 3–5-min incubation of the specific antibodies–marker conjugate and a tested sample is used in some test systems (Dykman & Khlebtsov, Citation2012; Wang et al., Citation2006; Xu et al., Citation2006; Yang et al., Citation2010) and provides improved sensitivity. In our developed method, the incubation of free specific antibodies with the sample made it possible to shift the detection limit by two orders of magnitude. Such high sensitivity can be claimed, given recent studies on the complex effect of mycotoxins (Cheli et al., Citation2015; Smith et al., Citation2016), when there is no excess of one toxin; however, the total contamination with different toxins can lead to negative effects.
Another advantage of this approach is the reduced consumption of specific immunoglobulins, which are significantly more expensive than antispecies antibodies. In addition, unlike labelled specific antibodies, a conjugate of GNPs with antispecies antibodies is a universal reagent that can be used in this ICA format to detect various compounds. This eliminates the need to conjugate a new specific antibody with GNPs and to characterize the resulting product for the development of each next test system.
4. Conclusions
For the first time, a highly sensitive immunochromatographic analysis for ZEA with a detection limit of 5 pg/mL in water-organic extracts of baby food was realized. The duration of the analysis was 17 min. In the proposed analysis format, the duration of the specific antibodies’ interaction with the sample’s antigen increases as a result of a preliminary 5-min incubation of these reagents. Another advantage of this approach is the reduction in the consumption of specific immunoglobulins. The developed test is able to compete with expensive analysis methods like HPLC without conceding sensitivity. The proposed approach is universal, does not require antibody labelling, and can be easily transferred to the immunochromatography of other low-molecular-weight compounds.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alldrick, A. (2014). Looking for the best compromise in rapid food mycotoxin tests: Speed, sensitivity, precision and accuracy. World Mycotoxin Journal, 7(4), 407–415. https://doi.org/10.3920/wmj2013.1685
- Barbas, C., Montepríncipe, U., Dams, A., & Majors, R. E. (2005). Separation of Aflatoxins by HPLC. Agilent Technologies, Inc. https://www.agilent.com/cs/library/applications/5989-3634EN.pdf.
- Barug, D. (2006). The mycotoxin Factbook: Food & feed Topics. Wageningen Academic Pub.
- Cheli, F., Giromini, C., & Baldi, A. (2015). Mycotoxin mechanisms of action and health impact:‘in vitro’or ‘in vivo’tests, that is the question. World Mycotoxin Journal, 8(5), 573–589. https://doi.org/10.3920/wmj2014.1864
- Chen, Y., Chen, Q., Han, M., Zhou, J., Gong, L., Niu, Y., Yuan, Z., Lidong, H., & Zhang, L. (2016). Development and optimization of a multiplex lateral flow immunoassay for the simultaneous determination of three mycotoxins in corn, rice and peanut. Food Chemistry, 213, 478–484. https://doi.org/10.1016/j.foodchem.2016.06.116
- Chiao, D.-J., Shyu, R.-H., Hu, C.-S., Chiang, H.-Y., & Tang, S.-S. (2004). Colloidal gold-based immunochromatographic assay for detection of botulinum neurotoxin type B. Journal of Chromatography B, 809(1), 37–41. https://doi.org/10.1016/j.jchromb.2004.05.033
- Dykman, L., & Khlebtsov, N. (2012). Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chemical Society Reviews, 41(6), 2256–2282. https://doi.org/10.1039/c1cs15166e
- Dzantiev, B. B., Byzova, N. A., Urusov, A. E., & Zherdev, A. V. (2014). Immunochromatographic methods in food analysis. TrAC Trends in Analytical Chemistry, 55, 81–93. https://doi.org/10.1016/j.trac.2013.11.007
- Erkekoğlu, P., Şahin, G., & Baydar, T. (2008). A special focus on mycotoxin contamination in baby foods: Their presence and regulations. FABAD Journal Pharmacy Science, 33, 51–66. http://dergi.fabad.org.tr/.
- Foubert, A., Beloglazova, N. V., & De Saeger, S. (2017). Comparative study of colloidal gold and quantum dots as labels for multiplex screening tests for multi-mycotoxin detection. Analytica Chimica Acta, 955, 48–57. https://doi.org/10.1016/j.aca.2016.11.042
- Frens, G. (1973). Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature Physical Science, 241(105), 20–22. https://doi.org/10.1038/physci241020a0
- Hao, K., Suryoprabowo, S., Song, S., Liu, L., & Kuang, H. (2018). Rapid detection of zearalenone and its metabolite in corn flour with the immunochromatographic test strip. Food and Agricultural Immunology, 29(1), 498–510. https://doi.org/10.1080/09540105.2017.1406461
- Heidtmann-Bemvenuti, R. (2011). Biochemistry and metabolism of mycotoxins: A review. African Journal of Food Science, 5(16), 861–869. https://doi.org/10.5897/ajfsx11.009
- Huang, Y., Xu, Y., He, Q., Chu, J., Du, B., & Liu, J. (2014). Determination of zearalenone in corn based on a biotin-avidin amplified enzyme-linked immunosorbent assay. Food and Agricultural Immunology, 25(2), 186–199. https://doi.org/10.1080/09540105.2012.759540
- Hussein, H. S., & Brasel, J. M. (2001). Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology, 167(2), 101–134. https://doi.org/10.1016/S0300-483X(01)00471-1
- Kafouris, D., Christofidou, M., Christodoulou, M., Christou, E., & Ioannou-Kakouri, E. (2017). A validated UPLC-MS/MS multi-mycotoxin method for nuts and cereals: Results of the official control in Cyprus within the EU requirements. Food and Agricultural Immunology, 28(1), 90–108. https://doi.org/10.1080/09540105.2016.1228834
- Kong, D., Liu, L., Song, S., Suryoprabowo, S., Li, A., Kuang, H., Wang, L., & Xu, C. (2016). A gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins. Nanoscale, 8(9), 5245–5253. https://doi.org/10.1039/c5nr09171c
- Linares, E. M., Kubota, L. T., Michaelis, J., & Thalhammer, S. (2012). Enhancement of the detection limit for lateral flow immunoassays: Evaluation and comparison of bioconjugates. Journal of Immunological Methods, 375(1-2), 264–270. https://doi.org/10.1016/j.jim.2011.11.003
- Liu, G., Han, Z., Nie, D., Yang, J., Zhao, Z., Zhang, J., Li, H., Liao, Y., Song, S., De Saeger, S., & Wu, A. (2012). Rapid and sensitive quantitation of zearalenone in food and feed by lateral flow immunoassay. Food Control, 27(1), 200–205. https://doi.org/10.1016/j.foodcont.2012.03.023
- Mak, W. C., Beni, V., & Turner, A. P. F. (2016). Lateral-flow technology: From visual to instrumental. TrAC Trends in Analytical Chemistry, 79, 297–305. https://doi.org/10.1016/j.trac.2015.10.017
- McNamee, S. E., Bravin, F., Rosar, G., Elliott, C. T., & Campbell, K. (2017). Development of a nanoarray capable of the rapid and simultaneous detection of zearalenone, T2-toxin and fumonisin. Talanta, 164, 368–376. https://doi.org/10.1016/j.talanta.2016.11.032
- Panferov, V. G., Safenkova, I. V., Varitsev, Y. A., Drenova, N. V., Kornev, K. P., Zherdev, A. V., & Dzantiev, B. B. (2016). Development of the sensitive lateral flow immunoassay with silver enhancement for the detection of Ralstonia solanacearum in potato tubers. Talanta, 152, 521–530. https://doi.org/10.1016/j.talanta.2016.02.050
- Peraica, M., Radic, B., Lucic, A., & Pavlovic, M. (1999). Toxic effects of mycotoxins in humans. Bulletin of the World Health Organization, 77(9), 754–766. http://www.who.int.
- Pestka, J. J. (1994). Application of immunology to the analysis and toxicity assessment of mycotoxins. Food and Agricultural Immunology, 6(3), 219–233. https://doi.org/10.1080/09540109409354833
- Pestka, J. J., Zhou, H.-R., Moon, Y., & Chung, Y. (2004). Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: Unraveling a paradox. Toxicology Letters, 153(1), 61–73. https://doi.org/10.1016/j.toxlet.2004.04.023
- Raeisossadati, M. J., Danesh, N. M., Borna, F., Gholamzad, M., Ramezani, M., Abnous, K., & Taghdisi, S. M. (2016). Lateral flow based immunobiosensors for detection of food contaminants. Biosensors and Bioelectronics, 86, 235–246. https://doi.org/10.1016/j.bios.2016.06.061
- Romagnoli, B., Ferrari, M., & Bergamini, C. (2010). Simultaneous determination of deoxynivalenol, zearalenone, T-2 and HT-2 toxins in breakfast cereals and baby food by high-performance liquid chromatography and tandem mass spectrometry. Journal of Mass Spectrometry, 45(9), 1075–1080. https://doi.org/10.1002/jms.1802
- Rubert, J., Soler, C., & Mañes, J. (2012). Application of an HPLC–MS/MS method for mycotoxin analysis in commercial baby foods. Food Chemistry, 133(1), 176–183. https://doi.org/10.1016/j.foodchem.2011.12.035
- Schatzmayr, G., & Streit, E. (2013). Global occurrence of mycotoxins in the food and feed chain: Facts and figures. World Mycotoxin Journal, 6(3), 213–222. https://doi.org/10.3920/wmj2013.1572
- Shim, W. B., Dzantiev, B. B., Eremin, S. A., & Chung, D. H. (2009). One-step simultaneous immunochromatographic strip test for multianalysis of ochratoxin A and zearalenone. Journal of Microbiology and Biotechnology, 19(1), 83–92. https://doi.org/10.4014/jmb.0802.105
- Smith, M.-C., Madec, S., Coton, E., & Hymery, N. (2016). Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins, 8(4), 94. https://doi.org/10.3390/toxins8040094
- Sotnikov, D. V., Zherdev, A. V., & Dzantiev, B. B. (2015). Development and application of a label-free fluorescence method for determining the composition of gold nanoparticle-protein conjugates. International Journal of Molecular Sciences, 16(1), 907–923. https://doi.org/10.3390/ijms16010907
- Urusov, A. E., Kostenko, S. N., Sveshnikov, P. G., Zherdev, A. V., & Dzantiev, B. B. (2011). Immunochromatographic assay for the detection of ochratoxin A. Journal of Analytical Chemistry, 66(8), 770–776. https://doi.org/10.1134/s1061934811080144
- Urusov, A. E., Petrakova, A. V., Gubaydullina, M. K., Zherdev, A. V., Eremin, S. A., Kong, D., Liu, L., Xu, C., & Dzantiev, B. B. (2017). High-sensitivity immunochromatographic assay for fumonisin B1 based on indirect antibody labeling. Biotechnology Letters, 39(5), 751–758. https://doi.org/10.1007/s10529-017-2294-5
- Urusov, A. E., Petrakova, A. V., Zherdev, A. V., & Dzantiev, B. B. (2016). «Multistage in one touch» design with a universal labeling conjugate for high-sensitive lateral flow immunoassays. Biosensors and Bioelectronics, 86, 575–579. https://doi.org/10.1016/j.bios.2016.07.027
- Urusov, A. E., Zherdev, A. V., & Dzantiev, B. B. (2010). Immunochemical methods of mycotoxin analysis. Applied Biochemistry and Microbiology, 46(3), 253–266. https://doi.org/10.1134/S0003683810030038
- Urusov, A. E., Zherdev, A. V., & Dzantiev, B. B. (2014). Use of gold nanoparticle-labeled secondary antibodies to improve the sensitivity of an immunochromatographic assay for aflatoxin B1. Microchimica Acta, 181(15-16), 1939–1946. https://doi.org/10.1007/s00604-014-1288-4
- Urusov, A. E., Zherdev, A. V., Petrakova, A. V., Sadykhov, E. G., Koroleva, O. V., & Dzantiev, B. B. (2015). Rapid multiple immunoenzyme assay of mycotoxins. Toxins, 7(2), 238–254. https://doi.org/10.3390/toxins7020238
- Van Egmond, H. P., Schothorst, R. C., & Jonker, M. A. (2007). Regulations relating to mycotoxins in food. Analytical and Bioanalytical Chemistry, 389(1), 147–157. https://doi.org/10.1007/s00216-007-1317-9
- Von Holst, C., & Stroka, J. (2014). Performance criteria for rapid screening methods to detect mycotoxins. World Mycotoxin Journal, 7(4), 439–447. https://doi.org/10.3920/wmj2014.1710
- Wang, S., Quan, Y., Lee, N., & Kennedy, I. R. (2006). Rapid determination of fumonisin B1 in food samples by enzyme-linked immunosorbent assay and colloidal gold immunoassay. Journal of Agricultural and Food Chemistry, 54(7), 2491–2495. https://doi.org/10.1021/jf0530401
- Wang, Y.-K., Shi, Y.-B., Zou, Q., Sun, J.-H., Chen, Z.-F., Wang, H.-A., Li, S.-Q., & Yan, Y.-X. (2013). Development of a rapid and simultaneous immunochromatographic assay for the determination of zearalenone and fumonisin B1 in corn, wheat and feedstuff samples. Food Control, 31(1), 180–188. https://doi.org/10.1016/j.foodcont.2012.09.048
- Wong, R., & Tse, H. (2009). Lateral flow Immunoassay. Humana Press.
- Xu, C., Wang, H.-A., Peng, C., Jin, Z., & Liu, L. (2006). Colloidal gold-based immumochromatographic assay for detection of diethylstilbestrol residues. Biomedical Chromatography, 20(12), 1390–1394. https://doi.org/10.1002/bmc.714
- Yang, H., Li, D., He, R., Guo, Q., Wang, K., Zhang, X., Huang, P., & Cui, D. (2010). A novel quantum dots-based point of care test for syphilis. Nanoscale Research Letters, 5(5), 875–881. https://doi.org/10.1007/s11671-010-9578-1
- Yang, X., Yang, M., Pang, B., Vara, M., & Xia, Y. (2015). Gold nanomaterials at work in biomedicine. Chemical Reviews, 115(19), 10410–10488. https://doi.org/10.1021/acs.chemrev.5b00193
- Yang, S., Zhang, H., Sun, F., Ruyck, K. D., Zhang, J., Jin, Y., Li, Y., Wang, Z., Zhang, S., De Saeger, S., & Zhou, J. (2017). Metabolic profile of zearalenone in liver microsomes from different species and its in vivo metabolism in rats and chickens using UHPLC-Q/TOF. Journal of Agricultural and Food Chemistry, 31(1), 180–188. https://doi.org/10.1016/j.foodcont.2012.09.048
- Zinedine, A., Soriano, J. M., Moltó, J. C., & Mañes, J. (2007). Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food and Chemical Toxicology, 45(1), 1–18. https://doi.org/10.1016/j.fct.2006.07.030