ABSTRACT
Rheumatoid arthritis (RA) is characterised by the infiltration of a number of proinflammatory cytokines into synovial fluid. Soya-cerebroside, an extract from Cordyceps militaris, inhibits synovial inflammation and reduces cartilage damage in an osteoarthritis model, although the role of soya-cerebroside in inflammatory cytokine expression in RA is uncertain. In this study, Gene Expression Omnibus (GEO) database records revealed higher levels of interleukin (IL)-1β, IL-6 and IL-8 in RA tissue compared with normal tissue. Elevated levels of these inflammatory cytokines were also higher in mice with collagen-induced arthritis (CIA) than in control mice. Soya-cerebroside effectively inhibited IL-1β, IL-6 and IL-8 expression in RA synovial fibroblasts, apparently by inhibiting the ERK, NF-κB and AP-1 signalling pathways. This anti-inflammatory agent shows promise for the treatment of RA.
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease that affects all body joints (Tang, Citation2019). Major symptoms of RA include cartilage degradation, osteophyte formation, bone remodelling, neoangiogenesis and synovial inflammation, which are associated with pain, physical disability, and markedly impaired quality of life (IJ et al., Citation2019; MacDonald et al., Citation2018; Mahajan & Mikuls, Citation2018). The synovium plays an important role in the pathogenesis of RA. The synthesis of chondrolytic enzymes and proinflammatory mediators by the inflamed synovium leads to cartilage destruction, which in turn enhances synovial inflammation (Kuo et al., Citation2017; Tang, Citation2019; Wu et al., Citation2018). RA synovial fibroblasts (RASFs) sustain arthritic pathology by excreting chondrolytic enzymes and inflammatory mediators (Hu et al., Citation2017; Huang et al., Citation2019; Lefevre et al., Citation2015).
The initiation and progression of RA disease is mediated by proinflammatory cytokines, including interleukin (IL)-1β, IL-6 and IL-8 (Abbasi et al., Citation2019). The advent of highly effective nonbiologic (conventional) and biologic disease-modifying agents have revolutionised the treatment of RA, although many patients are resistant to these therapies, are only partial responders, or cannot tolerate biologics (MacDonald et al., Citation2018). Importantly, these treatments fail to halt the progression of the disease, restore the original structure or facilitate a return to function of the damaged joint (MacDonald et al., Citation2018). Moreover, it is acknowledged that several safety concerns exist in regard to the available RA treatments (MacDonald et al., Citation2018). Researchers are increasingly interested in developing RA drugs from natural sources, in the hope that such agents would provide cartilage protection and be safe for long-term used in RA treatment.
The development of anti-inflammatory therapeutic agents from natural compounds looks promising (Azab et al., Citation2016; Cheung et al., Citation2016). The entomopathogenic fungus Cordyceps militaris is used in traditional Chinese medicine to treat inflammatory disorders (Brent et al., Citation2016). We have previously shown how soya-cerebroside, an extract from C. militaris, effectively inhibits inducible nitric oxide synthase and COX-2 production in macrophages (Chiu et al., Citation2016). Soya-cerebroside also inhibits monocyte chemoattractant protein-1 production in vitro and in vivo and thus inhibits synovial inflammation and reduces cartilage damage (Liu et al., Citation2017; Liu et al., Citation2019). We have also shown that soya-cerebroside suppresses vascular endothelial growth factor (VEGF)-regulated endothelial progenitor cell angiogenesis (Lee et al., Citation2020). In other research, another cerebroside isolated from C. militaris, cordycerebroside B, has exhibited marked inhibitory activity against protein tyrosine phosphatase 1B, indicating that C. militaris may possess hypoglycemic activity and be useful in type 2 diabetes (Sun et al., Citation2019). Moreover, six compounds isolated from C. militaris such as ergosterol, adenosine, cordycepin, ergosterol peroxide, tetracosanoic acid 2,3-dihydroxypropylester, and mannitol have shown strong procoagulant activity (Juanjuan et al., Citation2018), while a clinical study has revealed that oral administration of different dosages of C. militaris significantly decreased the activity of various cytokines, particularly inflammatory cytokines and chemokines, indicating that C. militaris can reduce excessive immune responses in diseases such as autoimmune disorders and cancers (Sun et al., Citation2014). Up until now, no reports have documented the effects of soya-cerebroside in RA. Our evidence suggests that soya-cerebroside has potential for treating RA.
2. Materials and methods
2.1. Materials
We purchased p-p85, p-Akt, p-ERK, p-p38, p-JNK, p-p65, p–c-Jun, p85, Akt, ERK, p38, JNK, p65, c-Jun, IL-1β, IL-6, IL-8 and β-actin from Santa Cruz Biotechnology (CA, USA).
2.2. Analysis of the Gene Expression Omnibus (GEO) dataset
Gene expression profiles of normal tissue samples and RA tissue samples were obtained from the GEO database and analysed for levels of IL-1β, IL-6 and IL-8 expression.
2.3. Cell culture
The MH7A cell line (human RA synovial fibroblasts [RASFs]) was obtained from Riken (Ibaraki, Japan), and consistent cell culture conditions were maintained according to the methods detailed in our previous reports (Hu et al., Citation2017; Su et al., Citation2019).
2.4. Western blot analysis
Extracted proteins were resolved by SDS-PAGE and transferred to Immobilon® PVDF membranes. Western blot analysis was performed according to our previous reports (Lee, Chen, et al., Citation2019; Lee, Wang, et al., Citation2019).
2.5. mRNA quantification
Total RNA was extracted from RASF using TRIzol reagent. Quantitative real-time PCR (qPCR) analysis was conducted according to our previous reports (Wang et al., Citation2019; Yang et al., Citation2019).
2.6. Collagen-induced arthritis (CIA) mouse model
CIA was elicited in mice according to the methods described in our previous publications (Huang et al., Citation2019; Su et al., Citation2015). The mice were sacrificed 42 days after arthritis induction. The ankle joints were removed and immunohistochemical (IHC) staining was performed as described in our previous reports (Huang et al., Citation2019; Liu et al., Citation2018).
2.7. Statistics
All values are presented as the mean ± standard deviation (S.D.). Differences between the two experimental groups were assessed for significance using the Student’s t-test. The difference was considered to be significant if the p value was < 0.05.
3. Results
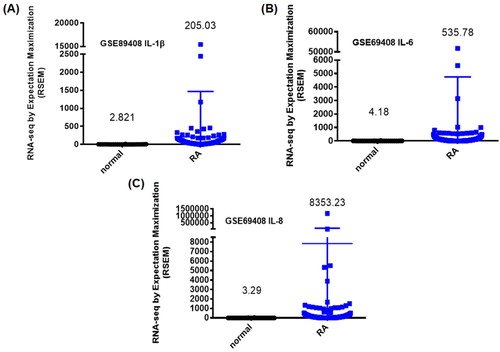
3.1. Upregulation of IL expression in RA tissue
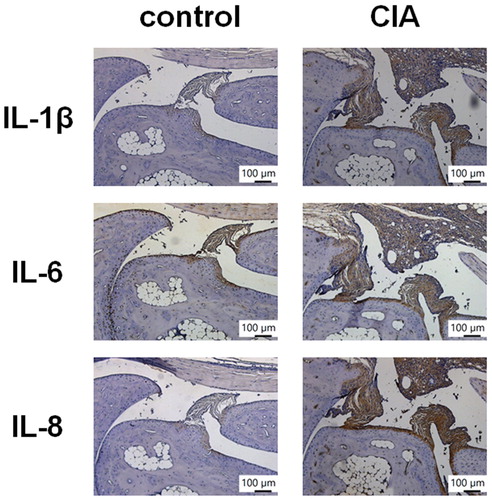
Increasing levels of inflammatory cytokine expression in synovial tissues are a critical step in RA disease progression (Burmester & Pope, Citation2017). Our analysis of the GEO database revealed higher levels of IL-1β, IL-6 and IL-8 expression in inflamed tissue from RA patients compared with normal tissue from healthy individuals (). IHC staining confirmed significantly higher levels of IL-1β, IL-6 and IL-8 expression in tissue from CIA mice compared with tissue from controls (), indicating that RA inflammatory activity correlates with disease progression.
3.2. Soya-cerebroside reduces levels of IL expression in human RASFs
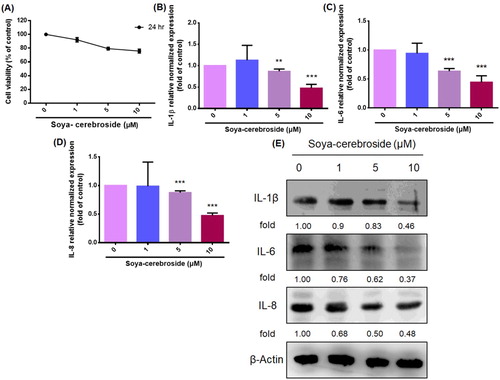
RASFs are among the principle cells involved in inflammation in the synovial microenvironment, responsible for secreting proinflammatory cytokines during RA development (Lefevre et al., Citation2015). The MTT assay revealed that soya-cerebroside (1–10 μM) did not affect the viability of the RASFs (A), so this concentration range was used for further experiments. Treatment of RASFs with soya-cerebroside concentration-dependently inhibited levels of IL-1β, IL-6 and IL-8 mRNA and protein expression (B-E). These results indicated that soya-cerebroside inhibits proinflammatory cytokine production in RASFs.
Figure 3. Soya-cerebroside inhibits IL-1β, IL-6 and IL-8 expression in RASFs. (A) RASFs were incubated with varying concentrations of soya-cerebroside (1–10 μM) for 24 h and cell viability was determined using the MTT assay. (B-E) RASFs were treated with soya-cerebroside (1–10 μM) for 24 h, and IL-1β, IL-6 and IL-8 expression were examined by qPCR and Western blot analysis. Data represent the mean ± S.D. *p < 0.05 compared with the control group.
3.3. Soya-cerebroside reduces IL production by inhibiting ERK, NF-κB and AP-1 signalling pathways
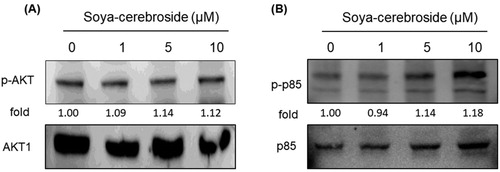
The MAPK (ERK, p38 and JNK) signalling pathway plays an important role during the inflammatory response (Yeung et al., Citation2018). Treatment of RASFs with soya-cerebroside inhibited ERK phosphorylation, but did not affect the phosphorylation of p38 or JNK (). Previous researchers have found that the PI3K/Akt signalling cascade upregulates IL-6 and IL-17 expression (Tsai et al., Citation2017; Yang et al., Citation2013). However, we failed to find any evidence showing that soya-cerebroside had any significant effect upon PI3K and Akt phosphorylation (), indicating that ERK signalling, but not p38 or JNK signalling, nor the PI3K/Akt pathway, is involved in the inhibitory effects of soya-cerebroside upon IL expression.
Figure 4. Soya-cerebroside inhibits ERK phosphorylation, but not p38 or JNK phosphorylation. RASFs were incubated with soya-cerebroside (1–10 μM) for 24 h. ERK, p38 and JNK phosphorylation was examined by Western blot analysis.
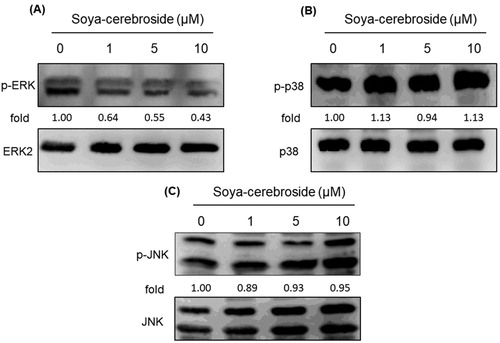
Figure 5. Soya-cerebroside does not inhibit PI3K or Akt phosphorylation. RASFs were incubated with soya-cerebroside (1–10 μM) for 24 h. PI3K and Akt phosphorylation was examined by Western blot analysis.
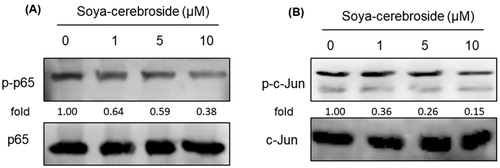
NF-κB and AP-1 are critical transcriptional factors that regulate the release of inflammatory cytokines during RA development (Yang et al., Citation2017; Yeung et al., Citation2018). We therefore examined whether NF-κB and AP-1 regulate the effects of soya-cerebroside. Treatment of RASFs with soya-cerebroside inhibited phosphorylation of p65 and c-Jun (), suggesting that NF-κB and AP-1 mediate the inhibitory effects of soya-cerebroside.
4. Discussion
RA is a systemic autoimmune and inflammatory disease characterised by synovial inflammation and joint degradation (Mahajan & Mikuls, Citation2018; Tang, Citation2019). RASFs in the synovial intimal lining play a crucial role in this process by secreting proinflammatory cytokines, including IL-1β, IL-6 and IL-8, which contribute to cartilage and bone degradation (Lefevre et al., Citation2015). Here, we analysed records from the GEO dataset and confirmed that IL-1β, IL-6 and IL-8 levels are higher in human RA tissue samples than levels in tissue from healthy individuals. This was mirrored by our findings that these proinflammatory cytokines were higher in CIA mice than in control mice. At non-cytotoxic concentrations, soya-cerebroside reduced the production of IL-1β, IL-6 and IL-8. Our data demonstrated that the ERK, NF-κB and AP-1 signalling pathways are involved in the inhibitory effects of soya-cerebroside upon IL-1β, IL-6 and IL-8 production, indicating that this compound is promising for RA treatment. We found that soya-cerebroside not only reduces the production of IL-1β, IL-6 and IL-8, but also inhibits the ERK, NF-κB and AP-1 signalling pathways. However, our evidence is not enough to elucidate any direct or indirect regulatory activity between the pathways. Whether soya-cerebroside inhibits IL-1β, IL-6 and IL-8 production via direct or indirect regulation of the ERK, NF-κB and AP-1 signalling pathways needs further investigation.
The MAPK and PI3K/Akt signalling pathways are the most commonly implicated in the control of inflammatory cytokine synthesis (Nishimura et al., Citation2020). Our investigation into the role of soya-cerebroside in RASFs revealed that this compound diminished ERK, but not p38 or JNK phosphorylation. Our lack of evidence for any significant effects of soya-cerebroside upon PI3K and Akt phosphorylation indicates that inhibition of ERK, but not p38 or JNK signalling, or the PI3K/Akt signalling pathway, is key to the inhibitory effects of soya-cerebroside upon IL-1β, IL-6 and IL-8 production.
NF-κB, a key transcription factor, regulates inflammatory responses (Sun, Citation2017). In this study, we found that treating RASFs with soya-cerebroside inhibited phosphorylation of NF-κB. AP-1 is another key regulator of arthritic joint destruction (Shiozawa & Tsumiyama, Citation2009). We found that soya-cerebroside inhibits AP-1 phosphorylation. Thus, the transcription factors NF-κB and AP-1 are implicated in the inhibitory effects of soya-cerebroside in IL-1β, IL-6 and IL-8 production. Similarly, other investigators have reported that the inhibitory effects of berberine upon IL-8 production are mediated through the NF-κB and AP-1 pathways (Li et al., Citation2014), while curcumin regulates hepatitis B virus infection via NF-κB and AP-1 activation (Hesari et al., Citation2018). Conversely, resveratrol reduces COX-2 production by inhibiting NF-κB and AP-1 signalling (Yang et al., Citation2017). This evidence suggests that NF-κB and AP-1 are critical transcriptional factors in inflammatory responses.
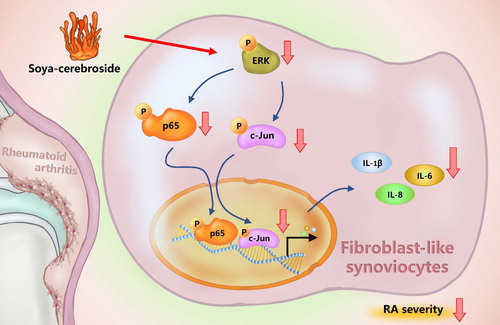
C. militaris has long been used in Chinese medicine to treat inflammatory disease (Brent et al., Citation2016). In our previous study, we isolated soya-cerebroside from C. militaris extract and found evidence of anti-inflammatory activity (Chiu et al., Citation2016). We have also demonstrated that soya-cerebroside reduces monocyte migration and reduces cartilage degradation in experimental inflammatory models (Liu et al., Citation2017; Liu et al., Citation2019). In this present study, our findings have revealed that soya-cerebroside inhibits IL-1β, IL-6 and IL-8 production in RASFs via the inhibition of the ERK, NF-κB and AP-1 signalling pathways (). The evidence supports the investigation of soya-cerebroside for the treatment of RA.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of Taiwan (MOST 107-2320-B-039-019-MY3 ), China Medical University Hospital (DMR-108-075) and by funding from China Medical University under the Higher Education Sprout Project, Ministry of Education, Taiwan (CMRC-CHM-3-1; CMRC-CHM-2-2). We would like to thank Iona J. MacDonald from China Medical University for her English language revision of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abbasi, M., Mousavi, M. J., Jamalzehi, S., Alimohammadi, R., Bezvan, M. H., Mohammadi, H., & Aslani, S. (2019). Strategies toward rheumatoid arthritis therapy; the old and the new. Journal of Cellular Physiology, 234(7), 10018–10031. https://doi.org/10.1002/jcp.27860
- Azab, A., Nassar, A., & Azab, A. N. (2016). Anti-inflammatory activity of natural products. Molecules, 21(10), 1321. https://doi.org/10.3390/molecules21101321
- Brent, C. S., Miyasaki, K., Vuong, C., Miranda, B., Steele, B., Brent, K. G., & Nath, R. (2016). Regulatory roles of biogenic amines and juvenile hormone in the reproductive behavior of the western tarnished plant bug (Lygus hesperus). Journal of Comparative Physiology B, 186(2), 169–179. https://doi.org/10.1007/s00360-015-0953-1
- Burmester, G. R., & Pope, J. E. (2017). Novel treatment strategies in rheumatoid arthritis. Lancet, 389(10086), 2338–2348. https://doi.org/10.1016/S0140-6736(17)31491-5
- Cheung, R. C. F., Ng, T. B., Wong, J. H., Chen, Y., & Chan, W. Y. (2016). Marine natural products with anti-inflammatory activity. Applied Microbiology and Biotechnology, 100(4), 1645–1666. https://doi.org/10.1007/s00253-015-7244-3
- Chiu, C. P., Liu, S. C., Tang, C. H., Chan, Y., El-Shazly, M., Lee, C. L., Du, Y.-C., Wu, T.-Y., Chang, F.-R., & Wu, Y.-C. (2016). Anti-inflammatory cerebrosides from cultivated cordyceps militaris. Journal of Agricultural and Food Chemistry, 64(7), 1540–1548. https://doi.org/10.1021/acs.jafc.5b05931
- Hesari, A., Ghasemi, F., Salarinia, R., Biglari, H., Tabar Molla Hassan, A., Abdoli, V., & Mirzaei, H. (2018). Effects of curcumin on NF-kappaB, AP-1, and Wnt/beta-catenin signaling pathway in hepatitis B virus infection. Journal of Cellular Biochemistry, 119(10), 7898–7904. https://doi.org/10.1002/jcb.26829
- Hu, S. L., Chang, A. C., Huang, C. C., Tsai, C. H., Lin, C. C., & Tang, C. H. (2017). Myostatin promotes interleukin-1beta expression in rheumatoid arthritis synovial fibroblasts through inhibition of miR-21-5p. Frontiers in Immunology, 8, 1747. https://doi.org/10.3389/fimmu.2017.01747
- Huang, C. C., Chiou, C. H., Liu, S. C., Hu, S. L., Su, C. M., Tsai, C. H., & Tang, C. H. (2019). Melatonin attenuates TNF-alpha and IL-1beta expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. Journal of Pineal Research, 66(3), e12560. https://doi.org/10.1111/jpi.12560
- IJ, M. D., Liu, S. C., Huang, C. C., Kuo, S. J., Tsai, C. H., & Tang, C. H. (2019). Associations between adipokines in arthritic disease and implications for obesity. International Journal of Molecular Sciences, 20(6). https://doi.org/10.3390/ijms20061505
- Juanjuan, Z., Wei, Z., Zhenhua, Y., Changqin, L., & Wenyi, K. (2018). Procoagulant constituents from Cordyceps militaris. Food Science and Human Wellness, 7(4), 282–286. doi: doi.org/10.1016/j.fshw.2018.11.001
- Kuo, S. J., Yang, W. H., Liu, S. C., Tsai, C. H., Hsu, H. C., & Tang, C. H. (2017). Transforming growth factor beta1 enhances heme oxygenase 1 expression in human synovial fibroblasts by inhibiting microRNA 519b synthesis. PLoS One, 12(4), e0176052. https://doi.org/10.1371/journal.pone.0176052
- Lee, H. P., Chen, P. C., Wang, S. W., Fong, Y. C., Tsai, C. H., Tsai, F. J., Chung, J.-G., Huang, C.-Y., Yang, J.-S., Hsu, Y.-M., Li, T.-M., & Tang, C.-H. (2019). Plumbagin suppresses endothelial progenitor cell-related angiogenesis in vitro and in vivo. Journal of Functional Foods, 52, 537–544. https://doi.org/10.1016/j.jff.2018.11.040
- Lee, H. P., Wang, S. W., Wu, Y. C., Lin, L. W., Tsai, F. J., Yang, J. S., Li, T.-M., & Tang, C.-H. (2020). Soya-cerebroside inhibits VEGF-facilitated angiogenesis in endothelial progenitor cells. Food and Agricultural Immunology, 31(1), 193–204. https://doi.org/10.1080/09540105.2020.1713055
- Lee, H. P., Wang, S. W., Wu, Y. C., Tsai, C. H., Tsai, F. J., Chung, J. G., Li, T.-M., & Tang, C.-H. (2019). Glucocerebroside reduces endothelial progenitor cell-induced angiogenesis. Food and Agricultural Immunology, 30(1), 1033–1045. https://doi.org/10.1080/09540105.2019.1660623
- Lefevre, S., Meier, F. M., Neumann, E., & Muller-Ladner, U. (2015). Role of synovial fibroblasts in rheumatoid arthritis. Current Pharmaceutical Design, 21(2), 130–141. https://doi.org/10.2174/1381612820666140825122036
- Li, X., Zhao, S. J., Shi, H. L., Qiu, S. P., Xie, J. Q., Wu, H., Zhang, B.-B., Wang, Z.-T., Yuan, J.-Y., & Wu, X.-J. (2014). Berberine hydrochloride IL-8 dependently inhibits invasion and IL-8-independently promotes cell apoptosis in MDA-MB-231 cells. Oncology Reports, 32(6), 2777–2788. https://doi.org/10.3892/or.2014.3520
- Liu, S. C., Chiu, C. P., Tsai, C. H., Hung, C. Y., Li, T. M., Wu, Y. C., & Tang, C. H. (2017). Soya-cerebroside, an extract of Cordyceps militaris, suppresses monocyte migration and prevents cartilage degradation in inflammatory animal models. Scientific Reports, 7. https://doi.org/Artn4320510.1038/Srep43205
- Liu, J. F., Lee, C. W., Tsai, M. H., Tang, C. H., Chen, P. C., Lin, L. W., Lin, C.-Y., Lu, C.-H., Lin, Y.-F., Yang, S.-H., & Chao, C.-C. (2018). Thrombospondin 2 promotes tumor metastasis by inducing matrix metalloproteinase-13 production in lung cancer cells. Biochemical Pharmacology, 155, 537–546. https://doi.org/10.1016/j.bcp.2018.07.024
- Liu, S. C., Tsai, C. H., Wu, T. Y., Tsai, C. H., Tsai, F. J., Chung, J. G., Huang, C.-Y., Yang, J.-S., Hsu, Y.-M., Yin, M.-C., Wu, Y.-C., & Tang, C.-H. (2019). Soya-cerebroside reduces IL-1 beta-induced MMP-1 production in chondrocytes and inhibits cartilage degradation: Implications for the treatment of osteoarthritis. Food and Agricultural Immunology, 30(1), 620–632. https://doi.org/10.1080/09540105.2019.1611745
- MacDonald, I. J., Liu, S. C., Su, C. M., Wang, Y. H., Tsai, C. H., & Tang, C. H. (2018). Implications of angiogenesis involvement in arthritis. International Journal of Molecular Sciences, 19(7), 2012. https://doi.org/10.3390/ijms19072012
- Mahajan, T. D., & Mikuls, T. R. (2018). Recent advances in the treatment of rheumatoid arthritis. Current Opinion in Rheumatology. https://doi.org/10.1097/BOR.0000000000000496
- Nishimura, R., Hata, K., Takahata, Y., Murakami, T., Nakamura, E., Ohkawa, M., & Ruengsinpinya, L. (2020). Role of signal transduction pathways and transcription factors in cartilage and joint diseases. International Journal of Molecular Sciences, 21(4), 1340. https://doi.org/10.3390/ijms21041340
- Shiozawa, S., & Tsumiyama, K. (2009). Pathogenesis of rheumatoid arthritis and c-Fos/AP-1. Cell Cycle, 8(10), 1539–1543. https://doi.org/10.4161/cc.8.10.8411.
- Su, C. M., Hsu, C. J., Tsai, C. H., Huang, C. Y., Wang, S. W., & Tang, C. H. (2015). Resistin promotes angiogenesis in endothelial progenitor cells through inhibition of MicroRNA206: Potential implications for rheumatoid arthritis. Stem Cells, 33(7), 2243–2255. https://doi.org/10.1002/stem.2024
- Su, C. M., Hu, S. L., Sun, Y., Zhao, J., Dai, C., Wang, L., Xu, G., & Tang, C. (2019). Myostatin induces tumor necrosis factor-alpha expression in rheumatoid arthritis synovial fibroblasts through the PI3K-Akt signaling pathway. Journal of Cellular Physiology, 234(6), 9793–9801. https://doi.org/10.1002/jcp.27665
- Sun, S. C. (2017). The non-canonical NF-kappaB pathway in immunity and inflammation. Nature Reviews Immunology, 17(9), 545–558. https://doi.org/10.1038/nri.2017.52
- Sun, Y., Shao, Y., Zhang, Z., Wang, L., Mariga, A. M., Pang, G., Geng, C., Ho, C.-T., Hu, Q., & Zhao, L. (2014). Regulation of human cytokines by Cordyceps militaris. Journal of Food and Drug Analysis, 22(4), 463–467. https://doi.org/10.1016/j.jfda.2014.01.025
- Sun, J., Xu, J., Wang, S., Hou, Z., Lu, X., An, L., & Du, P. (2019). A new cerebroside from cordyceps militaris with anti-PTP1B activity. Fitoterapia, 138, 104342. https://doi.org/10.1016/j.fitote.2019.104342
- Tang, C. H. (2019). Research of pathogenesis and novel therapeutics in arthritis. International Journal of Molecular Sciences, 20(7). https://doi.org/10.3390/ijms20071646
- Tsai, C. H., Liu, S. C., Wang, Y. H., Su, C. M., Huang, C. C., Hsu, C. J., & Tang, C. H. (2017). Osteopontin inhibition of miR-129-3p enhances IL-17 expression and monocyte migration in rheumatoid arthritis. Biochimica et Biophysica Acta (BBA) - General Subjects, 1861(2), 15–22. https://doi.org/10.1016/j.bbagen.2016.11.015.
- Wang, M., Chao, C. C., Chen, P. C., Liu, P. I., Yang, Y. C., Su, C. M., Huang, W.-C., & Tang, C.-H. (2019). Thrombospondin enhances RANKL-dependent osteoclastogenesis and facilitates lung cancer bone metastasis. Biochemical Pharmacology, 166, 23–32. https://doi.org/10.1016/j.bcp.2019.05.005
- Wu, T. J., Lin, C. Y., Tsai, C. H., Huang, Y. L., & Tang, C. H. (2018). Glucose suppresses IL-1beta-induced MMP-1 expression through the FAK, MEK, ERK, and AP-1 signaling pathways. Environmental Toxicology, 33(10), 1061–1068. https://doi.org/10.1002/tox.22618
- Yang, C. M., Chen, Y. W., Chi, P. L., Lin, C. C., & Hsiao, L. D. (2017). Resveratrol inhibits BK-induced COX-2 transcription by suppressing acetylation of AP-1 and NF-kappaB in human rheumatoid arthritis synovial fibroblasts. Biochemical Pharmacology, 132, 77–91. https://doi.org/10.1016/j.bcp.2017.03.003
- Yang, Y. C., Chiou, P. C., Chen, P. C., Liu, P. Y., Huang, W. C., Chao, C. C., & Tang, C. H. (2019). Melatonin reduces lung cancer stemness through inhibiting of PLC, ERK, p38, beta-catenin, and Twist pathways. Environmental Toxicology, 34(2), 203–209. https://doi.org/10.1002/tox.22674
- Yang, W. H., Liu, S. C., Tsai, C. H., Fong, Y. C., Wang, S. J., Chang, Y. S., & Tang, C. H. (2013). Leptin induces IL-6 expression through OBRl receptor signaling pathway in human synovial fibroblasts. PLoS One, 8(9), e75551. https://doi.org/10.1371/journal.pone.0075551
- Yeung, Y. T., Aziz, F., Guerrero-Castilla, A., & Arguelles, S. (2018). Signaling pathways in inflammation and anti-inflammatory therapies. Current Pharmaceutical Design, 24(14), 1449–1484. https://doi.org/10.2174/1381612824666180327165604