ABSTRACT
The experiment was conducted to evaluate the effects of different levels of cottonseed meal (CSM) in laying hens. A total of 175 hens, 60-wk-old, were allocated to five treatments. The control group was fed a corn-soybean meal diet, and the four experimental diets contained 19.5, 67.5, 115.5, and 163.5 g/kg CSM, in which free gossypol level was 20, 70, 120, 170 mg/kg, respectively. The results showed that no significant differences were observed on laying performance, egg production and organ indexes among all groups. Jejunum and ileum mucosa slgA were significantly lower than the control group when FG reached 120 mg/kg (P < 0.05). The ileum IL-2 was significantly decreased when FG reached 70 mg/kg (P < 0.05). The ileum TNF-α was increased linearly and the liver damage was aggravated with the increase of FG. In conclusion, FG can destroy intestinal immunity and liver tissue and dietary FG in laying hens should be limited within 70 mg/kg.
Introduction
Soybean meal is generally recognized as a high-quality protein feed-stuff (Kim, Citation2014; Taliercio et al., Citation2014). Cottonseed meal (CSM) is a very important protein feedstuff and can be used in animal diets to replace partial soybean meal (Swiatkiewicz et al., Citation2016). However, the application of CSM in the poultry diet is limited due to the presence of free gossypol (Tang et al., Citation2018). The concentration of free gossypol in CSM ranges from 200 to 5300 mg·kg−1. Several studies reported that FG can inhibit growth performance and increase mortality in broilers (Devanaboyina et al., Citation2007). Free gossypol can inhibit the activity of pepsin and trypsin in gastro-intestinal tract and reduce the digestibility of protein (Devanaboyina et al., Citation2007).
China's feed hygiene standard (GB 13078-2017) set the limit of free gossypol as 20 mg/kg in laying hens. The Germany and Britain allowance for free gossypol in the diet of laying hens is also 20 mg/kg. Lordelo et al. (Citation2007) found that there was no significant difference in laying performance with 200 and 400 mg/kg gossypol isomer diets However, there is still some divergence about the limits of free gossypol in diets of laying hens.
Laying hens are more sensitive to free gossypol than broilers (Devanaboyina et al., Citation2007). It was reported that excessive gossypol could reduce laying production (Panigrahi et al., Citation1989), deteriorate egg quality by causing yolk mottling (Phelps, Citation1966) and pinkish discolouration of albumen (Fitzsimmons et al., Citation1989; Devanaboyina et al., Citation2007). Thus, all of these indicators could be used to evaluate the FG limit in laying hen diets. Besides, the tolerance to dietary levels of FG in poultry varies widely depending on the age and strains. Jinghong hen is a brown-shell hen variety, which was obtained after 10 years of breeding in Beijing Huadu Yukou Poultry, Beijing, China. It has superior production performance, outstanding reproductive performance, practicality, adaptability and other advantages (Meng et al., Citation2017). To date, there was no report about the tolerance limit of FG level in Jinghong hen diets. On the other hand, there is a paucity of information about the effects of FG on laying hens at the later laying period. However, some study had shown different results with the above limit, so the limit level needs to be studied again, especially in the late stage of laying hens. Therefore, the objective of this study was to evaluate the effects of FG on laying performance, egg quality, intestinal mucosal immunization and liver histopathology of laying hens at the late laying period.
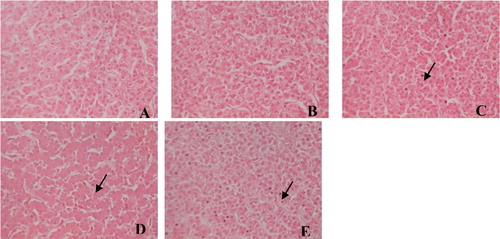
Figure 1. Hepatic histology in laying hens fed a control diet supplemented with 0 (A), 20 (B),70 (C), 120 (D) and 170 mg/kg FG(E) (haematoxylin-eosin staining, original magnification×40). Hepatic histology shows acidophil body (arrows) in mild hepatitis in FG 70 (C), FG 120 (D) and FG 170 (E) groups.
Materials and methods
Experimental design, birds and management
A total of 175 Jinghong hens, 60-wk-old, were randomly allocated to 5 groups with 7 replicates and 5 hens per replicate. The dietary treatments were corn-soybean meal-based diets supplemented with 0, 20, 70, 120, 170 mg/kg FG by adding cottonseed meal (FG content in CSM: 1030 mg/kg). The experiment lasted for 14 wk (including 2 wks of acclimation). The diets were formulated to meet the requirements of nutrients for Chinese feeding standard of chicken (NY/T 33-2004). Ingredients and nutrient composition of the diets were provided in . Cages were randomly located in a ventilated room with temperature between 21 and 24°C and 16 h/day of illumination (10–20 lx). Diets were offered twice daily for ad libitum intake and laying hens had free access to water throughout the experimental period. Eggs of each replicate were counted and weighted daily to calculate laying rate, average egg weight. Feed intake and FCR (feed/egg, g/g) were measured every 2 wks. Mortalities and health status were observed and recorded daily.
Table 1. Composition and nutrient levels of experimental diets (as-feed basis) (%).
The animal experiment was performed in the experiment farm of Academy of National Food and Strategic Reserves Administration (Changping District, Beijing, China). All experimental procedures were approved by the Animal Ethics Committee Guidelines (registration number: 2018P04) of Academy of National Food and Strategic Reserves Administration (Beijing, China).
Sampling
At the end of wk 12, 7 eggs (one egg from each replicate) from each treatment were randomly selected for determining egg quality and 7 hens per treatment were randomly selected. Blood samples were collected from the wing vein after food deprivation for 12 h. Carbon dioxide was used to make the bird stunning one by one, followed by exsanguination to ensure that the birds died. Liver, lung, kidney, heart and spleen were collected to calculate the organ indexes. Approximately 1 cm × 1 cm × 1 cm tissue samples were taken from the left lobe of liver and fixed in buffered formalin (4% formaldehyde; pH 7.4). Jejunum and ileum mucosa were scraped and then stored at −80°C to determine the content of sIgA, IL-2, IL-6, and TNF-α.
Analysis of experimental parameters
Feedstuffs of corn, soybean meal and CSM were collected and stored at −20°C for analysis. Crude protein was determined according to the method of AOAC (2002), and dry matter (DM) was determined by drying the samples at 105°C for 5 h. FG concentrations were determined by Chinese national standard GB 13086-91.
Eggs were weighed, and Haugh unit, yolk colour, eggshell thickness and eggshell strength were measured with a digital egg tester (DET-6000; Nsbel Co. Ltd, Kyoto, Japan). The egg shape index was calculated by measuring the transverse and longitudinal diameters of eggs with vernier calipers.
The contents of sIgA, IL-2, IL-6, and TNF-α in jejunum and ileum mucosa were determined by the ELISA assay kits (R&D Systems, Minneapolis, USA) according to the manufactures’ procedure.
The liver tissue was fixed with 10% phosphate-buffered formaldehyde and embedded in paraffin. Sections were prepared and stained with haematoxylin and eosin (Li et al., Citation2019).
Statistical analyses
The statistical analysis was performed with SPSS 17.0 software (SPSS Inc., Chicago, IL). The data were analyzed by one-way ANOVA and linear regression and quadratic analysis for the dietary treatment as the main source of variation. Significant differences among all treatments were compared by Turkey test. P ≤ 0.05 was considered as significant.
Results
Laying performance and egg quality
The effects of dietary supplementation of CSM on laying performance and egg quality were shown in and . No birds displayed symptoms of clinical illness during the experimental period. The diets supplemented with CSM had no significant effects on average daily feed intake (ADFI), egg production, average egg weight and FCR (P > 0.05). There were no significant effects on average egg sizes, yolk colour, eggshell thickness, eggshell strength, albumen height and egg-shaped indexes among all the groups (P > 0.05). And no mortality was observed in the whole feeding phases.
Table 2. Effects of different levels of free gossypol on laying performance in laying hens1.
Table 3. Effects of different levels of free gossypol on egg quality1.
Organ indexes
Organ indexes of laying hens fed CSM were shown in . The indexes of liver, lungs, kidneys, heart, and spleen were not affected by the experimental treatments (P > 0.05).
Table 4. Effects of different levels of free gossypol on organ index1.
Intestinal immunity
The effects of dietary supplementation of CSM on sIgA, IL-2, IL-6, and TNF-α in jejunum and ileum mucosa were shown in . The content of sIgA in jejunum mucosa was significantly lower in FG 120 group than that in control group and FG 20 group (P < 0.05). The content of sIgA and IL-2 in ileum mucosa decreased linearly with the increase of gossypol (P < 0.05). The content of sIgA in ileum mucosa was significantly lower than that in control group and FG20 group when hens fed the diet containing FG above 120 mg/kg (P < 0.05). Compared to control group, ileum IL-2 level was significantly lower when hens fed the diet containing FG above 70 mg/kg (P < 0.05). The jejunum and ileum mucosa IL-6 levels were not significantly affected by CSM addition (P > 0.05). Ileum TNF-α level increased linearly with free gossypol content increase (P < 0.05). The content of TNF-α in ileum mucosa was significantly higher when diet containing 120 mg/kg FG, compared with that in control group (P < 0.05).
Table 5. Effects of different levels of free gossypol on intestinal immunity1.
Liver histopathology
In the present study, liver histological changes were observed (). The liver histology in laying hens fed diet containing 20 mg/kg FG had abundant hepatocytes with no obvious abnormity comparing with that in control group. Acidophil bodies were found in livers fed diets containing 70, 120 or 170 mg/kg of FG.
Discussion
Free gossypol (FG) can passivate the activity of several enzymes in the digestive tract, leading to dyspepsia and growth retardation in animals (Swiatkiewicz et al., Citation2016). FG can inhibit gastrin secretion to cause abdominal distension, affecting the growth performance of animals.
The current study found that all laying hens appeared normal and no mortality occurred throughout the experimental period (data not shown). The laying hens fed the diet containing different level FG had no significant differences in laying performance, including ADFI, egg production, egg weight and FCR. The result was consistent with some previous studies, which indicated that ADFI and egg production were not affected when laying hens were fed diet containing 144 mg/kg FG (Davis et al., Citation2002) or 179 mg/kg FG (Yang, Citation2003). Wang et al. (Citation2017) showed that between days 22–42 and 1–42, ADG and ADFI had no significant difference among soybean meal, CSM (65.5 mg/kg FG), and fermented CSM (27.7 mg/kg FG) groups, but fermented CSM (55.3 mg/kg FG) significantly decreased ADG and increased FCR. The results of egg production and FCR were consistent with previous report of He et al. (Citation2015), which indicated that egg production and FCR consistent were not affected when laying hens were fed diet containing 100 mg/kg FG. Nevertheless, some studies reported inconsistent results. Mbahinzireki et al. (Citation2001) reported that poor palatability of cottonseed meal diets may lead to lower feed intake, and consequently decreased performance egg weight (He et al., Citation2015) were decreased when laying hens were fed diet containing 100 mg/kg FG. These inconsistent results might be due to the strain and age of bird and the duration of feeding time (Panigrahi & Morris, Citation1991). In this study, the strain of laying hens was Chinese Jinghong, and the production stage was the late egg-laying period (60∼74 wks). We assumed that the laying hens at the late laying period were not sensitive to external stimulation of FG.
In the present study, the high dietary FG did not cause adverse effects on egg quality. Haugh unit is an important index for evaluating freshness and protein quality of eggs. Our findings were similar with a study which showed that birds fed 40 mg/kg FG had no significant differences on haugh unit (Jiao et al., 2015), but were different with Yuan et al. (Citation2014). The reason could be attributed to differences in chemical composition or the rate of protein turnover between cottonseed meal and soybean meal materials (He et al., Citation2015). Besides, FG may result in yolk discolouration based on a chemical combination of gossypol with ferric iron (Fe+3) released from yolk proteins (Kemmerer et al., Citation1966). This effect was more obvious in stored eggs (Phelps, Citation1966). In this study, yolk discolouration was not observed even the hens fed diet containing 170 mg/kg FG. Similar findings were also reported in previous publications (He et al., Citation2015; Wang, Citation2016). Wang (Citation2016) reported that yolk discolouration was not recorded from hens fed diet containing 400 mg/kg FG during four weeks of experimental period. In addition, eggshell strength and eggshell thickness are two important indicators for eggshell quality. Eggshell strength ultimately affects the soundness of the shell. The weaker shelled eggs are more prone to have cracks and breakages following subsequent microbial contamination. This study showed no differences in eggshell strength and eggshell thickness among all the treatments, which were in accordance with the results of He et al. (Citation2015).
The relative weight of immune organs can be used to evaluate the immune status. In this study, there was no significant difference in the spleen index, and also had no significance in indexes of liver, lung, kidney and heart (P > 0.05). Our findings were similar to the previous publication by Zhu et al. (Citation2018), who reported the gossypol diet had no significant effects on liver index, spleen index and kidney index (P > 0.05).
Secretory IgA (slgA) is an acquired immune molecule, which can prevent harmful microorganisms from colonizing on the mucosa and then entering through the epithelium (Martin et al., Citation2017). In the present study, the content of sIgA in either jejunum or ileum mucosa was decreased dramatically when hens fed the diet containing FG in 120 mg/kg. Simultaneously, the content of TNF-α in ileum mucosa was increased by 16∼27% when hens were fed diet containing 70∼170 mg/kg FG. It is known that TNF-α is an important pro-inflammatory cytokine, and is a major tumourcidal effector of activating macrophage. The current result indicated that it tended to cause the inflammatory response when laying hens were fed diets containing FG higher than 70 mg/kg, and to damage the mucosal immune system when laying hens were fed diets containing FG higher than 120 mg/kg.
Autophagy is a stress reaction that arises when cells resist the undesirable external stimulus. The excessive autophagy impairs the morphology and functions of cells (Liu et al., Citation2018; Zhang et al., Citation2019). The ingestion of gossypol present in cottonseed and its products (cake and meal) may promote clinical poisoning of liver damage (Mena et al., Citation2004). In our study, acidophil bodies (also known as councilman bodies) were clearly illustrated in liver of hens fed diets containing FG over 70 mg/kg. Acidophil bodies are apoptotic hepatocytes, seen in a range of inflammatory, toxic and metabolic disorders, which are characterized as their shrunken size, dense eosinophilic cytoplasm and pyknotic nucleus (Alpert & Hart, Citation2016). Similarly, there is a similar report showing that chicks fed 400 mg/kg of gossypol had mild perivascular lymphoid aggregate formations and biliary hyperplasia in liver (Henry et al., Citation2001).
Conclusions
In summary, more than 120 mg/kg of dietary FG can decrease the jejunum and ileum mucosal sIgA to damage mucosal immune system in laying hens. More than 70 mg/kg of dietary FG can cause inflammatory response and hepatic apoptosis. Therefore, under the experimental condition, it is suggested that the content of FG in the diet should not exceed 70 mg/kg at the later laying period.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alpert, L., & Hart, J. (2016). The pathology of alcoholic liver disease. Clinics in Liver Disease, 20(3), 473–489. https://doi.org/10.1016/j.cld.2016.02.006
- Davis, A. J., Lordelo, M. M., & Dale, N. (2002). The use of cottonseed meal with or without added soap stock in laying hen diets. Journal of Applied Animal Research, 11, 127–133. https://doi.org/10.1093/japr/11.2.127.
- Devanaboyina, N., Rama, R. S. V., Panda, A. K., & Sastry, V. R. B. (2007). Cottonseed meal in poultry diets: A review. Journal of Poultry Science, 44(2), 119–134. https://doi.org/10.2141/jpsa.44.119
- Fitzsimmons, R. C., Newcombe, M., & Moul, I. E. (1989). The long-term effects of feeding ground and whole cottonseed to laying hens. Canadian Journal of Animal Science, 69(2), 425–431. https://doi.org/10.4141/cjas89-047
- He, T., Zhang, H. J., Wang, J., Wu, S. G., Yue, H. Y., & Qi, G. H. (2015). Application of low-gossypol cottonseed meal in laying hens’ diet. Pourltry Science, 10(4), 2456–2463. https://doi.org/10.3382/ps/pev247
- Henry, M. H., Pesti, C. M., Bakalli, R., Lee, J., Toledo, R. T., Eitnmiler, R. R., & Philips, R. D. (2001). The performance of broiler chicks fed diets containing extruded cottonseed meal supplemented with lysine. Poultry Science, 80(6), 762–768. https://doi.org/10.1093/ps/80.6.762
- Kemmerer, A. R., Heywang, B. W., Vavich, M. G., & Sheehan, E. T. (1966). Effect of sulphate on egg discoloration caused by gossypol. Poultry Science, 45(5), 1025–1028. https://doi.org/10.3382/ps.0451025
- Kim, S. W. (2014). Identification of a second major antigenic epitope in the α-subunit of soy β-conglycinin. Food and Agricultural Immunology, 25(3), 311–321. https://doi.org/10.1080/09540105.2013.791969
- Li, X. Y., He, S. Y., Gao, C. X., Deng, H., Liu, Y. F., Li, C., Li, Y., & Luo, Y. (2019). Isoorientin attenuates benzo[a]pyrene-induced liver injury by inhibiting autophagy and pyroptosis in vitro and vivo. Food and Agricultural Immunology, 30(1), 841–861. https://doi.org/10.1080/09540105.2019.1638888
- Liu, H. Y., Pei, X. L., Wang, J., Zhou, Y., Wang, L. M., & Qi, B. (2018). Effect of loach paste on the liver and immune organs of D-galactose-induced ageing mice. Food and Agricultural Immunology, 29(1), 316–331. https://doi.org/10.1080/09540105.2017.1376037
- Lordelo, M. M., Calhoun, M. C., Dale, N. M., & Dowd, M. K. (2007). Relative toxicity of gossypol enantiomers in laying and broiler breeder hens. Poultry Science, 86(3), 582–590. https://doi.org/10.1093/ps/86.3.582
- Martin, L., Eva, H., Katarina, B., Viera, K., Mikuláš, L., Okasana, I., Ľubomira, G., Klaudia, Č, Katarína, R., & Mikuláš, L. (2017). Inorganic or organic zinc and MUC-2, IgA, IL-17, TGF-β4 gene expression and sIgA secretion in broiler chickens. Food and Agricultural Immunology, 28(5), 801–811. https://doi.org/10.1080/09540105.2017.1313202
- Mbahinzireki, G. B., Dabrowski, K., Lee, K. J., EL-Saidy, D., & Wisner, E. R. (2001). Growth, feed utilization and body composition of tilapia (Oreochromis sp.) fed with cottonseed meal-based diets in a recirculating system. Aquaculture Nutrition, 27(3), 189–200. https://doi.org/10.1046/j.1365-2095.2001.00172.x
- Mena, H., Santos, J. E. P., Huber, J. T., Tarazon, M., & Calhoun, M. C. (2004). The effects of varying gossypol intake from whole cottonseed and cottonseed meal on lactation and blood parameters in lactating dairy cows. Journal of Dairy Science, 8(87), 2506–2518. https://doi.org/10.3168/jds.S0022-0302(04)73375-5
- Meng, G. H., Song, D., Li, L. B., Yang, C. J., Qu, Z. X., & Gao, Y. P. (2017). Dietary methionine requirement of Jing brown layer hens from 9 to 17 weeks of age. Journal of Animal Physiology and Animal Nutrition, 101(5), 925–935. https://doi.org/10.1111/jpn.12525
- Panigrahi, S., & Morris, T. R. (1991). Effects of dietary cottonseed meal and iron-treated cottonseed meal in different laying hen genotypes. British Poultry Science, 32(1), 167–184. https://doi.org/10.1080/00071669108417338
- Panigrahi, S., Plumb, V. E., & Machin, D. H. (1989). Effects of dietary cottonseed meal, with and without iron treatment, on laying hens. British Poultry Science, 30(3), 641–651. https://doi.org/10.1080/00071668908417187
- Phelps, R. A. (1966). Cottonseed meal for poultry: From research to practical application. World’s Poultry Science Journal, 22(2), 86–112. https://doi.org/10.1079/WPS19660016
- Swiatkiewicz, S., Arczewska-Wlosek, A., & Jozefiak, D. (2016). The use of cottonseed meal as a protein source for poultry: An updated review. World’s Poultry Science Journal, 72(3), 473–484. https://doi.org/10.1017/S0043933916000258
- Taliercio, E., Loveless, T., & Turano, M. J. (2014). Identification of epitopes of the A1aBx and A5A4B3 subunits of glycinin antigenic in three animal species. Food and Agricultural Immunology, 26(2), 271–281. https://doi.org/10.1080/09540105.2014.906566
- Tang, X., Xiang, R., Chen, S., Yang, S., Liu, H., Fang, R., & Li, A. (2018). Effects of fermented cottonseed meal and enzymatic hydrolyzed cottonseed meal on amino acid digestibility and metabolic energy in white leghorn rooster. Pakistan Journal of Zoology, 50(3), 957–962. https://doi.org/10.17582/journal.pjz/2018.50.3.957.962
- Wang, D. X. (2016). The impact on the production performance, egg quality and liver of laying hens exposed to the FG and CPFA. Yangzhou University.
- Wang, Y. W., Deng, Q. Q., Song, D., Wang, W. W., Zhou, H., Wang, L., & Li, A. K. (2017). Effects of fermented cottonseed meal on growth performance, serum biochemical parameters, immune functions, antioxidative abilities, and cecal microflora in broilers. Food and Agricultural Immunology, 28(4), 725–738. https://doi.org/10.1080/09540105.2017.1311308
- Yang, J. R. (2003). Effect of higher cottonseed meal diet formulated on the digestible amino acid basis on the performance, healthy and egg quality of laying hens. Shanxi Agricultural University.
- Yuan, C., Song, H. H., Zhang, X. Y., Jiang, Y. J., Zhang, A. T., Mahmoud, M. A., & Zou, X. T. (2014). Effect of expanded cottonseed meal on laying performance, egg quality, concentrations of free gossypol in tissue, serum and egg of laying hens. Animal Science Journal, 85(5), 549–554. https://doi.org/10.1111/asj.12169
- Zhang, Y., Fan, Y. Y., Gao, J. F., Xu, W. P., Xu, Z. P., Liu, Y. T., Li, Z., & Tao, L. M. (2019). A new 24-membered macrolide shows insecticidal activity against pieris rapae potentially through induction of programmed cell death. Food and Agricultural Immunology, 30(1), 727–742. https://doi.org/10.1080/09540105.2019.1626808
- Zhu, J. D., Chen, J., Chen, F., Liu, S. C., Pu, X. L., Cheng, Y. N., Zhu, A. X., & Wang, C. W. (2018). Effects of cottonseed meal on growth performance, organ index and organ morphology in mice. Heilongjiang Animal Science and Veterinary Medicine, 05(168-170), 266–267.
