ABSTRACT
The aim of this study was to evaluate the influence of Lactobacillus reuteri B1/1 on the local intestinal immune response in the cecum of chickens experimentally infected by C. jejuni CCM6189. Day-old chicken were divided into four groups: control (C), L. reuteri B1/1 (LR), C. jejuni CCM6189 (CJ), and combined L. reuteri B1/1 + C. jejuni CCM6189 (LRCJ). Cecal samples were collected during necropsy 12, 24, 48 hours after infection by C. jejuni (relative expression of NLRP3, caspase-1, interleukins, TLR4) and 4 as well as 7 day post infection (relative percentage of CD4+, CD8+, IgM). L. reuteri B1/1 significantly stimulated gene expression of NLRP3 inflammasome and other selected molecules, thereby increasing the capacity of the immune system to react more effectively to the infection of C. jejuni in chicken. In addition, an increase of IgM and the percentage of CD8+ influenced by L. reuteri was observed.
1. Introduction
The worldwide significance of campylobacteriosis as food-borne illness has motivated numerous studies to elucidate the causes of the disease as well as its prevention and treatment (Hochel et al., Citation2007). Nowadays, campylobacteriosis is one of the most commonly reported zoonosis as it has been since 2005, representing alone almost 70% of all the reported cases. Chicken meat is still considered to be the main source of campylobacteriosis (EFSA, Citation2018).
Campylobacter jejuni colonization of chicken is rapid and widespread, and results in long-term persistence and shedding. The chick innate immune system is inefficiently activated against C. jejuni colonization and the expression of relevant immune protein genes is low (Lacharme-Lora et al., Citation2017). Additionally, interaction of chicken’s immune system and C. jejuni play a main role in the pathogenesis and may contribute to the pathology.
Very important part of innate immunity is Nod-like receptor (NLR) protein family, pyrin domain-containing three (NLRP3) inflammasome, which is a multi-protein complex that recruits pro-caspase-1 via ASC (the adaptor molecule apoptosis-associated speck-like protein containing a CARD domain) and then proceeds to cleave the cytokine precursor’s pro-IL-1β and pro-IL-18 into mature IL-1β and IL-18 (Bo et al., Citation2020). However, activation of NLRP3 inflammasome in macrophages requires two steps: priming and activation. The priming step is provided by inflammatory stimuli such as TLR4 agonists which induce NF-κB-mediated NLRP3 and pro-IL-1β expression, and the activation step is triggered by recognition of PAMPs (pathogen-associated molecular patterns), which plays an important role in inflammation or DAMPs (danger-associated molecular patterns) (Yang et al., Citation2019). Furthermore, inflammasome-mediated processes are important during many microbial infections and the dysregulation of inflammasomes may result in impaired host-defence against bacterial pathogens (Li et al., Citation2016). During infections with C. jejuni, lipooligosaccharide (LOS) as one of PAMPs acts as a potent activator of TLR4-mediated innate immunity (Stephenson et al., Citation2013). Toll-like receptors (TLRs) are pattern recognition receptors that play a crucial role in “danger” recognition and are therefore essential components of an innate immune response (Shang et al., Citation2008).
B-lymphocytes as representatives of adaptive immune response could participate of NLRP3 in cytokine regulation, but this has not been well characterized (Ali et al., Citation2017). Moreover, there are only few studies about adaptive responses of chicken to C. jejuni infection (Lacharme-Lora et al., Citation2017).
Increased attention has been focused to the study of probiotic abilities of Lactobacillus strains. Lactobacillus is the largest genus of lactic acid bacteria (LAB), thus becoming the dominant bacterium in the animal gastrointestinal system. In general, it plays an essential role in maintaining the intestinal health balance (Heeney et al., Citation2018; Zhang et al., Citation2016). Similarly, several authors have observed that treatment of Lactobacillus species influenced activity of many relevant immune proteins during the interaction with pathogens in chickens (Clavijo & Flórez, Citation2018; Haghighi et al., Citation2008; Jia et al., Citation2019). Moreover, authors Howarth and Wang (Citation2013) observed that probiotics could influence intestinal inflammation, immune response and function also through an effect on inflammasomes.
Accordingly we decided to evaluate the influence of Lactobacillus reuteri B1/1 on the local innate (NLRP3 inflammasome, caspase-1, interleukins as well as TLR4) and local (CD4+, CD8+, IgM+) intestinal immune response in cecum of broiler chickens experimentally infected by C. jejuni CCM6189.
2. Materials and methods
2.1. Animals and experimental design
The State Veterinary and Food Administration of the Slovak Republic approved the experimental protocol number 836/17-221 and the animals were handled and sacrificed in a humane manner.
One hundred and twenty one day-old Campylobacter-free chicken of the Cobb 500 breed were placed in four gnotobiotic isolators (VELAZ s.r.o., Prague, Czech republic) and randomly divided into four experimental groups (n = 30): control (C), L. reuteri B1/1 (LR), C. jejuni CCM6189 (CJ), and combined L. reuteri B1/1 + C. jejuni CCM6189 (LRCJ).
Chicken were fed a special compound feed Altromin 0713 poultry started diet (Altromin GmbH & Co., Germany), without anticoccidials and probiotics, with the following composition: (g/kg) calcium 13.9; phosphorus 6.554.5; vitamin C 21.6; magnesium 1.7; vitamin B1 10.8; vitamin B2 7.2; vitamin B6 5.4; vitamin B12 0.014; nicotinic acid 24.6; pantothenic acid 12.6; folic acid 1.8; biotin 0.3, and the animals had unlimited access to water. The internal temperature of isolator was in the range 22–27°C, with a relative humidity of 40–80%, under a 12-h light/dark regimen. Lignocel 3-4S (VELAZ s.r.o., Prague, Czech Republic) bedding intended for barrier breeding was used.
L. reuteri B1/1 at the concentration of 109 CFU/0.2 ml in Ringer’s solution was administered individually per os to LR and LRCJ groups from day 1 to day 7 of the experiment. C. jejuni CCM6189 was administered orally individually on day 4 of the experiment by a single dose of 1 × 108 CFU/0.2 ml PBS to CJ and LRCJ experimental groups.
Chicken were euthanized with an intra-abdominal injection of xylazine (Rometar 2%, SPOFA, Czech Republic) and ketamine (Narkamon 5%, SPOFA, Czech Republic) at doses of 0.7 ml/kg body weight. Cecal samples for mRNA isolation of target genes (for measurement relative expression of NLRP3, caspase-1, TLR4 and interleukins genes) were collected during necropsy 12, 24, 48 hours after infection by C. jejuni (hpi) and for flow cytometry at 4 as well as 7 day post infection (to measure the relative percentage of CD4+, CD8+ and IgM+ lymphocytes).
2.2. Preparing of bacterial strains
2.2.1. Lactobacillus reuteri
Our study was carried out using Lactobacillus reuteri B1/1 isolated from the gut content of healthy pheasant at the Institute of Microbiology and Gnotobiology of the UVMP in Košice. In a previous study (Ryznerova et al., Citation2013) the strain was identified at the Centre of Biosciences of the Slovak Academy of the Institute of Animal Physiology in Košice by a matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS). The strain producing exopolysaccharides and resistant to gastric juice and bile salts also demonstrated inhibitory activity against indicated pathogenic strains under in vitro conditions (Borovská et al., Citation2012).
A spontaneous rifampicin-resistant mutant of this strain was isolated by inoculation onto de Man–Rogosa–Sharpe agar (MRS; Carl Roth GmbH + CO. KG, Karlsruhe, Germany) containing 30 µg/ml rifampicin-10 serial passages (Sigma Chemical Co., Great Britain).
2.2.2. Campylobacter jejuni
Campylobacter jejuni CCM6189 (supplied by the Culture Collection, Brno, Czech Republic) was prepared for inoculation to chickens at the Department of Microbiology and Immunology (Karaffová et al., Citation2017).
2.3. Homogenization of tissue and isolation of total RNA of NLRP3, interleukins, caspase-1 and TLR4 genes
Tissue samples (caudal parts of cecum) were cut into 20 mg pieces immediately placed in RNA Later solution (Qiagen, UK) and stored at –70°C before RNA purification as described in Karaffová et al. (Citation2017).
2.4. Relative expression of NLRP3, interleukins, caspase-1 and TLR4 genes in quantitative real-time PCR (qRT-PCR)
The mRNA levels of NLRP3, interleukins, caspase-1 and TLR4 genes were determined. In addition, mRNA relative expression of reference genes, coding GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and UB (ubiquitin) were selected based on expression stability using the geNorm program. The primer sequences, optimal annealing temperature and time for each primer used for qRT-PCR are listed in . All primer sets allowed DNA amplification efficiencies between 94% and 100%.
Table 1. List of primers used in qRT-PCR for target gene mRNA detection in chicks.
Amplification and detection of specific products were performed using the CFX 96 RT system (Bio-Rad, USA) and Maxima SYBR Green qPCR Master Mix (Thermo Scientific, USA). Subsequent qRT-PCR to detect relative expression of mRNA in selected parameters was carried out over 35 cycles under the following conditions: initial denaturation at 94°C for 3 min, subsequent denaturation at 93°C for 45 s, annealing (), final extension step 10 min at 72°C. A melting curve from 50°C to 95°C with readings at every 0.5°C was produced for each individual qRT-PCR plate. Analysis was performed after every run to ensure a single amplified product for each reaction. All reactions for real-time PCR were done in triplicate, and mean values of triplicates were used for subsequent analysis. We also confirmed that the efficiency of amplification for each target gene (including GAPDH, UB) was essentially 100% in the exponential phase of the reaction, where the quantification cycle (Cq) was calculated. The Cq values of the studied genes were normalized to an average Cq value of the reference genes (ΔCq), and the relative expression of each gene was calculated as 2–ΔCq.
2.5. Sequence data collection
The NLRP3 gene sequences of chicken origin were retrieved from the three public databases of nucleic acids including GenBank (http://www.ncbi.nlm.nih.gov/), Ribosomal database project (RDP, http://rdp.cme.msu.edu/), as well as Silva comprehensive ribosomal RNA database (Silva, http://www.arb-silva.de/) in January and February 2020, using the following search terms: chicken, broiler, chick, chicks, hen, poultry. Sequences shorter than 150 bp were removed from the data set to avoid uncertainties in comparing and classifying short sequences that have little or no sequence overlap. Possible chimeric sequences were identified using via Chimera Slayer and UCHIME in the Mothur package (Edgar et al., Citation2011; Schloss et al., Citation2009) and were also removed. The database entry information associated with each of the sequences was reviewed and non-poultry sequences were removed manually.
2.6. Flow cytometry of caeca
By the eighth day of the experiment (4 days post C. jejuni challenge) six individuals from each group were slaughtered and both ceca were removed and placed into cold-buffered Hank’s balance solution (HBSS, pH 7.2–7.3). Flow cytometric analysis of intraepithelial lymphocytes (IEL) was used to determine the relative percentages of T cells – CD4+, CD8+ and IgM+ cells. Lymphocytes were isolated and purified following a previously described methodology (Aguilar et al., Citation2001). The removed cecal sections were cut lengthwise and into 0.5 cm pieces, washed three times in HBSS and incubated 15 min in HBSS solution with 5 mm dithiothreitol (HBSS-DTT; 37 °C) by hand mixing in 5 min intervals. Dithiothreitol removed mucin from the intestinal mucosa. After incubation, the intestinal sections were rinsed three times in cold HBSS and incubated for 1 hour in HBSS with 0.1 mM EDTA (37°C) by mixing as described before. EDTA released the intraepithelial lymphocytes. After incubation, the supernatant was filtered through a nylon sieve (70 μm, BD, Germany) into a 50 ml conical test tube and placed on ice in a refrigerator. RPMI-1640 was added to test tube containing the ceca sections for a 15 min long pre-incubation to remove residues of previous media. The sections were then incubated to release LPL in RPMI-1640 with collagenase (Collagenase Type I, HP Biomedicals LLC, France; 15 mg.30 ml−1) for 1 hour at 37°C and with shaking at 5 min intervals. Supernatant was filtered through a nylon sieve and stored on ice. The tubes containing the isolated IEL were centrifuged for 10 min at 600g, the supernatant was removed, and the sediment was re-suspended, rinsed twice with HBSS (centrifugation for 5 min at 200g) and added to 1 ml HBSS. The number of lymphocytes was determined in a Bürker cell using a Türk solution (diluted 1:20). The concentration of lymphocytes was adjusted in all samples to 1 × 106/50 μl−1. Direct immunofluorescence was used for immunophenotyping of IEL by double staining. To 50 μl of lymphocyte suspension, 2 μl of labelled monoclonal mouse anti-chicken antibodies (SouthernBiotech, USA) were added (CD4 PE/CD8 FITC and IgM FITC). Isotype controls were used accordingly (mouse IgG1-FITC, IgG2b R-PE, or IgG2b FITC). The cells were incubated in the dark (room temperature, 15 min). After incubation, washing with 1 ml PBS was done (centrifuged 220g, 5 min). The supernatant was removed and the suspension was diluted at 200 μl PBS with 1% paraformaldehyde.
2.7. Flow cytometric analysis
Cells were measured and analysed on a FACScan flow cytometer (BD, Germany), with 488 nm air cooled argon ion laser. At least 10,000 cells were collected and evaluated in duplicate for each chicken sample. The proportions of individual subpopulations were expressed as relative percentages.
2.8. Statistical analysis
Statistical analysis of data was done using one-way ANOVA with Tukey’s post hoc analysis using Graph Pad Prism version 6.00. Differences between the mean values for different treatment groups were considered statistically significant at P < 0.05, P < 0.01, P < 0.001. Values in tables and figures are given as means with standard deviations (±SD).
3. Results
3.1. Relative expression of NLRP3, interleukins, caspase-1 and TLR4 by quantitative real-time PCR
The relative expression for NLRP3 gene was markedly upregulated in all samplings compared to other groups (P < 0.05; P < 0.001) ((a)).
Figure 1. Effect of probiotic L. reuteri B1/1 administration on relative expression level of (a) NLRP3, (b) IL-1β, (c) IL-18, (d) Casp-1, (e) TLR4 in the cecum of broiler chickens. Results at each time point are the median of 2–ΔCq. Means with different superscripts are significantly different abP < 0.05; acP < 0.01; adP < 0.001.
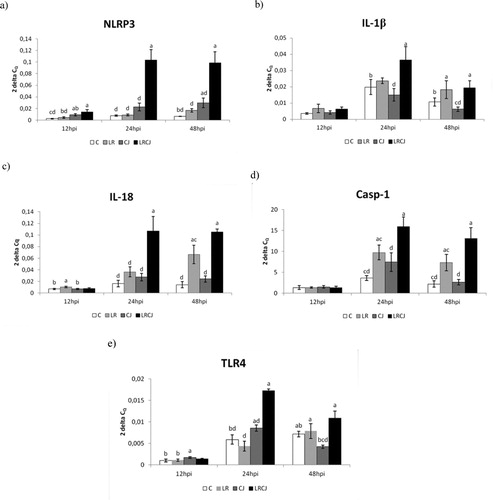
The relative expression for IL-1β gene was upregulated mainly in the combined group LRCJ compared to C (P < 0.05) and CJ (P < 0.001) 24 hours after infection. Similarly, at 48 hpi the relative expression of IL-1β was upregulated as LR as well as LRCJ group compared to the control (P < 0.05) and CJ groups (P < 0.01; P < 0.001) ((b)).
A significant level of relative expression for IL-18 gene was observed in the probiotic group LR compared to the control (P < 0.05) and CJ groups (P < 0.05) in the early phase of infection (12 hpi). However, at 24 hpi the highest relative expression of IL-18 was recorded in LRCJ group compared to the other groups (P < 0.001). The level of relative expression at 48 hpi for IL-18 was still the highest in the combined group compared to the control, CJ (P < 0.001) and LR groups (P < 0.01) ((c)).
The results for Casp-1 gene expression were very similar to relative expression of tested interleukins. After 24 hpi as well as 48 hpi the relative expression for Casp-1 gene was upregulated in the combined group compared to control, CJ group (P < 0.001) and LR group (P < 0.01) ((d)).
In the early phase of infection (12 hpi) relative expression for TLR4 gene was slightly upregulated in the infected group (CJ) compared to control and LR group (P < 0.05). On the other hand, during other sampling, gene expression was the highest in the LRCJ group compared to the other groups (P < 0.05; P < 0.01; P < 0.001) ((e)).
Altogether, gene expression of NLRP3, interleukins and caspase-1 as well as TLR4 was upregulated significantly upon L. reuteri B1/1 stimulation.
3.2. Immunophenotyping of lymphocytes
The percentages of CD4+, CD8+ and IgM cells are presented in .
Figure 2. Effect of probiotic L. reuteri B1/1 administration on relative percentage of (a) CD4+, (b) CD8+ and (c) IgM+ cells. Means with different superscripts are significantly different abP < 0.05; acP < 0.01; adP < 0.001.
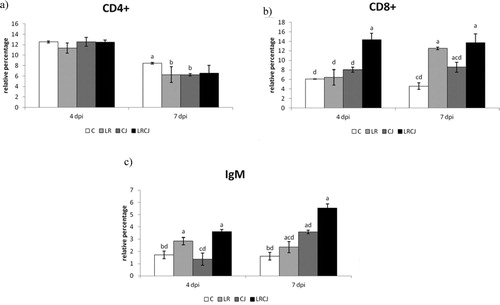
The relative percentage of CD4+ lymphocytes was significantly the highest during second sampling in control compared to the LR and CJ groups (P < 0.05). In general, proportions of CD4+ lymphocytes were not influenced by L. reuteri B1/1 administration ((a)).
The opposite trend was noted for the relative percentage of CD8+ lymphocytes, which was highest in the combined group (LRCJ) compared to the other groups (P < 0.001) during both samplings. In the LR group, a statistically significant higher proportion of CD8+ lymphocytes 7 days after infection in comparison with the control (P < 0.001) and infected groups (P < 0.01) was recorded ((b)).
In this study L. reuteri B1/1 showed a potency to markedly increase the relative percentage of IgM in cecum as compared to the control (P < 0.001) as well as to the treated groups (P < 0.001) ((c)).
4. Discussion
It has been shown that C. jejuni infection may have a relevant impact on animal health and welfare in intensive poultry production (Humphrey et al., Citation2014). In addition, in modern breeds of broiler, the effort to improve productivity and increase carcass weight in a short period probably has have a negative effect on T cell function, leading to a loss of immune regulation in the gut. In the case of C. jejuni infection, this imbalance leads to intestinal mucosal damage and diarrhea. Similarly, in the present study, we recorded downregulation of gene expression for all studied immunologically important molecules at early phase of infection (12 hpi). This may confirm the inability of immature immune system of chicken broiler to react promptly to the pathogen, thus allowing spread throughout the body. Furthermore, it could contribute to persistently high level of Campylobacter colonization in broiler gut (Hermans et al., Citation2012; Meade et al., Citation2009). In a recent study, Connerton et al. (Citation2018) observed suppressed effect of C. jejuni colonization on the kinetics of the pro-inflammatory response in 6 day-old chickens at the beginning of the infection. Conversely, certain in vivo studies observed an increase of proinflammatory immune response after C. jejuni infection (Li et al., Citation2008; Smith et al., Citation2008). This may be related to changes in the microbiota composition mediated by C. jejuni as well as different C. jejuni strains, dosages, breeds of chicken and age. However, it has been reported that LOS of campylobacter could alter NLRP3 inflammasome activation in mouse (Bouwman et al., Citation2014) or human (Hameed et al., Citation2019) macrophages during in vitro conditions.
Different Lactobacillus species are often used in probiotic products and regarded as safe. Moreover, they produce short chain fatty acids as well as exopolysaccharides, which have trophic effects on the intestinal mucosa. Many studies have reported that some L. reuteri strains can promote regulatory T cell development (Mu et al., Citation2018); increase secretory IgA level and mucins secretion in tissues (Wang et al., Citation2016); modulate the Th1-promoting capacity of DCs upon interaction with C-type lectins (Bene et al., Citation2017); maintain the balance in the production of pro- and anti-inflammatory cytokines (Isolauri, Citation2003).
Specifically, L. reuteri B1/1 was isolated from gut content of healthy pheasant and demonstrated promising immunomodulatory properties when tested in vitro conditions (Karaffová et al., Citation2020). Generally, probiotic bacteria can influence key biological signalling pathways of inflammation, which may be specific to an individual probiotic strain, including strains of the same bacterial species. Some studies suggest that probiotics are able to modulate intestinal inflammation and immune response of the host and also through action on inflammasomes (Howarth, Citation2012; Miettinen et al., Citation2012; Tohno et al., Citation2011). In addition, inflammasome-mediated processes are important in microbial infections, and its dysregulation may result in an impairment of the host defence response against bacterial pathogens (Guo et al., Citation2015).
In accordance with the previous statement, our results revealed significantly upregulation gene expression of the studied immunologically important molecules in combined LRCJ group 24 hpi. This may indicate prompt activation of caspase 1 with subsequent cleavage of pro-IL-1β and pro-IL-18 precursors into active forms and initiation of an inflammatory response to the presence of C. jejuni stimulated by the applied probiotic strain. Altogether, it confirms the ability of L. reuteri B1/1 to activate NLRP3 inflammasome in the cecum of broiler chickens. Similarly, Miettinen et al. (Citation2012) observed an increased ability of the probiotic strain Lactobacillus rhamnosus to activate NALP3 inflammasome in human macrophages. To our knowledge, no studies have been published to examine the effect of Lactobacillus on activation of NLRP3 inflammasome in chickens infected by C. jejuni.
In accordance with the detected upregulation of gene expression of studied immunologically important molecules, we observed a stimulating effect of L. reuteri B1/1 on the relative percentage of IgM in the combined group in both sampling (4 dpi, 7 dpi). Nevertheless, B-lymphocytes stimulated by CpG motifs resulted in NLRP3 and then caspase-1 activation followed by secretion of IgM. It also resulted in releasing inflammatory cytokines and chemokines, which are triggered by the activation of TLRs. Moreover, in terms of innate immunity function, TLR4 is one of the central and key TLRs, which specifically recognizes bacterial LPS, along with several other components of pathogens and endogenous molecules produced during abnormal situations, such as tissue damage (Vaure & Liu, Citation2014). Interestingly, Eisenbarth et al. (Citation2008) demonstrated inability of NLRP3 deficient mice to produce IgM antibodies. We suppose close relationship between NLRP3 activated by L. reuteri and IgM in chickens. In the Zhang et al. (Citation2008) study, combination of Lactobacillus acidophilus NCFM™ and L. reuteri ATCC 23272 significantly increased total serum IgM and intestinal IgM titer in gnotobiotic pigs after rotavirus infection. In addition, probiotic strains were found to be able to enhance intestinal mucosal immunity of chicken already at the early age (Yurong et al., Citation2005).
During enteric infections, intraepithelial lymphocyte compartment plays a very important protective role. Intestinal IELs reduce bacterial translocation, through secretion of soluble mediators such as antimicrobial peptides (AMPs) among others. Notably, most of intestinal IELs are composed primarily of CD8+ T cells. It was observed, that C. jejuni infection disrupts the intestinal epithelial barrier of chicken resulting in a loss of intercellular junctions and increased intestinal permeability (Awad et al., Citation2015). Logically, the most important role in the protection against C. jejuni is played by cytotoxic lymphocytes. Despite this fact, we observed no effect of sole Campylobacter infection on the proportion of CD8+ IEL lymphocytes on 4 dpi, or minimal effect on 7 dpi in comparison with combine LRCJ or LR group. We assume that in this case essential role may be played by the lag phase, which is probably an internal property of the chicken and is usually related to maternally derived immunity. Furthermore, in growing chickens, intestinal niche may undergo many physiological changes during the first weeks of life and could be altered by adding probiotics to the feed modulating the composition of intestinal microbiota, and also by the maturation of the mucosal immunity (Newell & Fearnley, Citation2003). Thus, it could contribute to the increased reactivity of IEL expressing the surface marker CD8+ in the combined group. These findings confirm ability of L. reuteri B1/1 to ameliorate proportion of cytotoxic T cells in the intraepithelial part of gut mucosa in response to presence of C. jejuni. In line with our results, Brisbin et al. (Citation2012) reported ability of different Lactobacillus species to modulate T cell response in chicken cecal tonsils. In contrast, administration of L. reuteri B1/1 did not show an effect on the percentage of CD4+ IEL lymphocytes. However, the mechanism of T cells immune responses to C. jejuni in chicken has not been well documented (Han et al., Citation2016).
5. Conclusion
The administration of L. reuteri B1/1 significantly stimulated gene expression of NLRP3 inflammasome and other immunologically important molecules in the cecum, thereby increasing the capacity of the innate immune system to react more effectively to the infection of C. jejuni in broiler chickens. In this respect, this study represents an enrichment of the existing knowledge about the effect of Lactobacillus on activation of NLRP3 inflammasome in chicken. Importantly, no studies about the effect of Lactobacillus on activation of NLRP3 inflammasome in chicken after infection by C. jejuni have been published. These findings are compatible with the observed increase of IgM+ and proportion of CD8+ IEL lymphocytes provoked by L. reuteri B1/1. On the other hand, the proportion of CD4+ IEL lymphocytes was not affected by either L. reuteri or the infection itself. Finally, it should not be forgotten that only coordinated cooperation between the individual components of the immune system leads to effective defence against infection, which can be modulated by administration of probiotic lactobacilli.
Acknowledgements
This work was supported by the Grant Agency for Science of Slovak Republic VEGA 1/0112/18 and Slovak Research and Developmental Agency APVV-15-0165.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aguilar, S., Beshah, G. I., Vengroski, E., Zarlenga, K. G., Jauregui, D., Cosio, L., Douglass, M., Dubey, L. W., Lunney, J. P., & K, J. (2001). Cytokine and lymphocyte profiles in miniature swine after oral infection with Toxoplasma gondii oocysts. International Journal for Parasitology, 31, 187–195. https://doi.org/10.1016/s0020-7519(00)00159-4
- Ali, M. F., Dasari, H., Van Keulen, V. P., & Carmona, E. M. (2017). Canonical stimulation of the NLRP3 inflammasome by fungal antigens links innate and adaptive B-lymphocyte responses by modulating IL-1β and IgM production. Frontiers in Immunology, 8, 1504. https://doi.org/10.3389/fimmu.2017.01504
- Awad, W. A., Molnár, A., Aschenbach, J. R., Ghareeb, K., Khayal, B., Hess, C., Liebhart, D., Dublecz, K., & Hess, M. (2015). Campylobacter infection in chickens modulates the intestinal epithelial barrier function. Innate Immunity, 21, 151–160. https://doi.org/10.1177/1753425914521648
- Bene, K. P., Kavanaugh, D. W., Leclaire, C., Gunning, A. P., MacKenzie, D. A., Wittmann, A., Young, I. D., Kawasaki, N., Rajnavolgyi, E., & Juge, N. (2017). Lactobacillus reuteri surface mucus adhesins upregulate inflammatory responses through interactions with innate C-type lectin receptors. Frontiers in Microbiology, 8, 321. https://doi.org/10.3389/fmicb.2017.00321
- Bo, N., Yilin, H., Haiyang, Y., & Yuan, Y. (2020). Acrylamide induced the activation of NLRP3 inflammasome via ROS-MAPKs pathways in kupffer cells. Food and Agricultural Immunology, 1, 45–62. https://doi.org/10.1080/09540105.2019.1696284
- Borovská, D., Nemcová, R., Mudroňová, D., & Šumichrastová, J. (2012). The use of polysaccharides from a standpoint of increasing the functionality of probiotic bacteria. Slovak Veterinary Journal, 37, 344–346.
- Bouwman, L. I., de Zoete, M. R., Bleumink-Pluym, N. M., Flavell, R. A., & van Putten, J. P. (2014). Inflammasome activation by Campylobacter jejuni. Journal of Immunology, 193, 4548–4557. https://doi.org/10.4049/jimmunol.1400648
- Brisbin, J. T., Parvizi, P., & Sharif, S. (2012). Differential cytokine expression in T-cell subsets of chicken caecal tonsils co-cultured with three species of Lactobacillus. Beneficial Microbes, 3, 205–210. https://doi.org/10.3920/BM2012.0014
- Clavijo, V., & Flórez, M. J. V. (2018). The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poultry Science, 97, 1006–1021. https://doi.org/10.3382/ps/pex359
- Connerton, P. L., Richards, P. J., Lafontaine, G. M., O’Kane, P. M., Ghaffar, N., Cummings, N. J., Smith, D. L., Fish, N. M., & Connerton, I. F. (2018). The effect of the timing of exposure to Campylobacter jejuni on the gut microbiome and inflammatory responses of broiler chickens. Microbiome, 6, 88. https://doi.org/10.1186/s40168-018-0477-5
- Crhanova, M., Hradecka, H., Faldynova, M., Matulova, M., Havlickova, H., Sisak, F., & Rychlik, I. (2011). Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infection and Immunity, 79, 2755–2763. https://doi.org/10.1128/IAI.01375-10
- De Boever, S., Vangestel, C., De Backer, P., Croubels, S., & Sys, S. U. (2008). Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Veterinary Immunology and Immunopathology, 122, 312–317. https://doi.org/10.1016/j.vetimm.2007.12.002
- Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C., & Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics, 27, 2194–2200. https://doi.org/10.1093/bioinformatics/btr381
- Eisenbarth, S. C., Colegio, O. R., O’Connor, W., Sutterwala, F. S., & Flavell, R. A. (2008). Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature, 453, 1122–1126. https://doi.org/10.1038/nature06939
- European Food Safety Authority. (2018). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA Journal, 16, 5500. https://doi.org/10.2903/j.efsa.2018.5500
- Grzegorzewska, A. K., Lis, M. W., & Sechman, A. (2016). Immunolocalization of leptin receptor and mrna expression of leptin and estrogen receptors as well as caspases in the chorioallantoic membrane (CAM) of the chicken embryo. Folia Biologica, 64, 79–87. https://doi.org/10.3409/fb64_2.79
- Guo, H., Callaway, J. B., & Ting, J. P. (2015). Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nature Medicine, 21, 677–687. https://doi.org/10.1038/nm.3893
- Haghighi, H. R., Abdul-Careem, M. F., Dara, R. A., Chambers, J. R., & Sharif, S. (2008). Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection. Veterinary Microbiology, 126, 225–233. https://doi.org/10.1016/j.vetmic.2007.06.026
- Hameed, A., Machado, L., Woodacre, A., & Gemma Marsden, G. (2019). Induction of inflammasome-dependent signalling in the human monocytic cell line THP-1 by Campylobacter lipooligosaccharides. Access Microbiology, 1, https://doi.org/10.1099/acmi.ac2019.po0564
- Han, Z., Willer, T., Pielsticker, C., Gerzova, L., Rychlik, I., & Rautenschlein, S. (2016). Differences in host breed and diet influence colonization by Campylobacter jejuni and induction of local immune responses in chicken. Gut Pathogens, 8, https://doi.org/10.1186/s13099-016-0133-1
- Heeney, D. D., Gareau, M. G., & Marco, M. L. (2018). Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Current Opinion in Biotechnology, 49, 140–147. https://doi.org/10.1016/j.copbio.2017.08.004
- Hermans, D., Pasmans, F., Messens, W., Martel, A., Van Immerseel, F., Rasschaert, G., Heyndrickx, M., Van Deun, K., & Haesebrouck, F. (2012). Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector Borne and Zoonotic Diseases, 12, 89–98. https://doi.org/10.1089/vbz.2011.0676
- Hochel, I., Slavíčková, D., Viochna, D., Škvor, J., & Steinhauserová, I. (2007). Detection of Campylobacter species in foods by indirect competitive ELISA using hen and rabbit antibodies. Food and Agricultural Immunology, 18, 151–167. https://doi.org/10.1080/09540100701666857
- Howarth, G. S. (2012). Commentary on prebiotic utility in colitis: Will inflammasomics hold the key? The Journal of Nutrition, 142, 1189–1190. https://doi.org/10.3945/jn.112.160754
- Howarth, G. S., & Wang, H. (2013). Role of endogenous microbiota, probiotics and their biological products in human health. Nutrients, 5, 58–81. https://doi.org/10.3390/nu5010058
- Humphrey, S., Chaloner, G., Kemmett, K., Davidson, N., Williams, N., Kipar, A., Humphrey, T., & Wigley, P. (2014). Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. mBio, 5, e01364–14. https://doi.org/10.1128/mBio.01364-14
- Isolauri, E. (2003). Therapy update: Probiotics for infectious diarrhoea. Gut, 52, 436–437. https://doi.org/10.1136/gut.52.3.436
- Jia, Y., Si, W., Hong, Z., Qu, M., Zhu, N., Liu, S., & Guanhong, L. (2019). Toll-like receptor 2-mediated induction of avian β-defensin 9 by Lactobacillus rhamnosus and its cellular components in chicken intestinal epithelial cells. Food and Agricultural Immunology, 30, 398–417. https://doi.org/10.1080/09540105.2019.1593325
- Karaffová, V., Marcinková, E., Bobíková, K., Herich, R., Revajová, V., Stašová, D., Kavuľová, A., Levkutová, M., Levkut, M., Lauková, A., Ševčíková, Z., & Levkut, M. S. (2017). TLR4 and TLR21 expression, MIF, IFN-β, MD-2, CD14 activation, and sIgA production in chickens administered with EFAL41 strain challenged with Campylobacter jejuni. Folia Microbiologica, 62, 89–97. https://doi.org/10.1007/s12223-016-0475-6
- Karaffová, V., Revajová, V., Nemcová, R., Ševčíková, Z., Levkutová, M., & Levkut, M. (2020). In vitro study of immune properties of new lactobacilli isolates from pheasant gut. Folia Veterinaria, 64, 39–47. https://doi.org/10.2478/fv-2020-0006
- Lacharme-Lora, L., Chaloner, G., Gilroy, R., Humphrey, S., Gibbs, K., Jopson, S., Wright, E., Reid, W., Ketley, J., Humphrey, T., Williams, N., Rushton, S., & Wigley, P. (2017). B lymphocytes play a limited role in clearance of Campylobacter jejuni from the chicken intestinal tract. Scientific Reports, 7, 45090. https://doi.org/10.1038/srep45090
- Li, Y. P., Ingmer, H., Madsen, M., & Bang, D. D. (2008). Cytokine responses in primary chicken embryo intestinal cells infected with Campylobacter jejuni strains of human and chicken origin and the expression of bacterial virulence-associated genes. BMC Microbiology, 8, 107. https://doi.org/10.1186/1471-2180-8-107
- Li, Y., Li, N., Yan, Z., Li, H., Chen, L., Zhang, Z., Fan, G., Xu, K., & Li, Z. (2016). Dysregulation of the NLRP3 inflammasome complex and related cytokines in patients with multiple myeloma. Hematology, 21, 144–151. https://doi.org/10.1179/1607845415Y.0000000029
- Malek, M., Hasenstein, J. R., & Lamont, S. J. (2004). Analysis of chicken TLR4, CD28, MIF, MD-2, and LITAF genes in a Salmonella enteritidis resource population. Poultry Science, 83, 544–549. https://doi.org/10.1093/ps/83.4.544
- Meade, K. G., Narciandi, F., Cahalane, S., Reiman, C., Allan, B., & O’Farrelly, C. (2009). Comparative in vivo infection models yield insights on early host immune response to Campylobacter in chickens. Immunogenetics, 61, 101–110. https://doi.org/10.1007/s00251-008-0346-7
- Miettinen, M., Pietilä, T. E., Kekkonen, R. A., Kankainen, M., Latvala, S., Pirhonen, J., Österlund, P., Korpela, R., & Julkunen, I. (2012). Nonpathogenic Lactobacillus rhamnosus activates the inflammasome and antiviral responses in human macrophages. Gut Microbes, 3, 510–522. https://doi.org/10.4161/gmic.21736
- Mu, Q., Tavella, V. J., & Luo, X. M. (2018). Role of Lactobacillus reuteri in human health and diseases. Frontiers Microbiology, 9, 757. https://doi.org/10.3389/fmicb.2018.00757
- Newell, D. G., & Fearnley, C. (2003). Sources of Campylobacter colonization in broiler chickens. Applied and Environmental Microbiology, 69, 4343–4351. https://doi.org/10.1128/aem.69.8.4343-4351.2003
- Rychlik, I., Karasova, D., Sebkova, A., Volf, J., Sisak, F., Havlickova, H., Kummer, V., Imre, A., Szmolka, A., & Nagy, B. (2009). Virulence potential of five major pathogenicity islands (SPI-1 to SPI-5) of Salmonella enterica serovar Enteritidis for chickens. BMC Microbiology, 9, 268. https://doi.org/10.1186/1471-2180-9-268
- Ryznerova, D. (2013). The study of the properties of the probiotic bacteria in terms of their biological effects and applications (Dissertation, The University of Veterinary Medicine and Pharmacy ).
- Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., Lesniewski, R. A., Oakley, B. B., Parks, D. H., Robinson, C. J., Sahl, J. W., Stres, B., Thallinger, G. G., Van Horn, D. J., & Weber, C. F. (2009). Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology, 75, 7537–7541. https://doi.org/10.1128/AEM.01541-09
- Shang, L., Fukata, M., Thirunarayanan, N., Martin, A. P., Arnaboldi, P., Maussang, D., Berin, C., Unkeless, J. C., Mayer, L., Abreu, M. T., & Lira, S. A. (2008). TLR signaling in small intestinal epithelium promotes B cell recruitment and IgA production in Lamina propria. Gastroenterology, 135, 529–538. https://doi.org/10.1053/j.gastro.2008.04.020
- Smith, C. K., Abuoun, M., Cawthraw, S. A., Humphrey, T. J., Rothwell, L., Kaiser, P., Barrow, P. A., & Jones, M. A. (2008). Campylobacter colonization of the chicken induces a proinflammatory response in mucosal tissues. FEMS Immunology and Medical Microbiology, 54, 114–121. https://doi.org/10.1111/j.1574-695X.2008.00458.x
- Stephenson, H. N., John, C. M., Naz, N., Gundogdu, O., Dorrell, N., Wren, B. W., Jarvis, G. A., & Bajaj-Elliott, M. (2013). Campylobacter jejuni lipooligosaccharide sialylation, phosphorylation, and amide/ester linkage modifications fine-tune human Toll-like receptor 4 activation. The Journal of Biological Chemistry, 288, 19661–11972. https://doi.org/10.1074/jbc.M113.468298
- Tohno, M., Shimosato, T., Aso, H., & Kitazawa, H. (2011). Immunobiotic Lactobacillus strains augment NLRP3 expression in newborn and adult porcine gut-associated lymphoid tissues. Veterinary Immunology and Immunopathology, 144, 410–416. https://doi.org/10.1016/j.vetimm.2011.09.010
- Vaure, C., & Liu, Y. (2014). A comparative review of toll-like receptor 4 expression and functionality in different animal species. Frontiers in Immunology, 5, 316. https://doi.org/10.3389/fimmu.2014.00316
- Wang, P., Li, Y., Xiao, H., Shi, Y., Le, G. W., & Sun, J. (2016). Isolation of Lactobacillus reuteri from Peyer’s patches and their effects on sIgA production and gut microbiota diversity. Molecular Nutrition & Food Research, 60, 2020–2030. https://doi.org/10.1002/mnfr.201501065
- Yang, Y., Wang, H., Kouadir, M., Song, H., & Shi, F. (2019). Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death & Disease, 10, 128. https://doi.org/10.1038/s41419-019-1413-8
- Yurong, Y., Ruiping, S., Shimin, Z., & Yibao, J. (2005). Effect of probiotics on intestinal mucosal immunity and ultrastructure of cecal tonsils of chickens. Archives of Animal Nutrition, 59, 237–246. https://doi.org/10.1080/17450390500216928
- Zhang, W., Azevedo, M. S., Gonzalez, A. M., Saif, L. J., Van Nguyen, T., Wen, K., Yousef, A. E., & Yuan, L. (2008). Influence of probiotic lactobacilli colonization on neonatal B cell responses in a gnotobiotic pig model of human rotavirus infection and disease. Veterinary Immunology and Immunopathology, 122, 175–181. https://doi.org/10.1016/j.vetimm.2007.10.003
- Zhang, Z., Liu, Z., Tao, X., & Wei, H. (2016). Characterization and sulfated modification of an exopolysaccharide from Lactobacillus plantarum ZDY2013 and its biological activities. Carbohydrate Polymers, 153, 25–33. https://doi.org/10.1016/j.carbpol.2016.07.084
