ABSTRACT
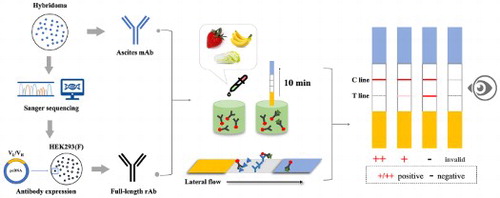
In this study, a rapid and reliable gold nanoparticles immunochromatographic strip (GNPs-ICS) assay based on a full-length recombinant antibody (rAb) was established for pyraclostrobin detection in food samples. First, a hybridoma PY-C7 secreting pyraclostrobin-specific monoclonal antibody (mAb) was produced. The variable region sequences of PY-C7-mAb were obtained by PCR and Sanger sequencing and used for the rAb expression in HEK293(F) cells. By GNPs-ICS assay, the visual LOD of pyraclostrobin standards was 0.1 mg L−1 (rAb) and 0.2 mg L−1 (mAb). Validated by recovery test, the visual LOQ of pyraclostrobin could meet the MRLs of Chinese food safety standard in strawberry, banana and Chinese cabbage samples by GNPs-mAb/rAb-ICS, in which the rAb showed better sensitivity and stability. Moreover, the GNPs-rAb-ICS showed comparable reliability with LC-MS/MS for actual sample detection. Above all, GNPs-ICS assay based on the full-length rAb was qualified for rapid detection of pyraclostrobin in food samples.
GRAPHICAL ABSTRACT
Introduction
Since the last century, the public has paid more and more attention to health, nutrition, food safety and environmental issues. In addition to controlling the sources of pollutants, how to carry out rapid and reliable detection of the analytes is a big challenge. Large-scale precision instruments such as chromatograph and mass spectrometer have high sensitivity and accurate results. They are currently used in research institutions and large enterprises. Although these methods are sensitive and reliable, they require precision instruments, professionals, high expenditure and complex preparation steps (Wang et al., Citation2005). Since the late last century, immunoassays based on antigen–antibody reaction are well developed to help assess food quality and food safety standards (Xu et al., Citation2009; Danks et al., Citation2010). Today, the most widespread rapid detection method is immunochromatographic strips (ICS) assay. As a kind of immunoassay, the ICS assay has been accepted as rapid, accurate, economical and easy-to-operate tools for detecting biomolecules in clinical diagnosis (Fu et al., Citation2011), and they are recently widely used in the food safety and environmental monitoring for trace amounts of chemicals (Ngom et al., Citation2010), especially for the detection of low-molecular-weight (LMW) compounds (Lee et al., Citation2012; Zhao et al., Citation2007) such as pesticides (Guo et al., Citation2009; Liu et al., Citation2017), mycotoxins (Wang et al., Citation2013; Puranik et al., Citation2020), antibiotics (Yu et al., Citation2019; Verheijen et al., Citation2010), feed additives (Gao et al., Citation2014).
Gold nanoparticles immunochromatographic strip (GNPs-ICS) assay, which uses colloidal gold as an indicator, enables to determine whether the substances to be examined exist in samples by naked eyes, without additional experimental steps (Dzantiev et al., Citation2014; Li et al., Citation2020). The core of the assay is the interaction of the antigen and the specific antibody with high affinity. An ideal antibody means that it can specifically bind to the analyte (antigen) with high affinity and it should have consistent performance. Taken the widely used mouse antibody as an example, there are mainly three generations until now. The first-generation antibody is polyclonal antibody which is low cost and short acquisition period, but has relatively poor specificity. As the second-generation antibody, the monoclonal antibody (mAb) is produced by hybridoma technology and commonly used because of high specificity and sensitivity. However, the hybridoma cells may lose the ability to secret effective mAbs in the process of cell culturing and storage, due to the complex genetic background and gene mutation/variation after long-term passage or repeated freezing and thawing (Liu et al., Citation2013; Bradbury et al., Citation2018). Hence, there are some problems in the preparation of GNPs-ICS assay, for example, differences in the batches of ascites antibodies, loss of effective mAb source, violating animal welfare requirements, core antibodies protected by patents and so on. Moreover, the discrepancy of specificity and sensitivity among mAb batches directly leads to high frequency of false positives or negatives in sample detection results from the GNPs-ICS assay.
In the recent decade, recombinant antibodies (rAbs) expressed in vitro have the potential to perform the same as their parental mAb from which they were derived. As a third-generation antibody, the sequence-defined rAb also has its unique advantages in the field of detection and diagnosis. It is easy to improve the sensitivity and reproducibility of the immunoassay with stepwise abandonment of experimental animals (Kavanagh et al., Citation2015; Groff et al., Citation2015; Farajnia et al., Citation2014). At present, rAbs towards LMW chemicals (haptens) are mainly obtained in three forms: (1) preparation of recombinant Fab or single chain variable fragment (ScFv) based on specific hybridoma cell lines (Xu et al., Citation2012; Zou et al., Citation2014); (2) screening of antibodies based on antibody libraries like phage antibody library (Hu et al., Citation2015); (3) mutation modification of antibody variable regions in vitro to obtain specific antibodies (Sheedy et al., Citation2007; Markus et al., Citation2014). Nonetheless, the hapten-specific rAbs obtained by these methods were not the full-length antibodies with constant regions. Some reports indicate that fragment antibodies are not as specific and sensitive as their parent mAb or full-length rAb (Yau et al., Citation1998; Qin & Li, Citation2014; Li et al., Citation2012; Picanço-Castro & Swiech, Citation2017). This is mostly because non-mammalian expression system such as Escherichia coli was used in the early stage, and correct folding, glycosylation and soluble expression of antibodies were difficult to achieve. Considering that mammalian expression systems have complete transcription, post-translational processing and secretion mechanisms, the full-length rAb has good biological activity, and are basically like natural antibodies in constructs and functions (Ames et al., Citation1995; Jones et al., Citation2003; Wang et al., Citation2020). In addition, the full-length antibodies could be modified by additional labels for detection in many immunoassays, while it was hard for the rAb fragments to reach the target (Jager et al., Citation2013). However, there is still lack of mammalian cells expressed full-length rAbs for the detection of LMW pollutants such as pesticides.
Pyraclostrobin is a novel strobilurin fungicide developed by BASF in Germany (1993). It inhibits mitochondrial respiration and suppresses the production of ATP by preventing electron transfer between cytochrome B and C1. Eventually, the pathogenic fungi cannot perform normal physiological metabolism to die (Lagunas-Allué et al., Citation2012). Pyraclostrobin has a broad antifungal activity spectrum, high efficiency and security, and it can treat almost all plant pathogenic fungal diseases. It can be mainly used in cereals, fruit and vegetables for protection against Stemphylium solani, Plasmopara viticola, Phytophthora infestans and so on (Yang et al., Citation2018). Pyraclostrobin is an environmentally friendly fungicide, which is less harmful to non-target organisms, but it has been reported that pyraclostrobin will affect the growth of silkworms and the quality of silk production (Zhang, Citation2007) and it also has a certain mitochondrial toxicity and neurovirulence in zebrafish (Li et al., Citation2019). Therefore, the use of pyraclostrobin should be controlled in agricultural production. The maximum residue limits (MRLs) are, for most fruits and vegetables, between 0.2 and 4.0 mg kg-1 in the EU (https://ec.europa.eu/food/plant/pesticides/eu-pesticidesdatabase/public/?event=pesticide.residue.CurrentMRL&language=EN) and wider range between 0.04 and 30 mg kg-1 in the USA (https://www.ecfr.gov/cgi-bin/text-idx?SID=b84715acdfbe3366fabe63ed0035210c&mc=true&node=sp40.26.180.c&rgn=div6#se40.26.180_1582). According to the latest national standards in China (GB 2763–2019), the MRLs in cereals are 0.2–1.0 mg kg-1, in oil crops are 0.05–0.4 mg kg-1, in vegetables are 0.02–20 mg kg-1, in fruits are 0.05–4 mg kg-1, in tea is 10 mg kg-1 and in other categories are 0.02–5 mg kg-1.
At present, instrumental analysis is competent in determining pyraclostrobin residues, such as liquid chromatography (LC) (De Melo Abreu et al., Citation2006), gas chromatography–mass spectrometry (GC-MS) (Lagunas-Allué et al., Citation2012), liquid chromatography–mass spectrometry (LC-MS) (Malhat et al., Citation2019; Zhang et al., Citation2012), ultrahigh-performance liquid chromatography–mass spectrometry (UPLC-MS) (Zhang et al., Citation2009). In 2008, Mercader et al. obtained a high-affinity monoclonal antibody and developed an enzyme-linked immunosorbent assays (ELISA) for pyraclostrobin detection with a limit of detection (LOD) below 0.1 μg L-1 (Mercader et al., Citation2008). In 2011, they synthesized many kinds of site-heterologous haptens for generating higher affinity pyraclostrobin-specific antibody (Mercader et al., Citation2011). In 2013, they combined ELISA with the QuEChERS pretreatment method for the analysis of pyraclostrobin residues in six kinds of fruits (Mercader et al., Citation2013). However, so far, few studies have been carried out on portable and on-site GNPs-ICS detection for pyraclostrobin residues.
This study aimed to establish a GNPs-ICS assay by a newly expressed full-length rAb against pyraclostrobin. Our work indicated that the developed rAbs-based GNPs-ICS assay was a fast, sensitive, reliable and portable method for the rapid detection of pyraclostrobin residues in both laboratory and on-site tests.
Material and methods
Reagents and materials
Pyraclostrobin standard was obtained from the Agro-Environmental Protection Institute, Ministry of Agriculture (Tianjin, China). N-hydroxysuccinimide (NHS), N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC), ovalbumin (OVA), bovine serum albumin (BSA), rabbit anti-mouse IgG (L+H) antibody, horseradish peroxidase (HRP)-labelled rabbit anti-mouse IgG (L+H) antibody, gold chloride, trisodium citrate dihydrate, pristane and Brilliant Blue G250 were purchased from Sigma-Aldrich (St. Louis, MO, USA). 3,3’,5,5'-Tetramethylbenzidine (TMB) substrate reaction solution was purchased from Biodragon Immunotechnologies (Beijing, China). DNA marker and prestained protein ladder were brought from Thermo Fisher Scitific Company. Sodium acetate, octanoic acid and sodium hydroxide were purchased from Zhongxing chemical regent company (Jinhua, Zhejiang, China). Polyethylene glycol (PEG, MW 20,000), polyvinylpyrrolidone (PVP, K-30), NaCl powder, Tween 20, sucrose and methanol were supplied by Sinopharm Chemical Reagent (Shanghai, China). Nitrocellulose (NC) membrane was provided by Sartorius (Germany). All kits, instruments and buffers used in this study were noted in the text.
Preparation and characterization of pyraclostrobin-specific mAb
The hapten of pyraclostrobin was synthesized according to Josep V. Mercaders’ research (Mercader et al., Citation2011) (). The immunizing conjugate PY-BSA (conjugates of pyraclostrobin hapten and BSA) and the coating conjugate PY-OVA (conjugates of pyraclostrobin hapten and OVA) were prepared by the carbodiimide method. The hybridoma cells which secreted pyraclostrobin-specific mAbs were produced by fusing myeloma cells (Sp2/0) and splenocytes which were from BALB/c mice immunized with PY-BSA. Indirect competitive ELISA (Ic-ELISA) procedures were applied for selecting excellent hybridoma. Briefly, the chessboard titration was used to optimize the working concentrations of PY-OVA and the pyraclostrobin-specific antibody (cell culture supernatant). Standard competition curves were acquired by plotting the inhibition rate against the logarithm of pyraclostrobin concentration. By multiple rounds of limiting dilution and ic-ELISA, the hybridoma cell line named PY-C7 that could stably produce pyraclostrobin-specific mAb was selected. It was supposed to subculture PY-C7 and freeze it into multiple tubes for longtime storage in liquid nitrogen. Irregularly resuscitate and subculture some tube of the frozen cell lines, and then limiting dilution was adopted with 96-well plates, monitoring the capacity for secreting mAbs and the sensitivity of recognizing pyraclostrobin by ELISA.
When preparing ascites mAbs, take one tube for cell resuscitation and subculturing, and inject it into the abdominal cavity of F1 hybrid mice. One week later, the extracted ascites from different batches of mice and cell lines could be collected and purified to obtain pyraclostrobin-specific mAbs following identification. The overall process was shown in Figure S1. In addition, the subtype of PY-C7-mAb was determined as IgG1 (heavy chain) and κ (light chain) by using a mouse mAb subtype identification ELISA kit (Beijing Biodragon Immunotechnologies).
Preparation of pyraclonstrobin-specific full-length rAb
In the process of limiting dilution and hybridoma cell line screening, some clones were found to be incapable of secreting the mAb of interest. To avoid this, the sequences of light and heavy chains variable regions (VL and VH for short) of PY-C7-mAb should be determined clearly. Based on the parental mAb’s sequences, a mammalian HEK293(F) cell system could be subsequently constructed to obtain the full-length rAb against pyraclonstrobin (PY-C7-rAb).
In brief, total RNA was extracted from PY-C7 cell line (RNAiso Plus, Takara 9109), the obtained DNA-free total RNA was immediately used as templates to synthesize cDNAs by reverse transcription (PrimeScript 1st strand cDNA synthesis Kit, Takara 2690A). The subtype-specific (κ and IgG) degenerated primers of VL and VH (Biointron Biological Inc, Taizhou, Jiangsu, China) () were used for PCR amplification (2×TransStart® FastPfu PCR SuperMix (-dye), TransGen Biotech, AS221-01). The PCR fragment was ligated into pMDTM19-T vector (pMD™19-T Vector Cloning Kit, Takara 6013), and then the construct was transformed into competent E. coli TOP10 (One ShotTM TOP10 Chemically Competent E. coli, Invitrogen by Thermo Fisher Scientific, C404010). Ten positive clones (ampicillin resistant) from each group were randomly selected to conduct colony PCR for sanger sequencing (M13F: CGCCAGGGTTTTCCCAGTCACGAC; M13R: AGCGGATAACAATTTCACACAGGA). The sequences were aligned to IgLκ and IgH sequences from the BLAST database and IMGT (http://www.imgt.org/IMGT_vquest/input). The analysis of complementarity determining regions (CDRs) was carried out by abYsis (http://www.abysis.org).
Table 1. Primer sequences for VH and VL gene amplification.
In the light of the exact sequences of VL and VH of PY-C7-mAb, one of the corresponding clones was cultured as specific PCR template. Upstream and downstream primers () were designed based on not only the sequences of variable regions of PY-C7-mAb but also the homologous sequences of the expression vector plasmids (Biointron Biological Inc, Taizhou, Jiangsu, China). Amplified products with the sequences of homologous arms (24 bp per segment) and variable regions were electrophoresed in 2% agarose gel for size verification (ChemiDocTMMP imaging system with Image lab 5.2 analysis software, Bio-RAD, USA) and recovered by agarose gel extraction (MiniBEST Agarose Gel DNA Extraction Kit, Takara 9762). As shown in , the purified PCR products were cloned into the linearized vectors (MCS with HindⅢ and EcoRⅠ) which contained signal peptide and mouse IgG1 constant region via homologous recombination (the sequences of homologous arms and MCS were shown in Figure S2) and transform into competent E. coli TOP10. The isolated single colonies were picked to undergo PCR (primers CMV-F: GATCGCCTGGAGACGCCATC; Seq-R: AGCGTAAAAGGAGCAACATAGT). The PCR products were evaluated by gel electrophoresis analysis.
Figure 2. Partial constructs of the expression vector and the illustration of homologous recombination.
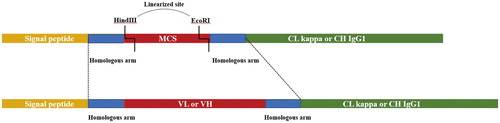
Table 2. Primers of PCR progress for obtaining homologous recombination fragments.a
Four colonies were randomly picked for Sanger sequencing via the primers above. The colony with correct sequences was transferred into LB medium with ampicillin to expand the culture, followed by plasmid extraction (QIAGEN Plasmid Midi Kit, QIAGEN 12143). HEK293(F) cells were suspension cultured and passaged with limited component medium (CD-293 medium, Biointron, Cat#BI293001). 100 mL volume transient transfection was performed containing the cells with the density of 3 × 106 per milliliter after 3rd passaged, 100 μg light chain and heavy chain plasmid with a ratio of 1:1, and 200 μL Lipofectamine. After that, the cell was cultured for 5 days at 37°C and centrifugation. Then, the supernatant was collected and followed 0.22 μm membrane filtration. The AKTA purifier with protein A (GE, Cat#17547402) was applied for antibody purification. Finally, the Glycine solution was used to elute the antibody. After dialysis and dissolution in PBS buffer, PY-C7-rAb was successfully obtained. The antibody’s performances of recognizing pyraclostrobin were characterized by ic-ELISA, and then the rAb was used for GNPs-ICS assay.
Preparation of gold nanoparticles
According to the previous procedure in our lab (Guo et al., Citation2009; Zhao et al., Citation2016), we prepared monodisperse colloidal gold with an average diameter of 28 nm. Under reflux conditions, 0.02% gold chloride solution (100 mL) was heated to its boiling point, and 1.5 mL of 1% trisodium citrate solution was added immediately while stirring rapidly. Once the solution turned blue and a while changed to bright wine red, it was supposed to boil for additional 5 minutes. The wavelength of maximum absorption of gold particles was 525 nm scanned by SpectraMax i3 microplate reader (Molecular Devices, Sunnyvale, USA) in the visible spectrum (400–600 nm). The obtained colloidal gold suspensions supplemented with 0.05% sodium azide could be stored at 4°C for several months.
Preparation of gold nanoparticles-labelled immunoconjugates
Visible spectrophotometry was used to determine the most stable amount of acetamiprid antibodies (GEOGHEGAN, Citation1988). The pH value of colloidal gold (8 mL) was adjusted from 5.0 to 9.5 with 0.1 M K2CO3, and then was divided into enough tubes for use later. The mAb/rAb which to be labelled was dialysed in 0.01 M PBS at 4°C overnight to remove excess salt ions. After setting gradient dilutions of the antibody solution (0 /10 /20 /40 /60 /80 /100 /120 mg L-1), the diluted antibody (0.1 mL) was respectively added to the prepared colloidal gold solution (1 mL). After stirring for 5 minutes, 0.1 mL of 10% NaCl solution was added to the mixed solution, and standing for 2 h. According to the colour rendering index (CRI) and the coefficient of variation (CV) of absorbance, the optimum pH was determined and the amount of antibody that can stabilize colloidal gold plus 10% is the optimum amount of labelling.
To make a batch of GNPs-labelled immunoconjugates, 1 mL of the optimum amount of antibody was added dropwise in 10 mL of colloidal gold solution. After mixing and standing for 1 h, 1.22 mL of 0.01 M PBS (pH 6.5, containing 10% (W/V) BSA and 1% (W/V) PEG) were added in the mixture. After standing for another 1 h, the mixture was centrifuged for 30 minutes (10 000 r/min), and the supernatant was discarded; and then the sediment was resuspended by 10 mL of 0.01 M PBS (pH 6.5, containing 1% W/V BSA and 0.1% W/V PEG). Centrifuge again and resuspend the sediment by 1 mL of 0.01 M PBS (pH 6.5, containing 0.5% W/V BSA and 5% V/V sucrose), store at 4°C.
GNPs-ICS test procedure
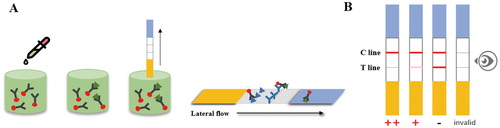
The strips assembly was referred to our previous work (Liu et al., Citation2019). During analyte testing, the GNPs-mAb/rAb immunoconjugates (25 μL) and analyte solution (25 μL) were premixed and the labelled antibodies could react with the analyte (pyraclostrobin existed) for 5 minutes. Then the mixture was dropped onto the sample pad and passed through the NC membrane for another 5 minutes. During lateral-flow chromatography process, GNPs-mAb/rAb that did not react with pyraclostrobin was captured by the T line, and the T/C lines showed corresponding colour changes. When the C line showed steady red, the more pyraclostrobin the sample contained, the weaker red appeared on the T line. Once the T line had no colour or showed faint red while the C line changed to red, indicating a pyraclostrobin positive sample. But the pyraclostrobin negative sample was defined by two lines with similar CRI, except for the invalid samples without red C lines ().
Figure 3. (A) Schematic illustration of the detection procedure by GNPs-ICS. (B) The decision criterion of GNPs-ICS assay. * (B) Negative samples (-), positive samples (+) and strongly positive samples (++) judged by naked eyes.
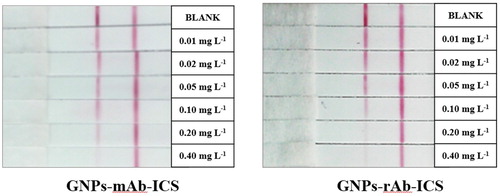
To determine the sensitivity of the assay, the pyraclostrobin mother liquor (100 mg L-1) was diluted to a series of standard solutions with gradient concentrations of 0.01, 0.02, 0.05, 0.10, 0.20, and 0.40 mg L-1 by 0.01 M PBS (involved in 20% methanol). Compared with the C line, the lowest concentration of pyraclostrobin that caused significantly weaker of the T line colour was considered as the visual sensitivity.
To identify the specificity of the strips, other strobilurin fungicide such as kresoxim-methyl, azoxystrobin and difenoconazole were tested with the concentration of 0.2 mg L-1.
Sample test by GNPs-ICS assay
Eliminate the matrix effects of samples is the key to sample detection. For reducing matrix effect, the samples were processed as follows. The homogenized strawberry (5 g), banana (5 g) and Chinese cabbage (5 g) samples (pyraclostrobin-free) were individually added with 10 mL of 0.01M PBS (20% methanol involved), and the extraction were set to gradient dilutions (1:2, 1:5, 1:10). Then, pH should be adjusted to 6.5–7.5, some samples extract was collected for matrix effect detection. A best fit dilution time was selected, according to the intensity and efficiency of colour developing. Six final concentrations of pyraclostrobin standard solutions were prepared by diluted sample extract with 0.01 M PBS (20% methanol involved) as the control.
To determine the accuracy of the GNPs-ICS assay, the strawberry, banana and Chinese cabbage samples were spiked with 0.25, 1.00 and 2.00 mg kg-1 of pyraclostrobin standard. After incubation in three samples for 2 h at RT, 10 mL 0.01M PBS (20% methanol involved) was added to the spiked samples. These samples were then vigorously shaken for 3 minutes, and sample extract was collected and tested by GNPs-mAb/rAb-ICS assay.
To further confirm the accuracy of the GNPs-ICS assay, the strawberry was selected for actual sample testing. The pretreatment of samples for GNPs-ICS assay was performed as mentioned above. For the reliability of the results, the samples were identified by liquid chromatography–tandem mass spectrometry (LC-MS/MS) and the pretreatment of sample was as follows. The homogenized strawberry sample (1 g) was accurately added 2 mL of acetonitrile and sharply shaken for 3 min. Then, 0.3 g of sodium chloride and 0.5 g of anhydrous magnesium sulfate were added into the sample and the mixture was evenly shaken for 1 min. After incubation for 30 min at RT, 1.5–2.0 mL of supernatant was removed, and 0.05 g of primary secondary amine (PSA) was added. After shaken for 1 minute, the mixture was centrifuged at 4°C 10000 rpm for 3 min and the supernatant was transferred into a 1.5 mL-MS sample bottle through a 0.22-μm organic filter membrane for instrument detection.
The analytical column was ACQUITY UPLC® BEH C18 column (2.1 × 100 mm, with a 1.7-μm particle size). The isocratic eluent included methyl alcohol and 0.1% formic acid at the flow rate of 300 μL min-1 with 10 μL sample injection volume. showed the ratio of gradient elution. MS conditions were electrospray ion source (ESI), positive ion scanning and multi-reaction monitoring (MRM). Other parameters were as follows: capillary voltage, 2.5 kV; source temperature, 150°C; desolvation temperature, 500°C; desolvation gas flow rate, 1000 L h-1; cone gas flow rate, 30 L h-1. MS/MS conditions for quantifying pyraclostrobin were: Q1: 388 / Q3: 194/163; DP: 20 V; CE: 18 V and EP: 6 V; CXP: 10 V.
Table 3. The ratio of gradient elution of pyraclostrobin detection by LC-MS/MS in strawberry.
Results and discussion
Characterization of different batches of pyraclostrobin-specific mAb
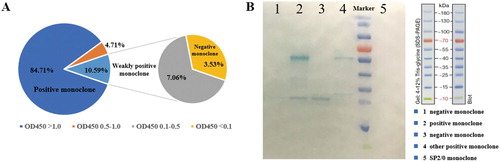
For a new batch of large-scale mAb’s production, the frozen hybridoma PY-C7 cell lines were resuscitated and subjected to limited dilution to a 96-well plate respectively, and the ELISA was performed to identify the affinity of the mAb in culture supernatant according to the OD450 value. It was clear that the supernatant of some monoclonal cell wells showed weak (OD450 < 0.5) or no recognition of pyraclostrobin (OD450 < 0.1, named negative monoclones), while the positive ones’ OD450 value was always more than 1.0 (A). The WB result shown that negative cell lines lost the heavy chains (∼50 kD) or both light chains (∼25 kD) and heavy chains (B).
Figure 4. (A) The pyraclostrobin-specific mAbs secretory capacity of resuscitated PY-C7 cell line in different monoclonal cell wells. (B) WB results of light or heavy chains of positive and negative monoclonal cell lines.
At the same time, three independent PY-C7 cell lines were resuscitated and used to produce three batches of ascites mAbs. However, the effective mAb concentrations were very different after purification. When we converted the different batches of purified ascites mAbs to the same working concentration, the half maximal inhibitory concentration (IC50) of recognizing pyraclostrobin ranged from 6.98 to 25.24 μg L-1 (see Figure S3). Therefore, the hybridoma cell line PY-C7 did have the risk of being non-functional to secret the mAb with no-affinity to pyraclostrobin.
Sanger sequencing of hybridoma PY-C7
Total RNA of PY-C7 was successfully extracted and the cDNA template was synthesized by reverse transcription. Using the subtype-specific degenerated primers of VL and VH of mouse antibody, the PCR procedure was carried out to obtain the VL and VH fragments of PY-C7 cell line, and the fragments were about 450–500 bp consistent with the theoretical situation (see Figure S4A). Some clones were identified using agarose gel electrophoresis (see Figure S4B) and then the picked 10 positive clones (about 600 bp) were sequenced and analysed. The sequences of VL and VH were as follows:
VL:
GACATTGTGCTGACCCAATCTCCAGCTTCTTTGGCTGTGTCTCTAGGGCAGAGGGCCACCATCTCCTGCAGAGCCAGCGAAAGTGTTGATAATTATGGCATTAGTTTTATGAACTGGTACCAACAGAAACCAGGACAGCCACCCAAACTCCTCATCTATGCTGCATCCAACCAAGGATCCGGGGTCCCTGCCAGGTTTAGTGGCAGTGGGTCTGGGACAGACTTCAGCCTCAACATCCATCCTATGGAGGAGGATGATAGTGCAATTTATTTCTGTCAGCAAAGTAAGGAGGTTCCTCCGACGTTCGGTGGAGGCACCAAGCTGGAAATCAAA (333)
GAAGTGGAGCTGGTCGAGTCTGGGGGTGGCTTAGTGAAGCCTGGAGGGTCCCTGAAACTCTCCTGTGCAGTCTCTGGATTCACTTTCAGTACCTATGCCATGTCTTGGGTTCGCCAGACTCCAGAGAAGAGGCTGGAATGGGTCGCATCCATTCGTAGTGGTGGTGACACCTACTATCCAGACAGTGTGAAGGGCCGATTCACCATCTCCAGAGATAATGCCAGGAACATTCTGTACCTGCAAATGAGCAGTCTGAGGTCTGAGGACACGGCCATGTATTACTGTACAAGACTAAGTCATTTCTACGTCTACGGGGACTACTGGGGCCAAGGCACCACTCTCACAGTCTCCTCA (354)
Expression of pyraclostrobin-specific full-length rAb in HEK293(F)
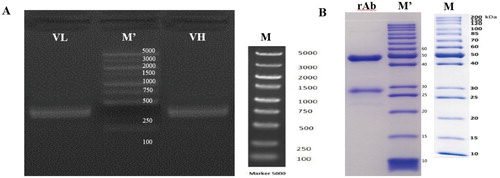
The fragments of VL and VH of PY-C7-mAb with homologous arms for cloning were verified by agarose gel electrophoresis. It was shown in (A) that the fragments were about 400 bp which was consistent with the theoretical design. Validated PCR fragments were constructed on an expression plasmid vector with the universal signal peptide and mouse IgG1 conserved constant region sequences. Figure S5 was the structures of PY-C7-rAb expression vectors which showed the sequences of antibody expression area containing homologous arms, signal peptides, variable and constant regions of light and heavy chain, and the primers (CMV-F/Seq-R) for verifying the clones. The correct expression plasmid vector was transfected into HEK293(F) for transient expression, and then the supernatant was collected and purified to obtain full-length rAbs. After the proteins were separated by gel electrophoresis, the light and heavy chains were about 25 and 50 KD, stained by Brilliant Blue G250 (B).
Figure 5. (A) PCR amplification of variable region fragments of PY-C7-mAb with homologous arms. (B) SDS-page of the full-length rAb expressed in mammalian cells HEK293(F). * (A) M’: 5000 KD Marker in actual map; M: standard 5000 KD Marker; (B) M’: 200 KD Marker in actual map; M: standard 200 KD Marker.
Ic-ELISA identification of pyraclostrobin-specific mAb/rAb
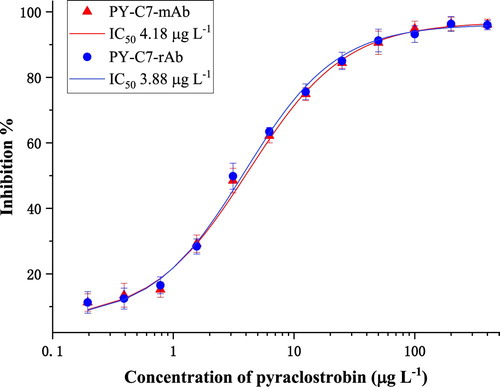
Ic-ELISA was used to determine the sensitivity of PY-C7-rAb and its parental PY-C7-mAb, which were from the same cell line resuscitated. The best working concentration of PY-OVA was 0.5 mg L-1, and that of antibody was 0.3 mg L-1 for both. As shown in , both PY-C7-mAb and PY-C7-rAb were able to well recognize pyraclostrobin, with close IC50 of 4.18 μg L-1 for PY-C7-mAb and 3.88 μg L-1 for PY-C7-rAb, respectively. Above all, ic-ELISA results demonstrated that PY-C7-rAb had almost the same sensitivity as PY-C7-mAb towards pyraclostrobin, so the rAb was used to establish the GNPs-ICS assay in the following study.
Preparation and characterization of GNPs-mAb/rAb immunoconjugates
Table S1 shows the CV of absorbance after the salt treatment of gold nanoparticles-labelled mAb/rAb solutions with different pH values. The CV values of absorbance were all less than 15% under different pH environments. Combined with CRI of T lines of the strips, pH 6.5 was supposed to be the optimal labeling solution.
As for the optimum doses of PY-C7-mAb/rAb for labelling, different dosage of antibodies was tested. The CV values of absorbance were also less than 15% before and after the salt treatment. Based on the CRI of T lines of the strips and the material consumption, the concentration 60 mg L-1 of PY-C7-mAb/rAb was the optimal labelling condition (see Table S2).
The sensitivity and specificity of GNPs-ICS assay
According to the decision principle, the visual LOD of GNPs-rAb-ICS was 0.1 mg L-1 and of GNPs-mAb-ICS was 0.2 mg L-1 (). The GNPs-ICS assay based on the rAb had a slightly better sensitivity to that with the mAb for semi-quantitative detection of pyraclostrobin standard solution.
Figure 7. GNPs-mAb/rAb-ICS assay for testing gradient-spiked pyraclostrobin standard solution. * The concentrations of pyraclostrobin standard were, in order, 0, 0.01, 0.02, 0.05, 0.10, 0.20, 0.40 mg L−1.
Other three strobilurin fungicides were tested and the CRI of each T line was close to that of the black control (). These cross-reaction results demonstrated that the strip assays were highly specific to pyraclostrobin.
Matrix effect of tested samples
The complex matrix in samples, containing pigments and saccharides, can interfere the sensitivity of GNPs-ICS assay. It could be solved by diluting the sample extract with the reaction buffer like PBS (or water instead), which was a simple and effective procedure without any complicated pretreatment for on-site detection.
As shown in Figure S6A, three different dilution times of the blank sample exaction had no significant effect on the CRI of the T lines with enough reaction time, whether GNPs-mAb-ICS assay or GNPs-rAb-ICS assay. Weighed the detection time, fivefold dilution time by which the colour development could be completed within 10 minutes was chosen. After that, six kinds of pyraclostrobin standard solutions at the final concentrations of 0.01–0.4 mg L-1 were respectively prepared by the extract from blank samples at fivefold dilution. As shown in Figure S6B, for three samples, the visual LODs of pyraclostrobin were about 0.2 mg L-1 detected by GNPs-mAb-ICS and 0.1 mg L-1 detected by GNPs-rAb-ICS. The results were basically consistent with the visual LOD of matrix-free pyraclostrobin standard above, which indicated that the matrix effects could be eliminated by the appropriate dilution time.
Recovery test
In this work, the recovery test was carried out to evaluate the accuracy of GNPs-ICS assay. It was noteworthy that in the premise of matrix effect elimination, the spiked concentrations of pyraclostrobin standard in recovery test were supposed to fall into the examination area of ICS to ensure the authenticity of the test. As mentioned above, 0.25, 1.00, 2.00 mg kg-1 of pyraclostrobin standards were individually added into strawberry, banana, Chinese cabbage samples and the test was repeated in triplicate. As shown in , it was clear that for the detection of pyraclostrobin in food samples by GNPs-mAb/rAb-ICS assays, the visual LOQ was about 1 mg kg-1 (except for strawberry detected by GNPs-mAb-ICS, about 2 mg kg-1), which could meet the requirements of Chinese MRLs (2 mg kg-1 in strawberries, 1 mg kg-1 in bananas and 5 mg kg-1 in Chinese cabbages). It also met the requirements of the pyraclostrobin MRLs in Chinese cabbage in the USA (5 mg kg-1) and European Union (1.5 mg kg-1). Moreover, results in showed that the GNPs-ICS based on rAbs showed an advantage in the terms of repeatability and sensitivity of assay performance.
Figure 9. The visual results of recovery tests of pyraclostrobin in strawberry, banana and Chinese cabbage samples detected by GNPs-mAb/rAb-ICS assays.
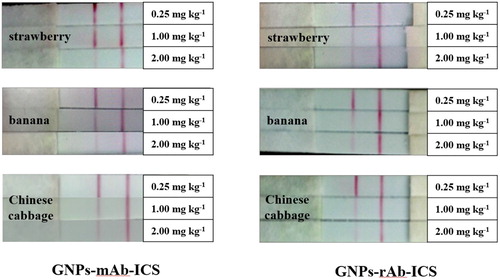
Table 4. Results of repeated recovery tests of pyraclostrobin in spiked food samples detected by GNPs-mAb/rAb-ICS assays.
Actual sample testing
To further confirm the accuracy of the assay, 10 kinds of real strawberry samples were collected from local fields and detected by GNPs-rAb-ICS according to the progress mentioned above. The LC-MS/MS was also used for quantification and the LOQ for pyraclostrobin was 0.005 mg kg-1. As shown in , the detection results of GNPs-rAb-ICS were practically satisfactory and consistent with those from LC-MS/MS.
Table 5. The detection of pyraclostrobin residue in actual strawberry samples by GNPs-ICS assay and LC-MS/MS.
Conclusion
In view of the instability of hybridoma cells during the passage and storage, this article first prepared a pyraclostrobin-specific full-length rAb. Based on the parental hybridoma cell line PY-C7, the sequences of VL and VH of PY-C7-mAb were successfully measured by Sanger Sequencing. Subsequently, the obtained variable regions were constructed on the expression plasmid with signal peptide and mouse IgG1 constant region, and then the full-length rAb was expressed by HEK293(F) cells. Verified by ic-ELISA, the sensitivity of the rAb was consistent with that of the parental mAb. Using the full-length rAb, GNPs-ICS assay was established to detect pyraclostrobin residues in strawberry, banana and Chinese cabbage samples, setting the GNPs-mAb-ICS as a reference. Satisfactorily, the visual LOQs of pyraclostrobin in food samples detected by the GNPs-mAb/rAb-ICS basically met the requirements of Chinese MRLs, and the GNPs-rAb-ICS showed better sensitivity and stability. Additionally, the GNPs-rAb-ICS assay showed comparable reliability with LC-MS/MS for actual strawberry samples. More critically, the use of full-length rAbs could reduce the chance of false negative or false positive detection errors, which might be usually caused by batch-to-batch variation of ascites mAbs. In brief, we provided an alternative biomaterial for immunochromatographic assay of small molecule pollutants, which could meet the needs of on-site rapid and reliable screening of samples. Furthermore, we would choose more sensitive labelling materials and quantitative methods to improve detection sensitivity and meet more stringent testing standards.
Supplemental Material
Download MS Word (2.4 MB)Acknowledgements
This study was financially supported by National Key R&D Program of China (2017YFF0210200).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Ames, R. S., Tornetta, M. A., Deen, K., Jones, C. S., Swift, A. M., & Ganguly, S. (1995). Conversion of murine Fabs isolated from a combinatorial phage display library to full length immunoglobulins. Journal of Immunological Methods, 184(2), 177–186. https://doi.org/10.1016/0022-1759(95)00086-P
- Bradbury, A. R. M., Trinklein, N. D., Thie, H., Wilkinson, I. C., Tandon, A. K., Anderson, S., Bladen, C. L., Jones, B., Aldred, S. F., Bestagno, M., Burrone, O., Maynard, J., Ferrara, F., Trimmer, J. S., Görnemann, J., Glanville, J., Wolf, P., Frenzel, A., Wong, J., … Dübel, S. (2018). When monoclonal antibodies are not monospecific: Hybridomas frequently express additional functional variable regions. mAbs, 10(4), 539–546. https://doi.org/10.1080/19420862.2018.1445456
- Danks, C., Chaudhry, M., Parker, L., Barker, I., & Banks, N. (2010). Development and validation of an immunoassay for the determination of tebuconazole residues in cereal crops. Food and Agricultural Immunology, 13(3), 151–159. https://doi.org/10.1080/09540100120075808
- De Melo Abreu, S., Caboni, P., Cabras, P., Garau, V. L., & Alves, A. (2006). Validation and global uncertainty of a liquid chromatographic with diode array detection method for the screening of azoxystrobin, kresoxim-methyl, trifloxystrobin, famoxadone, pyraclostrobin and fenamidone in grapes and wine. Analytica Chimica Acta, 573–574, 291–297. https://doi.org/10.1016/j.aca.2006.01.090
- Dzantiev, B. B., Byzova, N. A., Urusov, A. E., & Zherdev, A. V. (2014). Immunochromatographic methods in food analysis. TrAC Trends in Analytical Chemistry, 55, 81–93. https://doi.org/10.1016/j.trac.2013.11.007
- Farajnia, S., Ahmadzadeh, V., Tanomand, A., Veisi, K., Khosroshahi, S. A., & Rahbarnia, L. (2014). Development trends for generation of single-chain antibody fragments. Immunopharmacology and Immunotoxicology, 36(5), 297–308. https://doi.org/10.3109/08923973.2014.945126
- Fu, E., Liang, T., Houghtaling, J., Ramachandran, S., Ramsey, S. A., Lutz, B., & Yager, P. (2011). Enhanced sensitivity of lateral flow tests using a two-dimensional paper network format. Analytical Chemistry, 83(20), 7941–7946. https://doi.org/10.1021/ac201950g
- Gao, H., Han, J., Yang, S., Wang, Z., Wang, L., & Fu, Z. (2014). Highly sensitive multianalyte immunochromatographic test strip for rapid chemiluminescent detection of ractopamine and salbutamol. Analytica Chimica Acta, 839, 91–96. https://doi.org/10.1016/j.aca.2014.05.024
- Geoghegan, W. D. (1988). The effect of 3 variables on absorption of rabbit IgG to colloidal gold. Journal of Histochemistry & Cytochemistry, 36(4), 401–407. https://doi.org/10.1177/36.4.3346540
- Groff, K., Brown, J., & Clippinger, A. J. (2015). Modern affinity reagents: Recombinant antibodies and aptamers. Biotechnology Advances, 33(8), 1787–1798. https://doi.org/10.1016/j.biotechadv.2015.10.004
- Guo, Y., Liu, S., Gui, W., & Zhu, G. (2009). Gold immunochromatographic assay for simultaneous detection of carbofuran and triazophos in water samples. Analytical Biochemistry, 389(1), 32–39. https://doi.org/10.1016/j.ab.2009.03.020
- Hu, Z. Q., Li, H., Wu, P., Li, Y., Zhou, Z., Zhang, J., Liu, J., & Liao, Y. (2015). An affinity improved single-chain antibody from phage display of a library derived from monoclonal antibodies detects fumonisins by immunoassay. Analytica Chimica Acta, 867, 74–82. https://doi.org/10.1016/j.aca.2015.02.014
- Jager, V., Bussow, K., Wagner, A., Weber, S., Hust, M., Frenzel, A., & Schirrmann, T. (2013). High level transient production of recombinant antibodies and antibody fusion proteins in HEK293 cells. BMC Biotechnology, 13(1), 52. https://doi.org/10.1186/1472-6750-13-52
- Jones, D., Kroos, N., Anema, R., van Montfort, B., Vooys, A., van der Kraats, S., van der Helm, E., Smits, S., Schouten, J., Brouwer, K., Lagerwerf, F., van Berkel, P., Opstelten, D. J., Logtenberg, T., & Bout, A. (2003). High-level expression of recombinant IgG in the Human cell line PER.C6. Biotechnology Progress, 19(1), 163–168. https://doi.org/10.1021/bp025574h
- Kavanagh, O., Elliott, C. T., & Campbell, K. (2015). Progress in the development of immunoanalytical methods incorporating recombinant antibodies to small molecular weight biotoxins. Analytical and Bioanalytical Chemistry, 407(10), 2749–2770. https://doi.org/10.1007/s00216-015-8502-z
- Lagunas-Allué, L., Martínez-Soria, M., Sanz-Asensio, J., Salvador, A., Ferronato, C., & Chovelon, J. M. (2012). Degradation intermediates and reaction pathway of pyraclostrobin with TiO2 photocatalysis. Applied Catalysis B: Environmental, 115–116, 285–293. https://doi.org/10.1016/j.apcatb.2011.12.015
- Lagunas-Allué, L., Sanz-Asensio, J., & Martínez-Soria, M. (2012). Optimization and validation of a simple and fast method for the determination of fungicides in must and wine samples by SPE and GC/MS. Journal of AOAC International, 95(5), 1511–1519. https://doi.org/10.5740/jaoacint.11-402
- Lee, J., Kim, Y. A., Kim, M. Y., Lee, Y. T., Hammock, B. D., & Lee, H. (2012). Importance of membrane selection in the development of immunochromatographic assays for low-molecular weight compounds. Analytica Chimica Acta, 757, 69–74. https://doi.org/10.1016/j.aca.2012.10.052
- Li, F., Liu, Y., Li, Y., Li, Y., Xie, P., Ju, Q., Chen, L., & Li, G. (2012). Construction and development of a mammalian cell-based full-length antibody display library for targeting hepatocellular carcinoma. Applied Microbiology and Biotechnology, 96(5), 1233–1241. https://doi.org/10.1007/s00253-012-4243-5
- Li, H., Zhao, F., Cao, F., Teng, M., Yang, Y., & Qiu, L. (2019). Mitochondrial dysfunction-based cardiotoxicity and neurotoxicity induced by pyraclostrobin in zebrafish larvae. Environmental Pollution, 251, 203–211. https://doi.org/10.1016/j.envpol.2019.04.122
- Li, Y., Zhou, Y., Chen, X., Huang, X., & Xiong, Y. (2020). Comparison of three sample addition methods in competitive and sandwich colloidal gold immunochromatographic assay. Analytica Chimica Acta, 1094, 90–98. https://doi.org/10.1016/j.aca.2019.09.079
- Liu, L., Steven, S., Zheng, Q., Song, S., & Kuang, H. (2017). Development of an immunochromatographic strip for detection of acetamiprid in cucumber and apple samples. Food and Agricultural Immunology, 28(5), 767–778. https://doi.org/10.1080/09540105.2017.1312294
- Liu, J. L., Zabetakis, D., Acevedo-Vélez, G., Goldman, E. R., & Anderson, G. P. (2013). Comparison of an antibody and its recombinant derivative for the detection of the small molecule explosive 2,4,6-trinitrotoluene. Analytica Chimica Acta, 759, 100–104. https://doi.org/10.1016/j.aca.2012.10.051
- Liu, Y., Zhao, Y., Zhang, T., Chang, Y., Wang, S., Zou, R., Zhu, G., Shen, L., & Guo, Y. (2019). Quantum dots-based immunochromatographic strip for rapid and sensitive detection of acetamiprid in agricultural products. Frontiers in Chemistry, https://doi.org/10.3389/fchem.2019.00076
- Malhat, F., Saber, E., Elsalam Shokr, S. A., Ahmed, M. T., & El-Sayed Amin, A. (2019). Consumer safety evaluation of pyraclostrobin residues in strawberry using liquid chromatography tandem mass spectrometry (LC–MS/MS): An Egyptian profile. Regulatory Toxicology and Pharmacology, 108, 104450. https://doi.org/10.1016/j.yrtph.2019.104450
- Markus, H., Katrin, B., Katharina, D., Michaela, H., Jana, G., Tilman, S., Adrian, Z., Christian, S., Kepert, F., Brigitte, H., Michael, W., Hermann, B., Philipp, M., Michael, M., Constanze, K., Ulla, G., Dietmar, R., & Patrick, B. (2014). Assessment of chemical modifications of sites in the CDRs of recombinant antibodies. mAbs, 6(2), 327–339. https://doi.org/10.4161/mabs.27876
- Mercader, J. V., Agulló, C., Abad-Somovilla, A., & Abad-Fuentes, A. (2011). Synthesis of site-heterologous haptens for high-affinity anti-pyraclostrobin antibody generation. Organic & Biomolecular Chemistry, 9(5), 1443. https://doi.org/10.1039/c0ob00686f
- Mercader, J. V., Agulló, C., Esteve-Turrillas, F. A., Abad-Somovilla, A., & Abad-Fuentes, A. (2013). Immunoassays for pyraclostrobin analysis in processed food products using novel monoclonal antibodies and QuEChERS-based extracts. Food Control, 32(1), 42–48. https://doi.org/10.1016/j.foodcont.2012.12.003
- Mercader, J. V., Suárez-Pantaleón, C., Agulló, C., Abad-Somovilla, A., & Abad-Fuentes, A. (2008). Production and Characterization of monoclonal antibodies specific to the strobilurin Pesticide pyraclostrobin. Journal of Agricultural and Food Chemistry, 56(17), 7682–7690. https://doi.org/10.1021/jf801340u
- Ngom, B., Guo, Y., Wang, X., & Bi, D. (2010). Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: A review. Analytical and Bioanalytical Chemistry, 397(3), 1113–1135. https://doi.org/10.1007/s00216-010-3661-4
- Picanço-Castro, V., & Swiech, K. (2017). Recombinant glycoprotein production-methods and protocols. Humana Press.
- Puranik, N., Pal, V., Tripathi, N. K., & Goel, A. K. (2020). Development of a rapid immunochromatographic assay for detection of surface array protein (Sap), a potent biomarker of Bacillus anthracis. Food and Agricultural Immunology, 75(4), 613–617. http://doi.org/10.2478/s11756-019-00379-9.
- Qin, C., & Li, G. (2014). Mammalian cell display technology coupling with AID induced SHM in vitro: An ideal approach to the production of therapeutic antibodies. International Immunopharmacology, 23(2), 380–386. https://doi.org/10.1016/j.intimp.2014.09.017
- Sheedy, C., MacKenzie, C. R., & Hall, J. C. (2007). Isolation and affinity maturation of hapten-specific antibodies. Biotechnology Advances, 25(4), 333–352. https://doi.org/10.1016/j.biotechadv.2007.02.003
- Verheijen, R., Osswald, I., Dietrich, R., & Haasnoot, W. (2010). Development of a one step strip test for the detection of (dihydro)streptomycin residues in Raw Milk. Food and Agricultural Immunology, 12(1), 31–40. https://doi.org/10.1080/09540100099607
- Wang, Y., Yan, Y., Ji, W., Wang, H., Li, S., Zou, Q., & Sun, J. (2013). Rapid simultaneous quantification of zearalenone and fumonisin B1 in corn and wheat by lateral flow dual immunoassay. Journal of Agricultural and Food Chemistry, 61(21), 5031–5036. https://doi.org/10.1021/jf400803q
- Wang, S., Zhang, C., & Zhang, Y. (2005). Development of a flow-through enzyme-linked immunosorbent assay and a dipstick assay for the rapid detection of the insecticide carbaryl. Analytica Chimica Acta, 535(1–2), 219–225. https://doi.org/10.1016/j.aca.2004.12.009
- Wang, Z., Zhu, J., & Lu, H. (2020). Antibody glycosylation: Impact on antibody drug characteristics and quality control. Applied Microbiology and Biotechnology, 104(5), 1905–1914. https://doi.org/10.1007/s00253-020-10368-7
- Xu, Z., Dong, J., Wang, H., Li, Z., Beier, R. C., Jiang, Y., Lei, H., Shen, Y., Yang, J., & Sun, Y. (2012). Production and characterization of a single-chain variable fragment linked alkaline phosphatase fusion protein for detection of O,O-diethyl organophosphorus pesticides in a one-step enzyme-linked immunosorbent assay. Journal of Agricultural and Food Chemistry, 60(20), 5076–5083. https://doi.org/10.1021/jf300570q
- Xu, Z., Shen, Y., Beier, R. C., Yang, J., Lei, H., Wang, H., & Sun, Y. (2009). Application of computer-assisted molecular modeling for immunoassay of low molecular weight food contaminants: A review. Analytica Chimica Acta, 647(2), 125–136. https://doi.org/10.1016/j.aca.2009.06.003
- Yang, M., Zhang, J., Zhang, J., Rashid, M., Zhong, G., & Liu, J. (2018). The control effect of fungicide pyraclostrobin against freckle disease of banana and its residue dynamics under field conditions. Journal of Environmental Science and Health. Part. B, Pesticides, Food Contaminants, and Agricultural Wastes, 53(9), 615–621. https://doi.org/10.1080/03601234.2018.1473974.
- Yau, K., Tout, N. L., Trevors, J. T., Lee, H., & Hall, J. C. (1998). Bacterial expression and characterization of a picloram-specific recombinant Fab for residue analysis. Journal of Agricultural and Food Chemistry, 46(10), 4457–4463. https://doi.org/10.1021/jf980643m
- Yu, L., Liu, M., Wang, T., & Wang, X. (2019). Development and application of a lateral flow colloidal gold immunoassay strip for the rapid quantification of ciprofloxacin in animal muscle. Analytical Methods, 11(25), 3244–3251. https://doi.org/10.1039/C9AY00657E
- Zhang, Y. (2007). Novel methacrylic acid vinegar fungicide-pyraclostrobin. World Pesticides, 29(3), 47–48.
- Zhang, F., Wang, L., Zhou, L., Wu, D., Pan, H., & Pan, C. (2012). Residue dynamics of pyraclostrobin in peanut and field soil by QuEChERS and LC–MS/MS. Ecotoxicology and Environmental Safety, 78, 116–122. https://doi.org/10.1016/j.ecoenv.2011.11.003
- Zhang, K., Wong, J. W., Hayward, D. G., Sheladia, P., Krynitsky, A. J., Schenck, F. J., Webster, M. G., Ammann, J. A., & Ebeler, S. E. (2009). Multiresidue pesticide analysis of wines by dispersive solid-phase extraction and ultrahigh-performance liquid chromatography-tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 57(10), 4019–4029. https://doi.org/10.1021/jf9000023
- Zhao, J., He, S., Liu, W., Deng, A., Nan, T., Wang, B., Zhai, Z., & Li, Z. (2007). Development of a lateral flow dipstick immunoassay for the rapid detection of glycyrrhizic acid. Food and Agricultural Immunology, 17(3–4), 173–181. https://doi.org/10.1080/09540100601072875
- Zhao, Y., Yang, B., Liu, Y., Fang, Y., Si, F., Guo, Y., Cheng, J., & Zhu, G. (2016). Development and application of gold immunostrip for the detection of acetamiprid residue in tea samples. Chinese Journal of Pesticide Science, 18(2016), 337–343.
- Zou, L., Xu, Y., Li, Y., He, Q., Chen, B., & Wang, D. (2014). Development of a single-chain variable fragment antibody-based enzyme-linked immunosorbent assay for determination of fumonisin B1 in corn samples. Journal of the Science of Food and Agriculture, 94(9), 1865–1871. https://doi.org/10.1002/jsfa.6505