ABSTRACT
Metal ions are beneficial or toxic in part depending on their identity, valence state, and concentration. Antibodies against radioactive metal ions can be used in medical diagnosis and therapy, while antibodies against heavy metal ions are widely used in the trace element analysis of the immunoassay. This review focuses on the recent advances in antibody developments for metal ions and their applications. We exhaustively enumerated 18 chelate or ligand structures for metal hapten synthesis, which were proven to produce antibodies that cover almost 33 metal ions. The binding properties of these antibodies for their corresponding haptens were also discussed both in the structural foundation and molecular foundation. Furthermore, the representative applications of the antibodies used in medical diagnostics and therapy, and immunoassays were summarized. Finally, we illustrated the present challenges in high specific metal ion antibody preparation, and in immunoassay development. The future perspectives were also discussed.
1. Introduction
A deeper understanding of the pathogenic mechanisms of the immune system and urgent need for rapid and cost-effective analysis, greatly accelerate the use of antibodies in medical therapy and diagnostics, as well as in the hazard detection, which involve environmental health and food safety. Theoretically, antibodies can be generated against any type of target with immunogenicity, including macromolecules such as proteins, and small molecular compounds with suitable carriers, such as metal ions.
Metal ions play critical structural and functional roles in the biological process, including metabolism, osmotic regulation, catalysis, biomineralization, and signaling (Carter et al., Citation2014). Some heavy metal ions (e.g. Fe(III), Cu(II), Zn(II), Mn(II), Co(II), and Mo(VI)), are essential for the maintenance of human metabolism, while others, such as Hg(II), Pb(II), Cd(II), and As(III), have been proven to lack vital or beneficial effects (Quang & Kim, Citation2010). Furthermore, some radiometal ions with diverse nuclear properties (range of half-lives, particle emissions, and decay energies) are the key components in nuclear medicine, such as in diagnostic imaging (γ emitter, e.g. 67Ga, 99mTc, 111In, and 177Lu) and therapeutic applications (α or β emission, e.g. 90Y, 111In, 177Lu, 188Re, 212Pb, 213Bi, and 225Ac) (Comba et al., Citation2017). Metal ions are clearly ubiquitous, regardless of whether for the benefit or toxicity of life, probably quantifying the metal content is of fundamental importance, regardless of the field of environmental analysis, process control, biology, medicine, etc.
Antibody-based assays exhibit great advantages of high efficiency, reliability, rapidness, and low cost, when compared to instrument-intensive methods (e.g. atomic absorption spectroscopy and inductively coupled plasma mass spectrometry), for metal ion detection (Date et al., Citation2012; Khosraviani et al., Citation1998; Xu et al., Citation2011). This technique is theoretically applicable to any hazard target, in which suitable antibodies can be generated.
Historically, the antibody for metal ions was first developed in 1985, when Reardan et al. described their studies on the investigation of the recognition capability of an indium(III)-EDTA antibody against different metal–chelate complexes (Reardan et al., Citation1985). Since the last decades, the increase in the number and diversity of these antibodies has been astonishing, and these antibodies are extremely widespread in medical diagnosis and therapy (Chang et al., Citation2002; Chatal et al., Citation1994; Corneillie et al., Citation2006), particularly in the trace element analysis of immunoassay.
Antibody-based assays for metal ions have been previously reviewed (Blake et al., Citation1998; Blake et al., Citation2001a). We found that these reviews only focused on limited antibodies and immunoassays (3–5 antibodies against heavy metal ions, including Cd(II), Co(II), Pb(II), Hg(II), and U(VI)). However, since the past 15 years, this field has considerably grown, and a variety of antibodies against different metal ions have been developed, as well as dazzling development of assays. The aim of the present study is to provide the reader with an appreciation on how this field has developed in a relatively short time, with a special emphasis on the implications, in terms of scope and analytical performance.
The present review begins with a brief discussion of the antibody development against metal ions, including the preparation and recognition mechanism (binding properties), particularly to the chelators, since these reagents play a large and defining role in metal–chelate complex stability and antibody specificity. In addition, the sulfhydryl-containing ligand used for inducing antibodies recognized the mercuric ion as an independent epitope will be discussed separately, since these antibodies appear to be beyond the scope of the sufficient size and structural requirements for metal to induce the formation of specific antibodies. We will also present copious examples from the literature, in order to illustrate the scope of these metal-specific antibodies in areas such as immunoassays, medical diagnostics, and therapy. Finally, the future directions and applications will be addressed.
2. Brief history
Antibodies that bind to metal ions or metal ions complexes are rare, but it is often that there are magic examples of the range of possibilities in an immune response. In the early 1950s, a concept for the pathogenesis of chronic beryllium disease was presented by inflammation, which resulted from the reaction of beryllium and its specific antibody, and this was stimulated by the beryllium–protein antigen in the human body (Clarke, Citation1991). High levels of such beryllium immunoglobulins have been found in workers and the general public, who were exposed high beryllium content (Bencko et al., Citation1980). Subsequent studies have been proven to run the serological assays to detect such antibodies against beryllium (Clarke, Citation1991). Studies conducted in 1974 and 1978 revealed that myeloma patients with hypercupremia and hypercalcemia produced antibodies that bound copper and calcium (Baker & Hultquist, Citation1978; Lindgarde & Zettervall, Citation1974), and this further verified the possibility of metal antibodies induced by human immune systems.
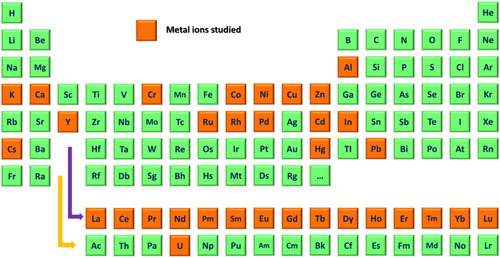
The first truly representative artificial system to study metal ions antibodies was constructed by metal–chelate immunogens in the 1980s, in which the Meares’ group did a series of studies for synthesizing complexes of metal–chelate antibodies for clinical medical radio-imaging analysis (Meares, Citation1986; Reardan et al., Citation1985). Antibodies directed against glutathione–metal complexes of Hg(II) have been reported in 1992 (Wylie et al., Citation1992). These have been demonstrated to be clearly that under appropriate conditions, certain metal ions can induce extremely specific antibodies. Based on this antibody, easier and faster immunoassays were developed to detect mercuric ions (no need for sample pretreatment with a chelator). Systemic researches were performed by Blake's group from the mid-1990s, which involved synthesizing antibodies to different heavy metal ions with metal–chelator antigens, the characterization of the binding properties of antibodies to metal–chelate complexes, and the development of antibody-based sensors for metal ions detection (Blake et al., Citation1996; Blake et al., Citation2001a; Blake et al., Citation2004; Darwish & Blake, Citation2002; Delehanty et al., Citation2003). Over the past decade, beyond those pioneering researchers already mentioned, a myriad of other groups that is far too long to list have made important contributions to the development of metal ion antibodies, especially in the development of antibody-based sensors or immunoassays for metal ions detection. To date, specific antibodies for 33 metal ions (), such as heavy metal ions (e.g. Cd(II), Cr(III), Pb(II), Hg(II), and Cu(II)) (Blake et al., Citation2001b; Chakrabarti et al., Citation1994; Lou et al., Citation2009; Mandappa et al., Citation2012), alkali metal ions (e.g. Cs(I) and K(I)) (Duta et al., Citation2004) and all of the lanthanides (Corneillie et al., Citation2003), have been reported in the scientific literature.
Figure 1. The orange colour-coded periodic table shows the present antibody development for 33 metal ions.
A rare earth-DOTA-binding antibody 2D12.5 shows the broad binding to NBD complexes of all the lanthanides.
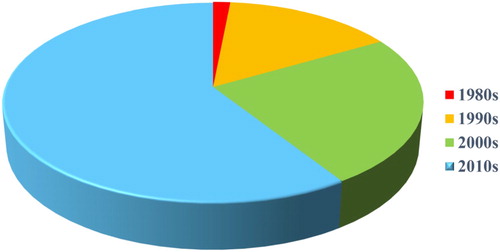
We counted a total of 66 antibodies against the reported metal ions, which were collected from the pioneering work of 1985 to present studies. It numbers were around only two in the early 1980s, and were close to 50 in the 2010s to date (). The increasing trend of antibody numbers clearly reflects the rapid development of such field.
Figure 2. The distribution diagram of 66 developed antibodies for metal ions at different timeframes. The antibody numbers collected represent the antibodies from different cell lines (monoclonal antibody) or serum (polyclonal antibody), which were reported in scientific literature. However, we consider that the actual amount of metal ions antibodies are well over this number.
3. Metal ions hapten
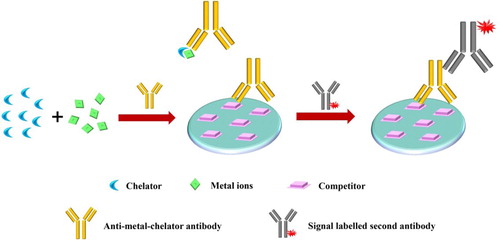
In general, size and structural complexity are the two important characteristics involved in immunogenicity, by the fact that vigorous immune responses are induced by large molecular weight proteins. However, molecules smaller than 5000 Da are usually conjugated as haptens to large molecular weight carriers to induce antibody formation (Westhoff et al., Citation1999). Metal ions have haptenic potential, but cannot be as a complete epitope, and causes immune responses (Westhoff et al., Citation1999). Most of the commonly used haptens for immunological studies have contained aromatic rings or charged, polar regions, which metal ions do not have. Empirically, some additional groups from other carriers/ligands are needed for metal ions to form an epitope. The most often used are chelating agents, which encapsulate metals in a cage-like structure through the coordination bonds between the chelate's electron-rich atoms and the electron-deficient metal. In order to combine the metal ions to a larger protein carrier, the chelating agents always have a functional group (referred to as bifunctional chelator), which enable it to form stable covalent bonds with the carrier protein (Blake et al., Citation1998). Designing metal complex-based immunogens requires correlating aspects of the coordination chemistry with in vivo behaviour. Immunogens with high thermodynamic stability and kinetic inertness should be as the prerequisite of producing antibody, which is of fundamental importance during immunization. Sometimes, metal ions can be easily released in vivo due to the transchelation by the endogenous metal ion competition or by endogenous ligands (Spang et al., Citation2016). The stability of a metal–chelator complex is determined by the properties of the metal ions as well as the structure of the chelating agent, and both have to be taken into consideration when developing a new antibody.
The acyclic (e.g. EDTA or DTPA) and macrocyclic chelator structure (e.g. DOTA or TETA) are the most two common family compounds for metal ions hapten synthesis. EDTA and its derivatives form hexacoordinate complexes with most metal cations, while DTPA appears to form octacoordinate complexes (Brechbiel et al., Citation1996; Maecke et al., Citation1989), which are used for generating antibodies against In(III), Hg(II), Cr(III), Cr(VI), Cd(II), Cu(II), Ni(II), and Pb(II). A DTPA derivative (CHX-A”-DTPA) with the benzyl group in the linking region and a rigid cyclohexylene bridge resulted in a 9100-fold affinity increasement in Pb(II) monoclonal antibody (Khosraviani & Blake, Citation2000). However, chelators derived from EDTA or DTPA did not coordinate U(VI) with a high enough affinity to preserve the metal–chelate complex during immunization. Thus a novel bifunctional derivative of 2,9-dicarboxy-1,10-phenanthroline (DCP) with an affinity of approximately 5 orders of magnitude greater than that determined for EDTA or DTPA, was developed to generate antibodies bound to the soluble DCP-UO22+ complex, with an equilibrium dissociation constant in the low nM range (Blake et al., Citation2004).
In general, macrocyclic ligands (e.g. DOTA or TETA) form thermodynamically and kinetically more stable complexes than acyclic chelators, and are available for stable Cu(II) incorporation. Cu(II) is susceptible to the reduction processes in vivo, producing a soft acid (Cu(I)), but this shows far less stability to complex ligand binding than that of Cu(II), resulting in the release of copper ions from the chelate complex (Wangler et al., Citation2011). A previous study pointed out that neither DTPA, nor backbone-substituted EDTA reagents could prevent the binding of copper to albumin in human serum (Meares, Citation1986). These macrocyclic chelators are summarized in for Cu(II) hapten synthesis, including the DOTA, NOTA, and TETA derivatives (Feng et al., Citation1998; Kong et al., Citation2012; Zhu, Miao, Qin, & Zhu, Citation2019).
Table 1. Haptens or bifunctional chelators used for metal ion antibody preparation
An artificial antigen for copper was synthesized by utilizing penicillin G sodium salt as a chelating agent (Xi et al., Citation2015; Xu et al., Citation2011), similar as the concept of mercuric ion coupled to the sulfhydryl group (–SH) in glutathione in the pioneer work of Wylie et al. in 1992 (Wylie et al., Citation1992), which induced antibodies against the certain metal ions, rather than the traditional concept that antibodies preferably bound to the cage-like metal–chelate structure (we will discuss in the chapter below). Copper ions coupled to penicillin G sodium salt through the –SH group, and formed a stable copper mercaptide of penicillenic acid (CMPA). Other unusual chelator structures have been designed to prepare antibodies for transition metal complexes (e.g. Rh(I) and Pd(II)) and Ru(II) (Kobayashi et al., Citation2020; Shreder et al., Citation1996; Yamaguchi et al., Citation2006).
Levy et al. isolated a specific anti-aluminium monoclonal antibody using a BSA–Al immunogen, based on the evidence on the ability of BSA to bind directly to Al(III) (Levy et al., Citation1998). Studies have shown at least three Al(III) bound per BSA molecule in the oxygen-containing octahedral sites (Jemil et al., Citation1992; Sadler et al., Citation1994).
3.1. Mercury hapten
In contrast to the metal-chelating hapten which the chelating compounds surround the metal with a cage-like structure, a kind of specific hapten containing sulfhydryl groups (e.g. glutathione (GSH) and 6-mercaptonicotinic acid (MNA)) was used to couple with mercuric ions to maximize the exposure of the metal ions (). In this case, it is likely that the metal would be perceived as a distinct entity by the immune system, and not simply as a component of a larger epitope. Thus specific antibodies bind mercuric ion itself without the need of conjugation to a large molecular weight carrier. These studies demonstrated that, under appropriate conditions, metal ions can serve as haptens toward which an antibody response can be directed. It should be noted that such antibody is rare, but is not alone, since antibodies that bound copper and calcium have been found in myeloma patients with hypercupremia and hypercalcemia (Baker & Hultquist, Citation1978; Lindgarde & Zettervall, Citation1974), as well as in orthopedic patients after the implantation of metallic devices (Yang & Merritt, Citation1994).
Table 2. Haptens or ligands used for mercury antibody preparation.
GSH, which is a tripeptide of a glutamic acid, cysteine, and glycine, that contains an – SH group of a single cysteine residue, makes it easy to tightly conjugate with mercuric ions as a monodentate ligand in a proper molecular environment (Wylie et al., Citation1992). Inspired by this concept, MNA contains a pyridine ring and mercapto group, which was later studied (Wang et al., Citation2012). It is expected that this would have greatly enhanced the immunogenicity due to the rigidity of the pyridine ring.
Prudenté et al. employed the intramolecular oxymercuration reaction to synthesize an organomercury hapten with structurally simple, but chemically robust. The corresponding antibody was capable of binding to both the organic forms and inorganic (Hg(II)) of mercury (Prudente et al., Citation2010).
4. Recognition mechanism of the metal ion antibody
A fundamental understanding of the binding properties associated with antibody–antigen interactions is of considerable importance in the design of antibody-based systems, particularly metal-containing ligand (metal–chelate complex and metal–peptide complex), which represents a special subset of hapten for small molecules. A previous study demonstrated that the antibody recognized metal–chelate haptens by only a limited number of molecular contacts, whereby both were direct ligations between the antibody and metal ion, and the interactions between the antibody and chelator participate (Delehanty et al., Citation2003; Love et al., Citation1993). The present study will discuss the binding properties from the structural foundation and molecular foundation level.
4.1. Structural foundation
Structural complementarity is the fundamental theory for any antibody–antigen recognition. Thus altering either the metal ions or chelator structure plays a major role in metal–chelate antibody affinity (Jones et al., Citation2002). The metal–chelate complex has various distinct conformations which primarily arise from the lability of the metal–ligand bonds, and that the bond lability is dependent upon the identity of the metal ions (Day & Reilley, Citation1964; Kula et al., Citation1963). Jones et al. investigated the structure/function comparison using a number of structurally similar metal–EDTA ligands to the anti-Cd(II)–EDTA antibodies (A4 and E5) (Jones et al., Citation2002). They found that antibody affinity correlated well with the extent of the total structural difference for these metal–EDTA complexes. When the chelator structure was changed (substituting CDTA for EDTA), E5 showed a more tight binding to the metal–CDTA complexes than that of metal-EDTA complexes. This similar phenomenon was also found in a study for a monoclonal antibody (2C12) that recognized the Pb(II)–DTPA complex, where the introduction of a benzyl group in the linking region or a rigid cyclohexylene bridge on the base of the DTPA structure, resulted in a 30-fold and 9100-fold affinity increasement, respectively (Khosraviani & Blake, Citation2000). In addition, they found that the way of chelate arms encircled the metal ion caused a major difference between the metal–EDTA haptens, further pointing out that the conformational changes of the chelate arms as these shift to accommodate different metal ions might be an important factor in antibody recognition.
In another study on the anti-metal–EDTA complex antibody (2A81G5), the metal-ion binding of the 2A81G5 antibody was found to correlate well with the size of the divalent metal ions, but with the exception of Hg(II) and Mg(II) (Blake et al., Citation1996). Furthermore, the role of metal ion size that effects antibody affinity seemed not only to correlate with the ionic radii but also the effect of each metal on the overall chelate complex conformation (Jones et al., Citation2002).
4.2. Molecular foundation
The molecular characterization of antibodies directed against haptens will further provide important insights into the binding properties study. In a study of the molecular features of indium(III)-4-[N’-(2-hydroxyethyl)thioureido]-L-benzyl-EDTA (In-EOTUBE) to its respective monoclonal antibody (CHA255), a histidine residue in the complementarity determining region of the heavy chain was been shown to directly coordinate the In(III) ion (Love et al., Citation1993). However, this histidine coordination was not present in the corresponding Fe(III) complex which resulted in a 24-fold lower affinity. This was apparently because the imidazole group of the histidine could not approach the chelated Fe(III) without contacting the relatively more enveloping carboxylate chelating groups of the EDTA moiety. Furthermore, hydrogen bonds from hapten to either the protein or buried water, the van der Waals interactions, and ion pairs (e.g. arginine, threonine, and tryprophan side chains to the carboxylate arms of the EDTA molecule) between the antibody and EOTUBE chelator contributed to the strong binding. That is to say, an antibody could provide a high degree of specificity for a certain metal ion, which could be the direct ligation of the metal ion using appropriately positioned amino acid side chains in the antigen binding site (Blake et al., Citation2004).
The participation of such histidine in direct metal ion ligation has also been found for other antibody interaction studies (Boden et al., Citation1995; Delehanty et al., Citation2003). If antibodies show a relatively high affinity for the metal-free chelator, it appears to be due to the majority of the amino acid residues in the antigen binding site, which are devoted to complementing the structure of the chelator itself, regardless of whether the metal ions are coordinated (Blake et al., Citation2004).
In an anti-Pb(II)-CHXDTPA antibody (2C12), hydroxyl-containing amino acid side chains (e.g. serine, threonine, and tyrosine) were found to be abundant in CDRs, which are highly likely to induce the selectivity to Pb(II) (Khosraviani & Blake, Citation2000).
Studies for the mercury-binding antibodies (react specifically with free mercuric ions) using GSH carriers demonstrated the unpaired cysteine residue in the CDR, which is unusual for murine antibodies, but this is an absolute requirement for mercury binding (Westhoff et al., Citation1999).
5. Applications of metal ions antibody
5.1. As specific recognition elements for immunoassay
Immunoassays or immunosensors are constructed to achieve precise quantitative detection of metal ions in the field, ranging from environment analysis to food safety (Xiao et al., Citation2018; Xing et al., Citation2014). Similar to the immunoassay for other small molecular compounds, a generally competitive format is the basis for the immunoassay for metal ions, which is determined by only one epitope (Chen et al., Citation2020; Galvidis et al., Citation2020; Huo et al., Citation2019). However, this has obvious difference from other small molecules, mostly requiring the addition of a molar excess of the chelator to convert the free metal ions in samples to metal–chelate complexes (except some anti-GSH/MNA-Hg(II) antibodies) (). This operation introduces a problem that the chelation itself would cause interference, since the chelator would extract all metals to different degrees and the anti-metal chelate antibodies identified to date show more or less the cross-reactivity to other chelated metals. In this case, methods for sample treatment to reduce interference are great of importance. In this section, we introduce some simple but great sample treatment strategies that could keep up with the specific demand without sacrificing the rapidity of the immunoassay. Furthermore, classic immune-based detection systems (e.g. microwell-based immunoassay, kinetic exclusion assay (KinExA); fluorescence polarization immunoassay (FPIA)) and popular lateral flow immunoassay (LFIA) and specific non-competitive immunoassay for metal ion sensing are also highlighted.
5.1.1. Sample treatment to reduce cross reactivity
In an immunoassay for Cd(II) in rice, the high natural occurrence of Mg(II) in rice results in a potential interference for Cd(II) determination. Although the identified antibody shows an affinity against Cd(II) with four orders of magnitude higher than that of Mg(II), the actual concentration demands for these two metal ions are very different, such as micromolar to millimolar containing concentration for Mg(II), while nanomolar toxic concentration for Cd(II). In this case, the large difference in concentrations is far enough to overcome the relatively good Cd(II) specificity of the antibody. A simple approach was explored to eliminate the Mg(II) interference through the judicious selection of the EDTA concentration based on the difference in complexation constants between Cd(II)-EDTA and Mg(II)-EDTA (Sasaki et al., Citation2008). However, this strategy would feel quite helpless in the face of other potentially interfering metal ions whose formation constants are comparable to that of the target metals.
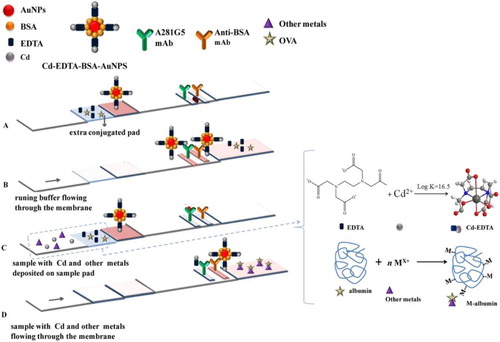
López Marzo et al. introduced an extra conjugation pad loading with ovalbumin (OVA) to mask other metal ions interference in the study of a lateral flow format, in order to detect Cd(II). In addition, the EDTA loaded on such pad would simultaneously chelate Cd(II), in which the formed Cd(II)-EDTA complexes are recognized by the antibody (). Based on this ingenious design, the lateral flow platform operates without any sample pretreatment step for Cd(II) detection (Marzo et al., Citation2013).
Figure 4. An integrated Cd(II) biosensor device with an extra conjugation pad loaded with ovalbumin (OVA) to mask other metal ion interference (Marzo et al., Citation2013).
5.1.2. Microwell-based immunoassay
Generally, enzyme-linked immunosorbent assay (ELISA) is the most representative system for microwell-based immunoassays. Overall, ELISA is a heterogeneous immunological technique that involves an enzyme–substrate system to quantify the concentration of an antibody or an antigen in the sample, and this mostly is operated in a 96-microwell plate (Berlina et al., Citation2019; Gao et al., Citation2020; Tighe et al., Citation2015). The most used variant of ELISA for metal ions quantification is the indirect competitive ELISA (ic-ELISA), which a second enzyme-labelled antibody (anti-species antibody) introduced to produce a chromogenic or fluorogenic signal. Almost all heavy metal ions that the specific antibodies developed were ever quantified by ic-ELISA, and these ic-ELISAs enzyme–substrate systems include HRP-TMB (He et al., Citation2011; Liu et al., Citation2009; Zhu et al., Citation2019), HRP-OPD (Gao et al., Citation2012) and ALP-pNPP (Mandappa et al., Citation2012).
Zhou et al. developed a multi-target ELISA using three types of heavy metal monoclonal antibodies (McAbs) in one ELISA system to analyze the individual and total concentration of Hg(II), Pb(II) and Cd(II) in water samples (Zhou et al., Citation2011). In this system, HRP and McAbs were simultaneously conjugated to colloidal gold nanoparticles (AuNPs) as detection probes. The high surface-to-volume ratio of AuNPs can load a large amount of HRP molecules, resulting in a higher sensitivity.
Unlike the previously mentioned ic-ELISA which is actually a plate-bound antigen format, immunoassays employ plate-bound antibody format, which is also referred to as one-step ELISA, and this has shown less operating time and higher sensitivity (Darwish & Blake, Citation2001). In this format, the antibody is directly immobilized on the plate, and an enzyme-labelled metal chelate conjugate is used to compete free metal ions in the sample, as well as for the generation of the signal. Darwish et al. successfully developed a one-step ELISA for the measurement of serum and blood cadmium levels with minimal sample pretreatment by acidification (Darwish & Blake, Citation2002).
5.1.3. Kinetic exclusion assay (KinExA)-based immunoassay
The KinExA is an automated and computer-controlled flow fluorimeter, which was previously designed to study protein binding interactions. These free, unbound proteins that are present in homogeneous solution reaction mixtures are captured and retained on a solid phase where a derivative of the ligand is pre-immobilized, while proteins that are already complexed with the soluble ligand in solutions are thereby kinetically excluded from binding to the immobilized ligand on the solid phase. The amount of captured protein is quantified using a fluorescently labeled antibody which specifically recognizes the captured protein (Blake et al., Citation1999). Unlike microwell-based immunoassay, the KinExA consists of a capillary flow cell fitted with a microporous screen and uniform beads, through which all unbound components in the solution are drawn under negative pressure. The high surface area of beads maximizes the opportunities for the capture of the free antibody area. Furthermore, the high flow rate of the molecule through the beads minimizes the diffusion limitations at the reaction surface, and the limited contact time of antibody and immobilized antigen (250–400 ms), which reduces the repeated combination of antibody binding to the immobilized metal–chelate complex, are theoretically supposed to create a more sensitive assay. This was confirmed by the study conducted by Blake et al., which ran a KinExA-based immunoassay to detect Cd(II), Co(II), U(VI) and Pb(II) with superior performance, when compared to ELISA (Blake et al., Citation2001a).
5.1.4. Fluorescence polarization immunoassay (FPIA)
FPIA, a homogeneous analysis technique, is mainly used for the rapid monitoring of small molecular compounds (Dong et al., Citation2019; Pennacchio et al., Citation2016; Wang et al., Citation2019), and this has been developed to detect Pb(II) and Cd(II) (Johnson, Citation1999; Johnson et al., Citation2002). In these studies, a fluorescent analogue of the metal–chelate complex (termed a tracer) was constructed to regulate the fluorescence polarization value through the transformation of the bound and unbound state to antibody molecules. This transformation was actually performed by the free metal–chelate complex presented in the sample to compete with the fixed amount of tracers for a fixed concentration of binding sites of the antibody. The structure of the tracer antigen conjugate is critical in FPIA development. The reactive intermediate chelators involve an anhydride moiety to react with the primary amine of a fluorescent dye, which are recommended.
5.1.5. Lateral flow immunoassay (LFIA)
Similar to the ELISA kit, which has been widely used in the field of testing and mobile laboratory use, LFIA probably is another backbone technology for rapid detection, particularly for on-site or point of care testing. The combination of capillarity of lateral flow, specific recognition of antibody–antigen and visual readouts of AuNPs or fluorescent materials, provides the LFIA incomparable abilities in terms of rapidity (10–15 min), small sample volume (100–200 μL) and visualization (visual inspection). Traditional LFIAs, which use AuNPs as signal readout probes, were successfully used to visually detect Cu(II), Cr(III)/Cr(VI), Cd(II), and Pb(II) at the ppb (parts per billion) level (Abe et al., Citation2011; Liu et al., Citation2012; Tang et al., Citation2010; Xing et al., Citation2013). A turn-on competitive LFIA using quantum dots and gold nano-stars shows better sensitivity for Cd(II) (Xiao et al., Citation2018). Furthermore, different signal detection systems coupled with the lateral flow strip, such as electrochemistry detection (Wang et al., Citation2016) and surface enhanced Raman scattering (SERS) (Fu et al., Citation2015), show that this not only has higher sensitivity and quantitative accuracy but also maintains the advantages that LFIA has.
5.1.6. Label-free and non-competitive immunoassay
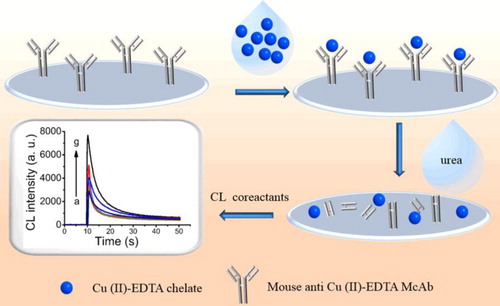
In contrast to the competitive immunoassay formats mentioned above, non-competitive immunoassays were also developed to detect metal ions. Fu's group reported two label-free chemiluminescence (CL) and fluorescent (FL) immunoassays for Cu(II) (Ouyang et al., Citation2016; Shu et al., Citation2017). In their system, the anti-Cu(II)-EDTA antibodies were directly immobilized on the solid phase surface (e.g. microwell plate) as an enrichment reagent. By using different dissociation reagent/strategy to release free Cu(II)-EDTA complex or single Cu(II), which further worked as specific signal transformation factors (e.g. Cu(II)-EDTA catalyses strongly luminol-H2O2 CL reaction and results in very intensive CL signal, while Cu(II) has efficient FL quenching to CdSe/ZnS quantum dots), ultra-facile and label-free immunoassays were developed (). These label free and non-competitive immunoassays overcame part to the defects of the traditional probe labeling platforms in terms of labour-intensive and time-consuming. However, the limited signal transformation mechanism from the metal ions or metal chelate complex probably limits such system development.
Figure 5. Label-free and non-competitive chemiluminescence immunoassays for Cu(II) (Ouyang et al., Citation2016).
Other immunoassays or immunosensors have been rapidly developed for metal ions analysis, including flow injection chemiluminescence immunoassay (Xu et al., Citation2015), microfluidic immunoassay (Date et al., Citation2013), microcantilever sensors (Velanki et al., Citation2007), surface plasmon resonance (SPR)-based competitive immunoassay (Kang et al., Citation2017) and electrochemiluminescent competitive immunoassay (Zhang et al., Citation2015).
5.2. As bispecific antibody (bsAb) for medical diagnostics and therapy
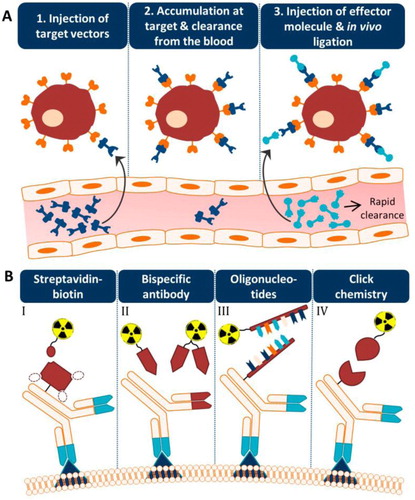
Metal–chelate antibodies are engineered with an antibody that binds specifically to pathogenic receptors (therapeutic target) to form a bispecific antibody (bsAb). This bsAb always combines with pretargeting strategy to arise medical targeted imaging and therapies, such as for cancer (Verhoeven et al., Citation2019). In pretargeting methodology for medical imaging, the targeting and imaging agents are decoupled by separating the targeting of a slow clearing antibody from the fast clearing radionuclide, resulting in superior signal/noise (tumour/nontumour) ratios. Briefly, the unlabeled bsAb was first administered to localize at the target site. Afterwards, the radiolabeled hapten (e.g. metal–chelate complex) was injected when the targeting agent has cleared from the blood (). In such a system, the radiolabeled hapten was small and fast cleared, avoiding the problematic long circulating lifetimes of radiolabeled antibodies in the bloodstream. The combination of high selectivity, high affinity (strong targeting), and reasonable clearance allow metal chelate hapten to be used for in vivo pretargeting strategies. Furthermore, there is no naturally occurring receptors for these haptens in living systems. Clinical testing was started with an anti-carcinoembryonic antigen (CEA) Fab coupled to an anti-In(III)-EOTUBE Fab through the administration of 20–40 mg of such a bsAb (Chang et al., Citation2002; Stickney et al., Citation1991). The clinical results showed a detection sensitivity of 95% for known tumor lesions, and particular to hepatic metastases, a site where traditional clinical studies using intact 111In-anti-CEA IgGs frequently had difficulties due to the high background activity in the liver (Abdelnabi et al., Citation1994; Divgi et al., Citation1991).
Figure 6. The concept of pretargeting. (A) The main process of the pretargeting methodology includes the injection of target vectors, the accumulation at the target and clearance from the blood, and the injection of the effector molecule and in vivo ligation. (B) Different systems for the radionuclide effector and antibody vector conjugation (Verhoeven et al., Citation2019).
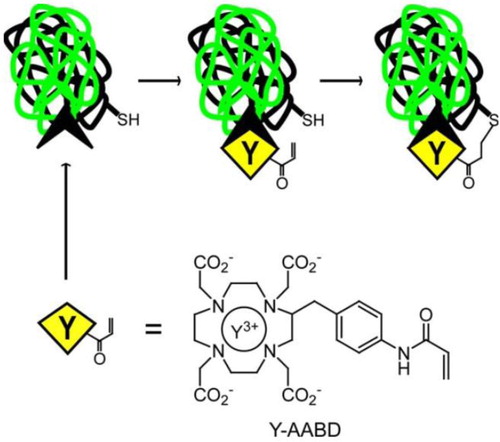
Furthermore, the irreversible binding pretargeting strategy based on engineering covalent ligand–receptor interactions was further explored to increase the bound lifetime of the anti-metal chelate antibody and its receptors, and finally maximize the targeting efficacy of such strategy (Corneillie et al., Citation2006). Since traditional anti-metal chelate antibody–ligand complex generally has a relatively weak affinity (Kd ≈ 1–10 nM), the half-life for dissociation of this antibody–ligand complex was far lower than (an order of magnitude lower) the half-lifetimes for decay of medically useful radiometals. That is to say, the metal–chelate ligand would not be bound to its antibody receptor long enough for all of the bound metals to decay. Based on Michael addition reaction, an engineered 2D12.5 Fab against Y-DOTA, incorporating a site-directed cysteine mutation at a site proximal to the binding site was used as the nucleophile to attack the acrylamide-based Michael acceptor (Y-AABD, a derivative of Y-DOTA incorporates a relatively unreactive acrylamide), yielding a permanent covalent bond ().
Figure 7. The Irreversible binding of anti-metal chelate antibody–ligand complex by a permanent covalent bond. The small metal–chelate complex (Y-AABD) was designed to have a weak electrophile (acrylamide) that selectively reacts to an engineered nucleophilic cysteine residue in a 2D12.5 antibody. The mutual reactivities of the acrylamide group and cysteine are significantly enhanced as the effective local concentration increased due to the high affinity binding interaction, yielding a permanent covalent bond between the engineered antibody and Y-AABD (Corneillie et al., Citation2006).
6. Summary and outlook
Antibodies for metal ions using artificial systems have been described since the mid-1980s. Since then, these have attracted considerable attention in medical diagnosis and therapy, particularly in the trace element analysis of immunoassays. A large variety of antibodies have been isolated from hapten immunization with the help of ligand compounds, including chelating agents, peptides, and even proteins, to form complete epitopes, and these have been developed over the years. Metal–chelate antibodies show specificity and affinity against certain metal ions, but in most cases, these also have more or less combinations to other metal–chelate complexes, and even to the chelator itself. This might create difficulties in the analysis of real samples that contain other metal ions in moderate to high concentrations. Furthermore, the requirement of an additional chelator to the samples increases the complexity of the analytical methods. These problems have improved in GSH-Hg antibody-based systems, since such antibody shows high specificity for individual Hg(II) which probably due to the specific combination of Hg(II) and –SH. However, this special phenomenon has not been observed in other metal ions antibodies, limiting the universality of such hapten compounds.
It is still a big challenge to reduce the interference of non-target metal ions in real sample immunodetections. Improving the specificity and affinity of antibodies against target metal ions is of great importance, which mainly included the hapten design and antibody modification. More stable and metal-exposed chelator structure or GSH-like ligands should be taken into the hapten design. In vitro affinity maturation or site directed mutagenesis can be used in antibody modification, on a basis of good insights into antibody–antigen interactions. Hence, a better understanding of the binding properties of metal ions antibodies is extremely significant in the hapten design and antibody modification. Computer-assisted molecular modeling (CAMM) is recommended for the hapten design.
For metal ions analysis, sample pretreatment strategies, including solid phase extraction and magnetic separation, have been proven to be effective tools to reduce the interference in real samples. More rapid sample pretreatment techniques and portable devices are the trend to increase the applicability of immunoassay technology. Furthermore, expanding antibody types for metal ions, such as the new emerging nanobody, perhaps invigorates this field. In some cases, the nanobody shows higher analytical or diagnostic performances that cannot be accomplished with traditional antibodies, and the unique composition of variable domains may provide a more accurate recognition against metal ions.
Abbreviations
| ALP | = | Alkaline phosphatase |
| AuNPs | = | Gold nanoparticles |
| BAD | = | S-2-(p-bromoacetamidobenzyl)-DOTA |
| BAT | = | S-2-(p-bromoacetamidobenzyl)-TETA |
| BSA | = | Bovine serum albumin |
| bsAb | = | Bispecific antibody |
| CAMM | = | Computer-assisted molecular modeling |
| CDR | = | Complementarity determining region |
| CDTA | = | trans-1,2-Diaminocyclohexane-N,N,N′,N′-tetraacetic acid |
| CEA | = | Carcinoembryonic antigen |
| CHX-A”-DTPA | = | N-[2-amino-3-p-isothiocyanatophenyl)propyl]-trans-cyclohexane-1,2-diamine-N,N′,N′,N′′,N′′-DTPA |
| CMPA | = | Copper mercaptide of penicillenic acid |
| DCP | = | 2,9-dicarboxy-1,10-phenanthroline |
| DOTA | = | 1, 4,7, 10-tetraazacyclododecane-N, N′, N'‘, N'''-tetraacetic acid |
| DTPA | = | Diethylenetriaminepentaacetic acid |
| EDTA | = | Ethylenediaminetetraacetic acid |
| ELISA | = | Enzyme-linked immunosorbent assay |
| EOTUBE | = | 4-[N′-(2-hydroxyethyl)thioureido]-L-benzyl-EDTA |
| FPIA | = | Fluorescence polarization immunoassay |
| GSH | = | Glutathione |
| HRP | = | Horseradish peroxidase |
| ic-ELISA | = | Indirect competitive ELISA |
| ITCBE | = | 1-(4-isothiocyanobenzyl)-ethylenediamine N, N, N′, N′-tetraacetic acid |
| KinExA | = | Kinetic Exclusion Assay |
| LFIA | = | Lateral flow immunoassay |
| McAb | = | Monoclonal antibody |
| MNA | = | 6-mercaptonicotinic acid |
| NBD | = | S-2-(4-nitrobenzyl)-DOTA |
| OPD | = | o-phenylenediamine |
| OVA | = | Ovalbumin |
| pNPP | = | 4-Nitrophenyl phosphate disodium salt hexahydrate |
| р-NH2-Bn-DTPA | = | 2-(4-aminobenzyl)-DTPA |
| р-SCN-Bn-DOTA | = | S-2-(4-isothiocyanatobenzyl)-DOTA |
| р-SCN-Bn-DTPA | = | 2-(4-isothiocyanatobenzyl)-DTPA |
| р-SCN-Bn-NOTA | = | S-2-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid |
| Pd complex 1 | = | η3-allyl-bis{4-(diphenylphosphino)benzoic acid} palladium(II) perchlorate |
| Rh complex 1 | = | (1,5-cyclooctadiene) {bis(2-diphenylphosphinoethyl) succinamido} rhodium(I) perchlorate |
| Ru complex 1 | = | Co(dmbpy)-(bpy)23+-methyl viologen hapten 1 |
| SERS | = | Surface enhanced Raman scattering |
| SPR | = | Surface plasmon resonance |
| TETA | = | 1, 4, 8, 11-tetraazacyclotetradecane-N, N′, N'‘, N'''-tetraacetic acid |
| TMB | = | Tetramethyl benzidine |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Abdelnabi, H., Doerr, R. J., Evans, N. H., Farrell, E. E., Chan, H. W., Balu, D., Gona, J., & Schweighardt, S. (1994). Radioimmunodetection of colorectal-carcinoma with In-111 labeled monoclonal-antibody IVP ZCE 025 (a tissue culture-produced anti-CEA mAB). Nuclear Medicine Communications, 15(2), 81–93. https://doi.org/10.1097/00006231-199402000-00004
- Abe, K., Nakamura, K., Arao, T., Sakurai, Y., Nakano, A., Suginuma, C., Tawarada, K., & Sasaki, K. (2011). Immunochromatography for the rapid determination of cadmium concentrations in wheat grain and eggplant. Journal of the Science of Food and Agriculture, 91(8), 1392–1397. https://doi.org/10.1002/jsfa.4321
- Baker, B. L., & Hultquist, D. E. (1978). Copper-binding immunoglobulin from a myeloma patient -studies of copper-binding site. Journal of Biological Chemistry, 253(23), 8444–8451.
- Bartos, A., Niedzielski, P., Buczylko, K., & Leszczynska, J. (2019). Nickel chelate complexes as a target for polyclonal antibodies raised in rabbits and mice. International Journal of Environmental Analytical Chemistry, 1691179.
- Bencko, V., Vasilieva, E. V., & Symon, K. (1980). Immunological aspects of exposure to emissions from burning coal of high beryllium content. Environmental Research, 22(2), 439–449. https://doi.org/10.1016/0013-9351(80)90156-5
- Berlina, A. N., Zherdev, A. V., & Dzantiev, B. B. (2019). ELISA and lateral flow immunoassay for the detection of food colorants: State of the art. Critical Reviews in Analytical Chemistry, 49(3), 209–223. https://doi.org/10.1080/10408347.2018.1503942
- Blake, D. A., Blake, R. C., Khosraviani, M., & Pavlov, A. R. (1998). Immunoassays for metal ions. Analytica Chimica Acta, 376(1), 13–19. https://doi.org/10.1016/S0003-2670(98)00437-1
- Blake, D. A., Chakrabarti, P., Khosraviani, M., Hatcher, F. M., Westhoff, C. M., Goebel, P., Wylie, D. E., & Blake, R. C. (1996). Metal binding properties of a monoclonal antibody directed toward metal-chelate complexes. Journal of Biological Chemistry, 271(44), 27677–27685. https://doi.org/10.1074/jbc.271.44.27677
- Blake, D. A., Jones, R. M., Blake, R. C., Pavlov, A. R., Darwish, I. A., & Yu, H. N. (2001a). Antibody-based sensors for heavy metal ions. Biosensors & Bioelectronics, 16(9–12), 799–809. https://doi.org/10.1016/S0956-5663(01)00223-8
- Blake, R. C., Pavlov, A. R., & Blake, D. A. (1999). Automated kinetic exclusion assays to quantify protein binding interactions in homogeneous solution. Analytical Biochemistry, 272(2), 123–134. https://doi.org/10.1006/abio.1999.4176
- Blake, R. C., Pavlov, A. R., Khosraviani, M., Ensley, H. E., Kiefer, G. E., Yu, H., Li, X., & Blake, D. A. (2004). Novel monoclonal antibodies with specificity for chelated uranium(VI): Isolation and binding properties. Bioconjugate Chemistry, 15(5), 1125–1136. https://doi.org/10.1021/bc049889p
- Blake, D. A., Pavlov, A. R., Yu, H. N., Kohsraviani, M., Ensley, H. E., & Blake, R. C. (2001b). Antibodies and antibody-based assays for hexavalent uranium. Analytica Chimica Acta, 444(1), 3–11. https://doi.org/10.1016/S0003-2670(01)01151-5
- Boden, V., Colin, C., Barbet, J., Ledoussal, J. M., & Vijayalakshmi, M. (1995). Complementary approach for the determination of histidine in the metal-binding site of an anti-DTPA-indium monoclonal-antibody. Enzyme Engineering Xii, 750, 284–287.
- Brechbiel, M. W., Gansow, O. A., Pippin, C. G., Rogers, R. D., & Planalp, R. P. (1996). Preparation of the novel chelating agent N-(2-aminoethyl)-trans-1,2-diaminocyclohexane-N,N’,N''-pentaacetic acid (H5CyDTPA), a preorganized analogue of diethylenetriaminepentaacetic acid (H5DTPA), and the structures of BiIII(CyDTPA)2- and BiIII(H2DTPA) complexes. Inorganic Chemistry, 35(21), 6343–6348. https://doi.org/10.1021/ic951326p
- Carter, K. P., Young, A. M., & Palmer, A. E. (2014). Fluorescent sensors for measuring metal ions in living systems. Chemical Reviews, 114(8), 4564–4601. https://doi.org/10.1021/cr400546e
- Chakrabarti, P., Hatcher, F. M., Blake, R. C., Ladd, P. A., & Blake, D. A. (1994). Enzyme-immunoassay to determine heavy-metals using antibodies to specific metal-EDTA complexes-optimization and validation of an immunoassay for soluble indium. Analytical Biochemistry, 217(1), 70–75. https://doi.org/10.1006/abio.1994.1084
- Chang, C. H., Sharkey, R. M., Rossi, E. A., Karacay, H., McBride, W., Hansen, H. J., Chatal, J. F., Barbet, J., & Goldenberg, D. M. (2002). Molecular advances in pretargeting radioimunotherapy with bispecific antibodies. Molecular Cancer Therapeutics, 1(7), 553–563.
- Chatal, J. F., Gestin, J. F., Faivrechauvet, A., Mease, R. C., Meinken, G., & Srivastava, S. C. (1994). Clinical-evaluation of 2 new bifunctional chelating-agents for immunoscintigraphy (IS) with indium-111-anti-CEA monoclonal antibody. Journal of Nuclear Medicine, 35(5), P12–P12.
- Chen, G., Jin, M. J., Ma, J., Yan, M. M., Cui, X. Y., Wang, Y. S., Zhang, X. Y., Li, H., Zheng, W. J., Zhang, Y. D., Abd El-Aty, A. M., Hacimuftuoglu, A., & Wang, J. (2020). Competitive bio-barcode immunoassay for highly sensitive detection of parathion based on bimetallic nanozyme catalysis. Journal of Agricultural and Food Chemistry, 68(2), 660–668. https://doi.org/10.1021/acs.jafc.9b06125
- Clarke, S. M. (1991). A novel enzyme-linked-immunosorbent-assay (ELISA) for the detection of beryllium antibodies. Journal of Immunological Methods, 137(1), 65–72. https://doi.org/10.1016/0022-1759(91)90394-U
- Comba, P., Jermilova, U., Orvig, C., Patrick, B. O., Ramogida, C. F., Ruck, K., Schneider, C., & Starke, M. (2017). Octadentate picolinic acid-based bispidine ligand for radiometal ions. Chemistry-a European Journal, 23(63), 15945–15956. https://doi.org/10.1002/chem.201702284
- Corneillie, T. M., Whetstone, P. A., Fisher, A. J., & Meares, C. F. (2003). A rare earth-DOTA-binding antibody: Probe properties and binding affinity across the lanthanide series. Journal of the American Chemical Society, 125(12), 3436–3437. https://doi.org/10.1021/ja029363k
- Corneillie, T. M., Whetstone, P. A., & Meares, C. F. (2006). Irreversibly binding anti-metal chelate antibodies: Artificial receptors for pretargeting. Journal of Inorganic Biochemistry, 100(5–6), 882–890. https://doi.org/10.1016/j.jinorgbio.2006.01.004
- Darwish, I. A., & Blake, D. A. (2001). One-step competitive immunoassay for cadmium ions: Development and validation for environmental water samples. Analytical Chemistry, 73(8), 1889–1895. https://doi.org/10.1021/ac0012905
- Darwish, I. A., & Blake, D. A. (2002). Development and validation of a one-step immunoassay for determination of cadmium in human serum. Analytical Chemistry, 74(1), 52–58. https://doi.org/10.1021/ac010510r
- Date, Y., Aota, A., Terakado, S., Sasaki, K., Matsumoto, N., Watanabe, Y., Matsue, T., & Ohmura, N. (2013). Trace-level mercury ion (Hg2+) analysis in aqueous sample based on solid-phase extraction followed by microfluidic immunoassay. Analytical Chemistry, 85(1), 434–440.
- Date, Y., Terakado, S., Sasaki, K., Aota, A., Matsumoto, N., Shiku, H., Ino, K., Watanabe, Y., Matsue, T., & Ohmura, N. (2012). Microfluidic heavy metal immunoassay based on absorbance measurement. Biosensors & Bioelectronics, 33(1), 106–112.
- Day, R. J., & Reilley, C. N. (1964). Nuclear magnetic resonance studies of metal aminopolycarboxylate complexes. Lability of individual metal ligand bonds in (ethylenedinitrilo)-tetraacetate complexes. Analytical Chemistry, 36(6), 1073–1076.
- Delehanty, J. B., Jones, R. M., Bishop, T. C., & Blake, D. A. (2003). Identification of important residues in metal - chelate recognition by monoclonal antibodies. Biochemistry, 42(48), 14173–14183. https://doi.org/10.1021/bi034839d
- Divgi, C. R., McDermott, K., Johnson, D. K., Schnobrich, K. E., Finn, R. D., Cohen, A. M., & Larson, S. M. (1991). Detection of hepatic metastases from colorectal-carcinoma using indium-111 (111In) labeled monoclonal-antibody (mAB) - MSKCC experience with mAB 111In-C110. Nuclear Medicine and Biology, 18(7), 705–710.
- Dong, B. L., Zhao, S. J., Li, H. F., Wen, K., Ke, Y. B., Shen, J. Z., Zhang, S. X., Shi, W. M., & Wang, Z. H. (2019). Design, synthesis and characterization of tracers and development of a fluorescence polarization immunoassay for the rapid detection of ractopamine in pork. Food Chemistry, 271, 9–17. https://doi.org/10.1016/j.foodchem.2018.07.147
- Duta, M., Asfari, Z., Hagege, A., Thuery, P., & Leroy, M. (2004). Synthesis and complexation studies of various precursors based on calix 4 arene-crown-5 and-6 for the immunoanalysis of potassium and caesium ions. Supramolecular Chemistry, 16(3), 205–215. https://doi.org/10.1080/10610270310001647001
- Feng, X., Pak, R. H., Kroger, L. A., Moran, J. K., DeNardo, D. G., Meares, C. F., DeNardo, G. L., & DeNardo, S. J. (1998). New anti-Cu-TETA and anti-Y-DOTA monoclonal antibodies for potential use in the pre-targeted delivery of radiopharmaceuticals to tumor. Hybridoma, 17(2), 125–132. https://doi.org/10.1089/hyb.1998.17.125
- Fu, Q. Q., Liu, H. W. L., Wu, Z., Liu, A., Yao, C. Z., Li, X. Q., Xiao, W., Yu, S. T., Luo, Z., & Tang, Y. (2015). Rough surface Au@Ag core-shell nanoparticles to fabricating high sensitivity SERS immunochromatographic sensors. Journal of Nanobiotechnology, 13.
- Galvidis, I. A., Eremin, S. A., & Burkin, M. A. (2020). Development of indirect competitive enzyme-linked immunoassay of colistin for milk and egg analysis. Food and Agricultural Immunology, 31(1), 424–434. https://doi.org/10.1080/09540105.2020.1733935
- Gao, W., Nan, T. G., Tan, G. Y., Zhao, H. W., Wang, B. M., Li, Q. X., & Meng, F. Y. (2012). Development of a sensitive monoclonal antibody-based enzyme-linked immunosorbent assay for the analysis of cadmium ions in water, soil and rape samples. Food and Agricultural Immunology, 23(1), 27–39. https://doi.org/10.1080/09540105.2011.589045
- Gao, Y., Zhou, Y. Z., & Chandrawati, R. (2020). Metal and metal oxide nanoparticles to enhance the performance of enzyme-linked immunosorbent assay (ELISA). Acs Applied Nano Materials, 3(1), 1–21. https://doi.org/10.1021/acsanm.9b02003
- He, H., Tang, B., Sun, C., Yang, S. G., Zheng, W. J., & Hua, Z. C. (2011). Preparation of hapten-specific monoclonal antibody for cadmium and its ELISA application to aqueous samples. Frontiers of Environmental Science & Engineering in China, 5(3), 409–416. https://doi.org/10.1007/s11783-011-0349-8
- Huo, J. Q., Barnych, B., Li, Z. F., Wan, D. B., Li, D. Y., Vasylieva, N., Knezevic, S. Z., Osipitan, O. A., Scott, J. E., Zhang, J. L., & Hammock, B. D. (2019). Hapten synthesis, antibody development, and a highly sensitive indirect competitive chemiluminescent enzyme immunoassay for detection of dicamba. Journal of Agricultural and Food Chemistry, 67(20), 5711–5719.
- Jemil, S., Fatemi, A., Williamson, D. J., & Moore, G. R. (1992). A Al-27 NMR investigation of Al3+ binding to small carboxylic-acids and the proteins albumin and transferrin. Journal of Inorganic Biochemistry, 46(1), 35–40. https://doi.org/10.1016/0162-0134(92)80061-Y
- Johnson, D. K. (1999). A fluorescence polarization immunoassay for cadmium(II). Analytica Chimica Acta, 399(1-2), 161–172. https://doi.org/10.1016/S0003-2670(99)00587-5
- Johnson, D. K., Combs, S. M., Parsen, J. D., & Jolley, M. E. (2002). Lead analysis by anti-chelate fluorescence polarization immunoassay. Environmental Science & Technology, 36(5), 1042–1047. https://doi.org/10.1021/es011114t
- Jones, R. M., Yu, H. N., Delehanty, J. B., & Blake, D. A. (2002). Monoclonal antibodies that recognize minimal differences in the three-dimensional structures of metal-chelate complexes. Bioconjugate Chemistry, 13(3), 408–415. https://doi.org/10.1021/bc0155418
- Kang, G. F., Wang, Y. Z., Bai, Y. F., Chen, Z. Z., & Feng, F. (2017). Surface plasmon resonance based competitive immunoassay for Cd2+. Rsc Advances, 7(70), 44054–44058. https://doi.org/10.1039/C7RA07635E
- Khosraviani, M., & Blake, R. C. (2000). Binding properties of a monoclonal antibody directed toward lead-chelate complexes. Bioconjugate Chemistry, 11(2), 267–277. https://doi.org/10.1021/bc9901548
- Khosraviani, M., Pavlov, A. R., Flowers, G. C., & Blake, D. A. (1998). Detection of heavy metals by immunoassay: Optimization and validation of a rapid, portable assay for ionic cadmium. Environmental Science & Technology, 32(1), 137–142. https://doi.org/10.1021/es9703943
- Kobayashi, Y., Murata, K., Harada, A., & Yamaguchi, H. (2020). A palladium-catalyst stabilized in the chiral environment of a monoclonal antibody in water. Chemical Communications, 56(10), 1605–1607. https://doi.org/10.1039/C9CC08756G
- Kong, T., Li, X. B., Liu, G. W., Xie, G. H., Wang, Z., Zhang, Z. G., Zhang, Y., Sun, J., & Tang, J. (2012). Preparation of specific monoclonal antibodies against chelated copper ions. Biological Trace Element Research, 145(3), 388–395. https://doi.org/10.1007/s12011-011-9206-7
- Kula, R. J., Sawyer, D. T., Chan, S. I., & Finley, C. M. (1963). Nuclear magnetic resonance studies of metal-ethylenediaminetetraacetic acid complexes. Journal of the American Chemical Society, 85(19), 2930–2936. https://doi.org/10.1021/ja00902a016
- Levy, R., Shohat, L., & Solomon, B. (1998). Specificity of an anti-aluminium monoclonal antibody toward free and protein-bound aluminium. Journal of Inorganic Biochemistry, 69(3), 159–163. https://doi.org/10.1016/S0162-0134(97)10013-7
- Lindgarde, F., & Zettervall, O. (1974). Characterization of a calcium-binding igG myeloma protein. Scandinavian Journal of Immunology, 3(3), 277–285. https://doi.org/10.1111/j.1365-3083.1974.tb01258.x
- Liu, G. L., Wang, J. F., Li, Z. Y., Liang, S. Z., & Wang, X. N. (2009). Immunoassay for cadmium detection and quantification. Biomedical and Environmental Sciences, 22(3), 188–193. https://doi.org/10.1016/S0895-3988(09)60044-1
- Liu, X., Xiang, J. J., Tang, Y., Zhang, X. L., Fu, Q. Q., Zou, J. H., & Lin, Y. H. (2012). Colloidal gold nanoparticle probe-based immunochromatographic assay for the rapid detection of chromium ions in water and serum samples. Analytica Chimica Acta, 745, 99–105. https://doi.org/10.1016/j.aca.2012.06.029
- Lou, Y., Yang, F. L., Zhu, X. X., & Liu, F. Q. (2009). Production of a specific monoclonal antibody against mercury-chelate complexes and its application in antibody-based assays. Food and Agricultural Immunology, 20(1), 23–33. https://doi.org/10.1080/09540100802626479
- Love, R. A., Villafranca, J. E., Aust, R. M., Nakamura, K. K., Jue, R. A., Major, J. G., Radhakrishnan, R., & Butler, W. F. (1993). How the anti-(metal chelate) antibody CHA255 is specific for the metal-ion of its antigen-X-ray structures for 2 Fab’ hapten complexes with different metals in the chelate. Biochemistry, 32(41), 10950–10959. https://doi.org/10.1021/bi00092a004
- Maecke, H. R., Riesen, A., & Ritter, W. (1989). The molecular-structure of indium-DTPA. Journal of Nuclear Medicine, 30(7), 1235–1239.
- Mandappa, I. M., Ranjini, A., Haware, D. J., Manjunath, M. N., & Manonmani, H. K. (2012). Immunoassay for the detection of lead ions in environmental water samples. International Journal of Environmental Analytical Chemistry, 92(3), 334–343. https://doi.org/10.1080/03067319.2010.525790
- Marzo, A. M. L., Pons, J., Blake, D. A., & Merkoci, A. (2013). All-integrated and highly sensitive paper based device with sample treatment platform for Cd2+ immunodetection in drinking/tap waters. Analytical Chemistry, 85(7), 3532–3538. https://doi.org/10.1021/ac3034536
- Meares, C. F. (1986). Chelating-agents for the binding of metal-ions to antibodies. Nuclear Medicine and Biology, 13(4), 311–318.
- Ouyang, H., Shu, Q., Wang, W. W., Wang, Z. X., Yang, S. J., Wang, L., & Fu, Z. F. (2016). An ultra-facile and label-free immunoassay strategy for detection of copper (II) utilizing chemiluminescence self-enhancement of Cu(II)-ethylenediaminetetraacetate chelate. Biosensors & Bioelectronics, 85, 157–163. https://doi.org/10.1016/j.bios.2016.05.007
- Pennacchio, A., Varriale, A., Scala, A., Marzullo, V. M., Staiano, M., & D'Auria, S. (2016). A novel fluorescence polarization assay for determination of penicillin G in milk. Food Chemistry, 190, 381–385. https://doi.org/10.1016/j.foodchem.2015.05.127
- Prudente, C. K., Sirios, R. S., & Cote, S. (2010). Synthesis and application of organomercury haptens for enzyme-linked immunoassay of inorganic and organic mercury. Analytical Biochemistry, 404(2), 179–185. https://doi.org/10.1016/j.ab.2010.05.021
- Quang, D. T., & Kim, J. S. (2010). Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chemical Reviews, 110(10), 6280–6301. https://doi.org/10.1021/cr100154p
- Reardan, D. T., Meares, C. F., Goodwin, D. A., McTigue, M., David, G. S., Stone, M. R., Leung, J. P., Bartholomew, R. M., & Frincke, J. M. (1985). Antibodies against metal-chelates. Nature, 316(6025), 265–268. https://doi.org/10.1038/316265a0
- Sadler, P. J., Tucker, A., & Viles, J. H. (1994). Involvement of a lysine residue in the N-terminal Ni2+ and Cu2+ binding-site of serum albumins comparison with Co2+, Cd2+ and Al3+. European Journal of Biochemistry, 220(1), 193–200. https://doi.org/10.1111/j.1432-1033.1994.tb18614.x
- Safi, S., Asfari, Z., Leroy, M., Basset, C., Quemeneur, E., Vidaud, C., & Hagege, A. (2009). Design and characterization of immunogens for raising antibodies directed towards chelated alkali metals. The Analyst, 134(2), 256–260. https://doi.org/10.1039/B810356A
- Sasaki, K., Oguma, S., Glass, T., Namiki, Y., Sugiyama, H., Ohmura, N., & Blako, D. A. (2008). Simple method to reduce interference from excess magnesium in cadmium immunoassays. Journal of Agricultural and Food Chemistry, 56(17), 7613–7616. https://doi.org/10.1021/jf8011147
- Sasaki, K., Oguma, S., Namiki, Y., & Ohmura, N. (2009). Monoclonal antibody to trivalent chromium chelate complex and its application to measurement of the total chromium concentration. Analytical Chemistry, 81(10), 4005–4009. https://doi.org/10.1021/ac900419c
- Shreder, K., Harriman, A., & Iverson, B. L. (1996). Molecular recognition of a monoclonal antibody (AC1106) cross-reactive for derivatives of Ru(bpy)32+ and Ru(phen)32+. Journal of the American Chemical Society, 118(13), 3192–3201. https://doi.org/10.1021/ja952014o
- Shu, Q., Liu, M. L., Ouyang, H., & Fu, Z. F. (2017). Label-free fluorescent immunoassay for Cu2+ ion detection based on UV degradation of immunocomplex and metal ion chelates. Nanoscale, 9(34), 12302–12306. https://doi.org/10.1039/C7NR04087C
- Spang, P., Herrmann, C., & Roesch, F. (2016). Bifunctional gallium-68 chelators: Past, present, and future. Seminars in Nuclear Medicine, 46(5), 373–394. https://doi.org/10.1053/j.semnuclmed.2016.04.003
- Stickney, D. R., Anderson, L. D., Slater, J. B., Ahlem, C. N., Kirk, G. A., Schweighardt, S. A., & Frincke, J. M. (1991). Bifunctional antibody - a binary radiopharmaceutical delivery system for imaging colorectal-carcinoma. Cancer Research, 51(24), 6650–6655.
- Sun, H. B., Wang, M. M., Wang, J. L., Tian, M., Wang, H., Sun, Z. W., & Huang, P. L. (2015). Development of magnetic separation and quantum dots labeled immunoassay for the detection of mercury in biological samples. Journal of Trace Elements in Medicine and Biology, 30, 37–42. https://doi.org/10.1016/j.jtemb.2015.01.009
- Tang, Y., Zhai, Y. F., Xiang, J. J., Wang, H., Liu, B., & Guo, C. W. (2010). Colloidal gold probe-based immunochromatographic assay for the rapid detection of lead ions in water samples. Environmental Pollution, 158(6), 2074–2077. https://doi.org/10.1016/j.envpol.2010.03.001
- Tighe, P. J., Ryder, R. R., Todd, I., & Fairclough, L. C. (2015). ELISA in the multiplex era: Potentials and pitfalls. Proteomics Clinical Applications, 9(3–4), 406–422. https://doi.org/10.1002/prca.201400130
- Velanki, S., Kelly, S., Thundat, T., Blake, D. A., & Ji, H. F. (2007). Detection of Cd(II) using antibody-modified microcantilever sensors. Ultramicroscopy, 107(12), 1123–1128. https://doi.org/10.1016/j.ultramic.2007.01.011
- Verhoeven, M., Seimbille, Y., & Dalm, S. U. (2019). Therapeutic applications of pretargeting. Pharmaceutics, 11(9), 9. https://doi.org/10.3390/pharmaceutics11090434
- Wang, Y. L., Li, Z. F., Barnych, B., Huo, J. Q., Wan, D. B., Vasylieva, N., Xu, J. L., Li, P., Liu, B. B., Zhang, C. Z., & Hammock, B. D. (2019). Investigation of the small size of nanobodies for a sensitive fluorescence polarization immunoassay for small molecules: 3-phenoxybenzoic acid, an exposure biomarker of pyrethroid insecticides as a model. Journal of Agricultural and Food Chemistry, 67(41), 11536–11541.
- Wang, Y. L., Wang, L. M., Wang, S. Y., Yang, M. M., Cai, J., & Liu, F. Q. (2016). ‘Green’ immunochromatographic electrochemical biosensor for mercury(II). Microchimica Acta, 183(9), 2509–2516.
- Wang, Y., Yang, H., Pschenitza, M., Niessner, R., Li, Y., Knopp, D., & Deng, A. (2012). Highly sensitive and specific determination of mercury(II) ion in water, food and cosmetic samples with an ELISA based on a novel monoclonal antibody. Analytical and Bioanalytical Chemistry, 403(9), 2519–2528.
- Wangler, B., Schirrmacher, R., Bartenstein, P., & Wangler, C. (2011). Chelating agents and their use in radiopharmaceutical sciences. Mini-Reviews in Medicinal Chemistry, 11(11), 968–983.
- Westhoff, C. M., Lopez, O., Goebel, P., Carlson, L., Carlson, R. R., Wagner, F. W., Schuster, S. M., & Wylie, D. E. (1999). Unusual amino acid usage in the variable regions of mercury-binding antibodies. Proteins, 37(3), 429–440. https://doi.org/10.1002/(SICI)1097-0134(19991115)37:3<429::AID-PROT10>3.0.CO;2-P
- Wylie, D. E., Lu, D., Carlson, L. D., Carlson, R., Babacan, K. F., Schuster, S. M., & Wagner, F. W. (1992). Monoclonal-antibodies specific for mercuric ions. Proceedings of the National Academy of Sciences of the United States of America, 89(9), 4104–4108. https://doi.org/10.1073/pnas.89.9.4104
- Xi, T., Zhan, X. J., Xing, H. B., Cao, C. X., & Zhou, P. (2015). Synthesis and characterization of artificial antigens for copper and application for development of an indirect competitive enzyme-linked immunosorbent assay. Analytical Letters, 48(9), 1411–1425. https://doi.org/10.1080/00032719.2014.984192
- Xiao, M., Fu, Q. Q., Shen, H. C., Chen, Y., Xiao, W., Yan, D. G., Tang, X. J., Zhong, Z. Y., & Tang, Y. (2018). A turn-on competitive immunochromatographic strips integrated with quantum dots and gold nano-stars for cadmium ion detection. Talanta, 178, 644–649. https://doi.org/10.1016/j.talanta.2017.10.002
- Xing, C. R., Feng, M., Hao, C. L., Xu, L. G., Kuang, H., Wang, L. B., & Xu, C. L. (2013). Visual sensor for the detection of trace Cu(II) ions using an immunochromatographic strip. Immunological Investigations, 42(3), 221–234. https://doi.org/10.3109/08820139.2012.752378
- Xing, C. R., Liu, L. Q., Zhang, X., Kuang, H., & Xu, C. L. (2014). Colorimetric detection of mercury based on a strip sensor. Analytical Methods, 6(16), 6247–6253. https://doi.org/10.1039/C3AY42002G
- Xu, M. X., Chen, M. T., Dong, T. T., Zhao, K., Deng, A. P., & Li, J. G. (2015). Flow injection chemiluminescence immunoassay based on resin beads, enzymatic amplification and a novel monoclonal antibody for determination of Hg2+. The Analyst, 140(18), 6373–6378. https://doi.org/10.1039/C5AN01131K
- Xu, W., Xie, P., Fan, L. Y., Cao, C. X., Xi, T., & Zhou, P. (2011). Synthesis and characteristics of a novel artificial hapten using the copper mercaptide of penicillenic acid from penicillin G for immunoassay of heavy metal ions. Science China-Life Sciences, 54(9), 813–821. https://doi.org/10.1007/s11427-011-4220-8
- Yamaguchi, H., Hirano, T., Kiminami, H., Taura, D., & Harada, A. (2006). Asymmetric hydrogenation with antibody-achiral rhodium complex. Organic & Biomolecular Chemistry, 4(19), 3571–3573. https://doi.org/10.1039/b609242j
- Yang, J., & Merritt, K. (1994). Detection of antibodies against corrosion products in patient after Co-Cr total joint replacements. Journal of Biomedical Materials Research, 28(11), 1249–1258. https://doi.org/10.1002/jbm.820281102
- Yang, J., & Merritt, K. (1996). Production of monoclonal antibodies to study corrosion products of Co-Cr biomaterials. Journal of Biomedical Materials Research, 31(1), 71–80.
- Zhan, X. J., Xi, T., & Zhou, P. (2015). Preparation of a polyclonal antibody against the cadmium-DTPA complex and its application for determination of cadmium. Food and Agricultural Immunology, 26(6), 794–803.
- Zhang, Y., Li, X. B., Liu, G. W., Wang, Z., Kong, T., Tang, J. J., Zhag, P., Yang, W., Li, D. N., Liu, L., Xie, G. H., & Wang, J. G. (2011). Development of ELISA for detection of mercury based on specific monoclonal antibodies against mercury-chelate. Biological Trace Element Research, 144(1–3), 854–864.
- Zhang, J., Wang, M. B., Yao, X., Deng, A. P., & Li, J. G. (2015). Highly sensitive electroluminescence immunoassay for Hg(II) ions based on the use of CdSe quantum dots, the methylmercury-6-mercaptonicotinic acid-ovalbumin conjugate, and a specific monoclonal antibody. Microchimica Acta, 182(3-4), 469–477.
- Zhou, Y., Tian, X. L., Li, Y. S., Zhang, Y. Y., Yang, L., Zhang, J. H., Wang, X. R., Lu, S. Y., Ren, H. L., & Liu, Z. S. (2011). A versatile and highly sensitive probe for Hg(II), Pb(II) and Cd(II) detection individually and totally in water samples. Biosensors & Bioelectronics, 30(1), 310–314. https://doi.org/10.1016/j.bios.2011.08.034
- Zhu, X. X., Hu, B. S., Lou, Y., Xu, L. N., Yang, F. L., Yu, H. N., Blake, D. A., & Liu, F. Q. (2007). Characterization of monoclonal antibodies for lead-chelate complexes: Applications in antibody-based assays. Journal of Agricultural and Food Chemistry, 55(13), 4993–4998. https://doi.org/10.1021/jf070787d
- Zhu, X. X., Miao, X. Y., Qin, X. Y., & Zhu, X. H. (2019). Design of immunogens: The effect of bifunctional chelator on immunological response to chelated copper. Journal of Pharmaceutical and Biomedical Analysis, 174, 263–269. https://doi.org/10.1016/j.jpba.2019.06.001