ABSTRACT
We investigated if Red ginseng extract (RGE) suppressed monosodium urate crystal (MSU)-induced NLRP3 activation and could be a potential therapeutic agent for gout. The effects of RGE on acute gouty inflammation were investigated using the air-pouch model. RGE significantly suppressed acute gout inflammation in the air-pouch model, based on the decreased number of WBCs in the air-pouch lavage fluid. In vitro, RGE inhibited MSU-induced IL-1β production in THP-1 cells. Twenty-four gout patients in intercritical period were randomized either to red ginseng tablet (RGT) or placebo group. The assembly of NLRP3 inflammasomes was inhibited via reduced ASC expression and oligomerization. Patients taking RGT for 3 months showed significantly reduced NLRP3 expression compared to baseline. In conclusion, RGE suppressed acute gout inflammation by suppressing NLRP3 inflammasomes in animal models. Clinically, RGT suppressed NLRP3 expression in gout patients. The data suggest that red ginseng may exert an additive benefit in gout treatment.
Introduction
Gout is a chronic, systemic disease caused by monosodium urate crystals (MSU) which are present in the articular and periarticular structures following oversaturation of serum uric acid levels (Dalbeth et al., Citation2016). Although the first description of the disease can be traced to the ancient era, the exact molecular pathogenesis of gout is being currently under evaluation. Acute inflammation of the gout is initiated by IL-1β production in the monocytes/macrophages, which recruit neutrophils and further amplify the inflammation (So & Martinon, Citation2017). Detection of MSU crystals by monocytes/macrophages triggers the assembly of intracellular molecular machinery known as NLRP3 inflammasomes and activation of caspase-1, which leads to the synthesis of mature IL-1β from pro IL-1β. Therefore, the NLRP3 inflammasomes play an essential role and targeting NLRP inflammasome is a novel treatment strategy for gout (Stamp & Dalbeth, Citation2019).
Korean red ginseng (Panax Ginseng C.A Meyer) is the most widely known herbal medicines. Its anti-inflammatory property is well known, and its efficacy in various inflammatory diseases has been described (Jhun et al., Citation2014; Lee et al., Citation2018; Liu et al., Citation2019; Xu et al., Citation2018; Zaafan et al., Citation2019). Notably, the cumulative evidence suggests that red ginseng regulates NLRP3 inflammasome activation (Yi, Citation2019; Yoon et al., Citation2015). Although the danger-associated molecular patterns (DAMP) reported in previous studies were not focused on MSU, the therapeutic potential of RGE in suppressing MSU-induced NLRP3 inflammasome appears promising.
In addition, red ginseng reduced serum urate levels in patients with renal failure by increasing uric acid excretion (Peng & Guo, Citation2010). Considering that hyperuricemia is prerequisite for gout development, it would be ideal if red ginseng could reduce serum uric acid level as well as it suppresses MSU-induced NLRP3 inflammasome activation.
Thus, in the current study, we investigated whether RGE treatment affected on serum uric acid levels and/or suppressed of MSU-induced acute inflammation in a rodent animal model. Also, we performed in vitro experiments using human monocyte cell to elucidate the underlying mechanism. Further, we investigated if tablets made of red ginseng extracts (RGT) could be beneficial to intercritical gout patients in two aspects: (1) RGT reduces the serum uric acid level and (2) RGT suppresses NLRP3 inflammasome-related inflammatory markers.
Materials and methods
Korean red ginseng extract
Korean red ginseng extract (RGE)s were manufactured from the roots of a 6-year-old fresh Panax ginseng and provided by Korea Ginseng Corporation (Daejeon, Republic of Korea). RGE yields 4.37% saponins; the main components of ginsenosides were Rb1 (12.6%), Rb2 (6.2%), Rc (6.9%), Rd (3.4%), Re (6.4%), Rf (2.1%), Rg1 (15.8%), and Rg3 (1.4%). The identified constituents are well standardized and qualified by the Korea Ginseng Corporation.
Red ginseng tablets and placebo tablets
Red ginseng tablets were manufactured from the roots of a 6-year-old fresh Panax ginseng and provided by Korea Ginseng Corp (Daejeon, Republic of Korea). A dosage level of 2 g Panax ginseng C.A. Meyer root material consists of ginsenoside Rg1+Rb1+Rg3 4.5 mg per 1 g of material was prescribed per day. Subjects were told to take 2 tablets at once and two times per day, which makes 4 tablets a day for 3months trial. The placebo tablets were also manufactured and provided by Korea Ginseng Corp (Daejeon, Republic of Korea). They consisted of cellulose and had the same size, weight, shape, and colour as the ginseng tablets. Study participants were given a 100-d supply of either the ginseng or placebo tablets to take home after allocation.
Macrophage differentiation and immunoblotting analysis
Human monocyte cell line, THP-1 cells (ATCC, VA, US) were maintained in RPMI 1640 medium supplemented with 10% FBS (Thermo). THP-1 cells of 5 × 106 were stimulated with 100 nM phorbol myristate acetate (PMA) (Sigma, MO, US) for 3 h. The medium was changed with medium containing 100 ng/mL lipopolysaccharide (LPS) (Chondrex, Redmond, WA, US) for 2 h and RGE (10, 30, 100, 300 and 1000 μg/mL) was added. Cells were treated with 150 μg/mL of MSU. After 6 h, the cells were harvested for immunoblot analysis. Protein concentration of cell lysates was measured using the Bradford assay (Bio-Red, Herculed, CA, US). For Western blot hybridization, the membrane was preincubated with blocking buffer for 2 h and then incubated with primary antibodies against NLRP3, pro-Caspase-1, pro-IL-1β, ASC (all from Cell Signaling Technology, MA, US) and β-actin (Sigma) for 16 h at 4°C. After washing, horseradish peroxidase-conjugated secondary antibodies were added, and the membranes were incubated for 2 h at room temperature. After washing, the hybridized bands were detected using SuperSignal® West Pico Chemiluminescent substrate (Thermo) and Amersham Imager 600 (GE Healthcare, IL, US). Concentrated supernatant was diluted with 4× Laemmli buffer (Genedepot, TX, US), separated by SDS-PAGE. After transfer to PVDF membrane, it was incubated with primary antibodies against cleaved caspase-1 (Cell Signaling Technology). For ASC oligomerization assay, cells were washed with PBS and treated with 2 mM disuccinimidyl suberate (DSS, No-Weigh™ Format, Thermo) for 20 min at room temperature. Cells were washed with ice cold PBS, then pellet was lysed in RIPA buffer. Anti-ASC antibodies (Cell Signaling Technology) were used for the immunoblot.
Flow cytometry
Cells in the lavage fluid were washed with 1% bovine serum albumin (Thermo, MA, US) containing PBS buffer, then stained with APC conjugated anti-Ly6G and PE conjugated anti-CD11b antibodies (all Biolegend, CA, US) for 30 min at 4°C dark condition. After washing, cells were analysed by LSRII fortessa (BD) and FACS DIVA software (version 10). Gating strategy is as follows; granulocytes in FSC-A vs. SSC-A; neutrophils in CD11b-PE vs. Ly6G-APC. The absolute number of neutrophil was re-calculated by multiplying the proportion of dual positive cells and the absolute cell number of white blood cells.
Enzyme-linked immunosorbent assay (ELISA)
The level of uric acid in the sera of rat and mice were determined using FUJI DRI-CHEM SLIDE™ and DRI-CHEM ANALYZER™ (Tokyo, Japan). Procedures were followed by the manufacturer’s instruction. The level was determined using human IL-1β development kit for THP-1 experiments and murine IL-1β development kit for air pouch fluid, respectively, which were purchased from R&D systems (MN, US); Absorbance at 450 nm was measured using an ELISA microplate reader (Molecular Devices, CA, US) according to the manufacturer’s instructions. For clinical trial, we used sera from the subjects to measure the levels of human IL-1β, IL-6, TNF-α and TGF-β, using kits purchased from R&D systems (Minneapolils, MN, US) according to the manufacturer’s instructions. Absorbance at 450 nm was measured using an ELISA microplate reader (Molecular Devices, CA, US).
Real-time reverse transcriptase polymerase chain reaction (RT–PCR)
Samples were incubated with TRIzol (Thermo) and RNA was extracted. A RevertAid™ First Strand cDNA Synthesis Kit (Thermo) was used to synthesize cDNA from 200 ng of the extracted RNA. The following primers were used for RT–PCR: human IL1B forward: 5′-CCCAACTGGTACATCAGCAC-3′, reverse: 5′-GGAAGACACAAATTGCATGG-3′; NLRP3 forward: 5′-TTCGACATCTCCTTGGTCCT-3′, reverse: 5′-AGCTGACCAACCAGAGCTTC-3′; Caspase-1(CASP1) forward: 5′-AAATCGAACCTTGCGGAAA-3′, reverse: 5′-GCTTTCTGCTCTTCCAACACC-3′; ASC forward: 5′-TTTTCAAGCTGGCTTTTCGT-3′, reverse: 5′-AGTTTCACACCAGCCTGGAA- 3′; BETA ACTIN forward: 5′-GGACTTCGAGCAAGAGATGG-3′, reverse: 5′-TGTGTTGGGGTACAGGTCTTTG-3′. The results were calculated using LightCycler96 (Basel, Switzerland). The mRNA levels of various target genes were normalized to the levels of β-actin (BETA ACTIN) mRNA. Primers were acquired from Macrogen (Seoul, Korea).
Animals
All procedures involving animals were performed in accordance with the
Laboratory Animals Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Guidelines and Policies for Rodent Experimentation provided by the Institutional Animal Care and Use Committee of the School of Medicine of The Catholic University of Korea.
RGE-treated rat and mouse hyperuricemia model
Male Sprague Dawley rats of 5week-old (OrientBio, Seongnam, Korea) were fed with RGE containing diet (0.5%, 1%, 2% RGE with AIN-76A) for 4weeks. Potassium oxonate (KO) (250 mg/kg) was orally administered for 5 days during the week 4. Serum uric acid levels were determined by ELISA at the end of week 4 and 3 h after KO intraperitoneal injection as well. Male C57BL/6 mice aged 8 weeks (OrientBio, Seongnam, Korea) were orally administered with RGE (60 mg/mouse) for 4 weeks. Allopurinol (5 mg/kg) was orally administered for 4 weeks as positive control. Serum uric acid levels were measured by ELISA.
Acute inflammation in vivo model – acute air pouch model
Male C57BL/6 mice of 8-week-old (OrientBio, Seongnam, Korea) were provided with standard chow and water (mineral water, 5% RGE or 10% RGE) ad libitum. The back skin of mice was shaved and 3 mL of air was injected subcutaneously to form an air pouch. Three days after the first injection, an additional 3 mL of air was injected into the pouch. Three days later, we injected 3 mg of MSU crystals (Invivogen, CA, US) in 500 μL phosphate-buffered saline (PBS) into the air pouch and the control group was injected with 500 μL of PBS. Six hours after injection of MSU crystal or vehicle, mice in each group were sacrificed and air pouch lavage fluid was obtained with 5 mL of PBS. The number of white blood cells (WBCs) was counted with a cell counter (ADAM, NH, US) and the percentage of neutrophils was determined by flow cytometry. The level of IL-1β in the lavage fluid was measured by ELISA.
Study population for the clinical trial
Male gout patients who visited Seoul St Mary’s hospital during March 2017 and January 2018 were enrolled. Patients were in intercritical period and did not show any sign of acute gout attack. Patients with chronic renal disease (estimated glomerular filtration rate (eGFR) < 60 mL/min) were excluded. Age, disease duration, medication information was obtained.
Clinical trial protocol
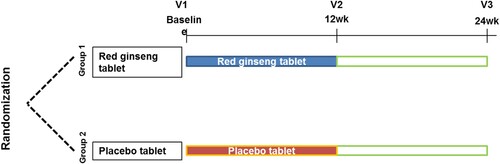
Subjects were randomly allocated to either RGT or placebo groups in a double blinded manner. The subjects took either RGT or placebo for 3 months in addition to their standard treatment. Blood and urine sampling was performed at baseline, 3 months (for primary effect) and 6 months (long term effect) and outcomes were determined. This study was approved by institutional review board of Seoul St Mary’s hospital (KC17HESI0483). Trial registration no. KCT0003130 registered on 28th August 2018 in Clinical Research Information Service (CRIS), Republic of Korea.
Outcomes for the clinical trial
Because this is a pilot study, the sample size (the number of patients) was not estimated by primary outcome. We tried to investigate whether taking RGT for 3months could (i) reduce serum uric acid level, (ii) prevented acute gout attack, (iii) suppress baseline NLRP3 inflammasomes activation during intercritical period. To this end, we measured serum uric acid level and calculated uric acid clearance. We also collected data of acute flare. Serum BUN, creatinine, AST, ALT was measured. Peripheral mononuclear cell (PBMC)s were isolated and used for PCR analysis of NLRP3 inflammasomes related molecules.
Statistical analysis
Statistical analysis was performed using SPSS (version 24, IBM) and GraphPad Prism (Version 5, GraphPad software). For animal experiments, Kruskal–Wallis test with post-hoc analysis was used to compare continuous variables. Repeated measures analysis of variance (ANOVA) was used to compare vehicle and RGE-treated groups at each time point. For the clinical trial, Mann Whitney U test or student’s t test was used to compare continuous variables in two groups where appropriate. Chi square test was used for categorical variables. Repeated measure analysis of variance (ANOVA) was used to compare placebo and RGT groups at three time points. P<0.05 was considered statistically significant.
Results
RGE suppressed MSU-induced IL-1β production by THP-1 cells in vitro
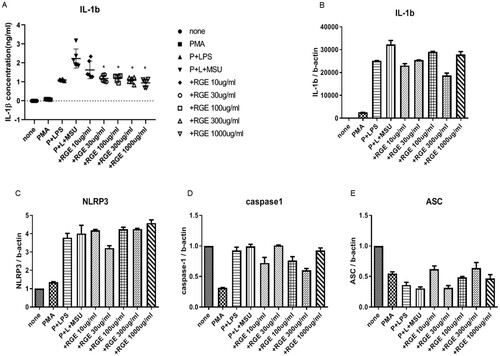
As RGE treatment suppressed MSU-induced acute inflammation in vivo, first, we tested if RGE inhibited MSU-induced IL-1β production by monocytes in vitro. MSU stimulation in LPS primed IL-1β synthesis by THP-1 cells and the level of IL-1β in the supernatant was measured using ELISA. Notably, RGE suppressed IL-1β in a dose-dependent manner ((A)). As RGE did not suppress pro IL-1β mRNA expression ((B)), we hypothesized that IL-1β was suppressed in the post-transcriptional stage by RGE. We also found that the transcription of NLRP3, ASC, and pro-caspase-1 was not affected by RGE ((C–E)).
Figure 1. Effect of red ginseng extract (RGE) on monosodium urate (MSU) crystal-induced IL-1β production and expression of NLRP3 inflammasome-related molecules. THP-1 cell lines were preactivated with PMA and lipopolysaccharide and stimulated with MSU (150 µg/mL) for 6 h. Various concentrations of RGE were used during MSU stimulation. The supernatant was obtained and analysed via ELISA to measure IL-1β (A). The cells were harvested and the mRNA expression was determined with PCR analysis for IL-1β (B), NLRP3 (C), caspase-1 (D), and ASC (E).
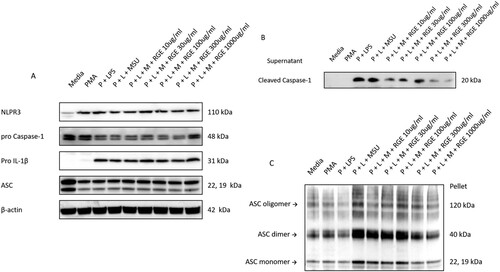
RGE suppressed ASC oligomerization and subsequent NLRP3 inflammasome activation
Immunoblotting analysis confirmed that pro-IL-1β and procaspase-1 protein expression was not suppressed by RGE ((A)), and active caspase-1 expression in the supernatant was reduced by RGE ((B)). Therefore, we hypothesized that the inhibition occurred via suppression of NLRP3 inflammasome activation by RGE. We observed that ASC expression and subsequent oligomerization, which are critical for NLRP3 inflammasome activation were suppressed by RGE ((C)). We also investigated if this inhibition was associated with AMPK phosphorylation. However, RGE treatment did not affect AMPK activation in the setting of MSU-induced NLRP3 activation (data not shown).
Figure 2. Effect of red ginseng extract (RGE) on monosodium urate (MSU)-induced NLRP3 inflammasome activation. THP-1 cell lines were preactivated with PMA and lipopolysaccharide and stimulated with MSU (150 µg/mL) for 6 h. Various concentrations of RGE were used at the time of MSU stimulation. The cells were harvested and the protein expression was determined via immunoblotting analysis of NLRP3, procaspase-1, pro IL-1β, and ASC (A). The supernatant was obtained and used for immunoblotting analysis to measure the cleaved caspase-1 level (B). ASC expression and oligomerization were determined via immunoblotting analysis (C).
RGE treatment did not affect uric acid level in rodents
Next, we investigated if RGE affected the serum uric acid concentrations in rodents because the serum uric acid level is critical in gout. Rats were fed with a standard chow diet or a 0.5%/1%/2% RGE containing diet ad libitum. Serum uric acid levels were measured at baseline and 3 weeks thereafter. As the rodents express uricase enzyme, the uricase inhibitor potassium oxonate (KO) (250 mg/kg) was orally administered for 5 days during the week 4. Serum uric acid levels were measured 3 h after KO intraperitoneal injection as well. Mice were orally administered with RGE (60 mg/mouse). Allopurinol (5 mg/kg) was orally administered for 4 weeks as positive control. We found that RGE treatment did not affect the serum uric acid level either in rats or mice, whereas allopurinol significantly reduced the serum uric acid level in mice (Supplementary Fig. 1).
RGE treatment suppressed acute gout inflammation in the air pouch model
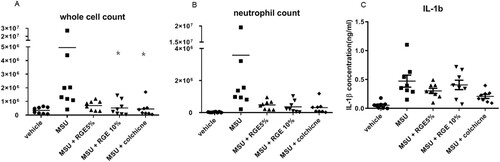
Next, we investigated if RGE treatment suppressed MSU-induced acute inflammation in vivo. Mice were fed with 0/5%/10% RGE containing water ad libitum. Mice exposed to 10% RGE solution showed a significantly lower whole cell count similar to the colchicine-treated group ((A)). However, the neutrophil count or the level of IL-1β in the pouch fluid was not significantly different between the groups ((B,C)).
Figure 3. Effect of red ginseng extract (RGE) on acute inflammation in air pouch model. Mice were fed with 0/5/10% RGE containing water ad libitum for 3 weeks (n = 8 per group). Air pouch formation was induced on the subcutaneous tissue dorsally by injecting air. Monosodium urate (MSU) crystals (3 mg/pouch) were injected into the air pouch. After 6 h, the lavage fluid was obtained and the whole cell counts (A) and neutrophil counts (B) were determined. The IL-1β level was analysed using ELISA (C).
Clinical trial with red ginseng tablets in gout patients
As we observed therapeutic potential of red ginseng in in vitro and animal models, we performed a clinical trial with RGT which has been manufactured as health functional food by Korean ginseng corporation based on the preclinical data with RGE. Gout patients in intercritical period were enrolled and randomized either to RGT or placebo group. A total of 24 patients were enrolled, 12 patients were allocated to each group by randomization in a double-blind manner (). 10 patients per each group finally completed 3 months of investigational product taking period (). Baseline characteristics of the subjects are described in . There was no significant difference in age, disease duration, body mass index, medications between the two groups.
Table 1. Baseline characteristics of the study subjects.
When uric acid level (Supplementary Fig 2A, 2B) and uric acid clearance (Supplementary Fig 2C, 2D) was addressed, there was no significant difference between the two groups. Taking RGT for 3 months did not affect the uric acid level/clearance in our gout patients who showed stable uric acid level.
Next, we investigated if the RGT could reduce the chance of acute gout attack, however, there were equally 2 patients per group who experienced mild flare, which makes no difference between the groups.
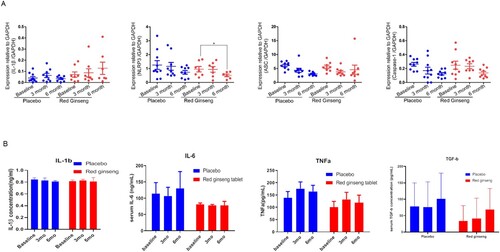
To investigate if RGT affects low grade inflammation or NLRP3 inflammasome expression in intercritical period, we obtained PBMCs and measured mRNA expression of NLRP3 inflammasome components and pro- or anti-inflammatory cytokines including IL-1β, IL-6, TNF-α and TGF-β. In addition, we determined serum levels of the cytokines. RGT did not affect either mRNA expression of cytokines of PBMCs or serum cytokine levels. Caspase-1 expression showed a tendency to decrease with RGT treatment, however, statistical significance was not achieved. Of note, we did observe NLRP3 expression was significantly suppressed with RGT treatment at 6 month ().
Figure 6. Expression of NLRP3 inflammasome components and serum cytokines level after 3 months’ treatment of red ginseng tablet or placebo. (A) mRNA expression of IL-1β, NLRP3, ASC, Caspase-1 measured by RT-PCR (B) serum levels of IL-1β, IL-6, TNF-α and TGF-β measured by ELISA.
With respect to safety, adverse events were investigated and compared between the two groups. Two patients in the placebo arm complained febrile sensation and one of them was dropped out. One patient in the placebo arm had appendicitis and one patient experienced cellulitis over the knee area during the trial period. There was no patient-reported adverse event in RGT group. In addition, one patient in the placebo group showed elevated ALT after taking placebo tablets for 3 months.
Discussion
In the current study, we showed RGE suppressed MSU-induced NLRP3 inflammasome activation via suppressing ASC oligomerization. Also, at least in this experimental setting, we did not find any evidence that RGE could reduce serum uric acid level. Therefore, therapeutic potential of RGE for gout is involved with anti-inflammatory function.
In vitro, we found that MSU-induced IL-1β production was suppressed by RGE. As the mRNA expression of IL-1β was not affected, we assumed that the suppressive effect of RGE was involved with signal 2 (NLRP3 inflammasome activation) rather than signal 1 (NF-kb) (Martinon et al., Citation2006). Indeed, we observed that ASC expression and subsequent oligomerization which is essential for NLRP3 inflammasome activation was suppressed by RGE treatment. Unfortunately, in this study, we could not elucidate how RGE suppress ASC and future research should be warranted. In this context, we investigated if the effect of RGE was associated with AMPK pathway. Recently, McWherter et al. (Citation2018) demonstrated that MSU-induced NLRP3 activation was affected by AMPK pathway, and phosphorylation of AMPK could ameliorate MSU-induced IL-1β production. Also, ginsenoside Rg2 was shown to inhibit adipogenesis in high-fat diet induced mice through the AMPK pathway (Liu et al., Citation2019). Previously, molecules that suppress NLRP3 inflammasome activation by inhibiting ASC oligomerization were reported, but none of them clearly suggested the inhibitory mechanisms (Chung et al., Citation2019; Leu et al., Citation2019; Wang et al., Citation2019). Syk-dependent (Lin et al., Citation2015) or Cl– channel (Green et al., Citation2018) dependent mechanisms have been suggested, which should be clarified in the future research.
In acute air pouch model, RGE significantly suppressed MSU- induced WBC recruitment in the air pouch. Although, neutrophil count and concentration IL-1β did not reach statistical significance, the same trend was observed. The reason why the efficacy in vivo was not as apparent as it was in vitro seems to be the amount and concentration of the RGE needed. We found that relatively large amount of RGE was needed to observe the anti-inflammatory effects in gout model. Initially, we planned to treat RGE by manufacturing 0.5%, 1% and 2% RGE containing chow according to previous studies and check the effect but it turned to be ineffective up to 2% (data not shown). Then, we tried higher concentration by solution as described by Kim et al. (Citation2014) and confirmed RGE could suppress the acute inflammation. When calculated by the daily consumption of the chow and water, the actual RGE dose taken was 60–120 mg/day by 2% RGE chow. In contrast, 200–350 mg/day of RGE was estimated to be administered to 10% RGE solution-fed mice. Therefore, we assumed that enough amount of RGE is needed to reduce MSU-induced inflammation.
Another issue is that recent studies found that the function of saponin and non-component of RGE could be different even opposite. While saponin fraction of RGE was consistently shown to inhibit inflammation including NLRP3 inflammasome activation (Chen et al., Citation2016; Han et al., Citation2018; Xu et al., Citation2018; Yoon et al., Citation2015), non-saponin fraction of RGE has controversial results. Han et al. (Citation2017) reported that non-saponin fraction of RGE prime activation of NLRP3 inflammasome, while Ann et al. (Citation2019) demonstrated that non-saponin fraction of RGE suppressed TLR4 expression and attenuated cytokine production. Considering signal 1 in MSU-induced NLRP3 inflammasome activation, the overall effect seems to suppress the activation as shown in our study. It would be intriguing if we could perform the same analysis using only saponin fraction of RGE in the future research to see whether saponin only fraction has better therapeutic potential.
In the clinical trial, we observed that taking RGT twice per day for 3 months reduced NLRP3 expression in PBMC of the intercritical gout patients. This is a proof-of-concept study that investigated the therapeutic potential of RGT which previous observations suggested. As mentioned, red ginseng extracts or saponin fraction of red ginseng was reported to inhibit NLRP3 inflammasome activation (Chen et al., Citation2016; Han et al., Citation2017, Citation2018; Jhun et al., Citation2014; Kim et al., Citation2014; Yoon et al., Citation2015). However, to the best of our knowledge, this is the first study which implicated red ginseng in gout patients to investigate its efficacy.
There have been only few studies that investigated whether the red ginseng had the urate lowering effect. One Chinese study supported the notion and showed that Panax notoginseng reduced the level of serum uric acid in patients with chronic renal failure (Peng & Guo, Citation2010). However, we could not observe those findings were recapitulated in gout patients. As we excluded patients with chronic renal disease, the difference in subjects might result in the difference. Or the amount of red ginseng might be insufficient to urate lowering effect in our trial.
Also, our data could not demonstrate that RGT could prevent acute gout attack. This may be due to a small number of subjects. Indeed, we observed that RGT significantly suppressed NLRP3 expression of the PBMCs of the intercritical gout patients. Although, it was not proved in the current study, we suggest that reduced NLRP3 expression should affect acute gout attack. Considering its anti-inflammatory effect shown in the previous studies, appropriately powered study may show a positive result.
Indeed, previous studies showed that red ginseng suppressed IL-1β production by inhibiting NLRP3 inflammasome in vitro (Kim et al., Citation2014). The results can be implicated in acute gout arthritis rather than intercritical gout. However, unfortunately, we could not investigate if the RGT could exert anti-inflammatory effect in acute gout setting. Based on the in vitro findings that red ginseng extracts reduced IL-1β production by inhibiting NLRP3 inflammasome activation, it is highly likely that RGT can reduce acute inflammation. However, the current study was not designed to investigate the therapeutic efficacy in acute gout setting. Also, the current strategy to suppress acute gout attack with non-steroid anti-inflammatory drug or steroid with/without colchicine is so efficient that it may be hard to demonstrate the additive effect of RGT, especially in a small number of subjects like the current pilot study. Rather, RGT treatment may have a role in preventing acute gout flare by reducing NLRP3 expression.
To our disappointment, RGT failed to suppress the inflammatory cytokine levels. However, gout patients have mostly basal level of those cytokine and it may be hard to demonstrate “lower” serum level in intercritical period.
In conclusion, RGE effectively suppressed MSU-induced acute inflammation by inhibiting NLRP3 inflammasome activation in preclinical and in vitro studies. In gout patients, RGT decreased NLRP3 expression in PBMCs of gout patients in intercritical periods. These results suggest that red ginseng maybe implicated as adjunctive therapeutic in gout management.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available upon reasonable request
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/09540105.2021.2017684).
Additional information
Funding
References
- Ahn, H., Han, B.-C., Kim, J., Kang, S. G., Kim, P.-H., Jang, K. H., So, S. H., Lee, S.-H., & Lee, G.-S. (2019). Nonsaponin fraction of Korean red ginseng attenuates cytokine production via inhibition of TLR4 expression. Journal of Ginseng Research, 43(2), 291–299. https://doi.org/10.1016/j.jgr.2018.03.003
- Chen, W., Wang, J., Luo, Y., Wang, T., Li, X., Li, A., Li, J., Liu, K., & Liu, B. (2016). Ginsenoside Rb1 and compound K improve insulin signaling and inhibit ER stress-associated NLRP3 inflammasome activation in adipose tissue. Journal of Ginseng Research, 40(4), 351–358. https://doi.org/10.1016/j.jgr.2015.11.002
- Chung, Y. H., Kim, H. Y., Yoon, B. R., Kang, Y. J., & Lee, W. W. (2019). Suppression of Syk activation by resveratrol inhibits MSU crystal-induced inflammation in human monocytes. Journal of Molecular Medicine, 97(3), 369–383. https://doi.org/10.1007/s00109-018-01736-y
- Dalbeth, N., Merriman, T. R., & Stamp, L. K. (2016). Gout. The Lancet, 388(10055), 2039–2052. https://doi.org/10.1016/S0140-6736(16)00346-9
- Green, J. P., Yu, S., Martín-Sánchez, F., Pelegrin, P., Lopez-Castejon, G., Lawrence, C. B., & Brough, D. (2018). Chloride regulates dynamic NLRP3-dependent ASC oligomerization and inflammasome priming. Proceedings of the National Academy of Sciences, 115(40), E9371–E9380. https://doi.org/10.1073/pnas.1812744115
- Han, B.-C., Ahn, H., Lee, J., Jeon, E., Seo, S., Jang, K. H., Lee, S.-H., Kim, C. H., & Lee, G.-S. (2017). Nonsaponin fractions of Korean red ginseng extracts prime activation of NLRP3 inflammasome. Journal of Ginseng Research, 41(4), 513–523. https://doi.org/10.1016/j.jgr.2016.10.001
- Han, X., Song, J., Lian, L.-H., Yao, Y.-L., Shao, D.-Y., Fan, Y., Hou, L.-S., Wang, G., Zheng, S., Wu, Y.-L., & Nan, J.-X. (2018). Ginsenoside 25-OCH3-PPD promotes Activity of LXRs to ameliorate P2X7R-mediated NLRP3 inflammasome in the development of hepatic fibrosis. Journal of Agricultural and Food Chemistry, 66(27), 7023–7035. https://doi.org/10.1021/acs.jafc.8b01982
- Jhun, J., Lee, J., Byun, J.-K., Kim, E.-K., Woo, J.-W., Lee, J.-H., Kwok, S.-K., Ju, J.-H., Park, K.-S., Kim, H.-Y., Park, S. H., & Cho, M.-L. (2014). Red ginseng extract ameliorates autoimmune arthritis via regulation of STAT3 pathway, Th17/Treg balance, and osteoclastogenesis in mice and human. Mediators of Inflammation, 2014, 1–13. https://doi.org/10.1155/2014/351856
- Kim, J., Ahn, H., Han, B.-C., Lee, S.-H., Cho, Y.-W., Kim, C. H., Hong, E.-J., An, B.-S., Jeung, E.-B., & Lee, G.-S. (2014). Korean red ginseng extracts inhibit NLRP3 and AIM2 inflammasome activation. Immunology Letters, 158(1-2), 143–150. https://doi.org/10.1016/j.imlet.2013.12.017
- Lee, Y. K., Choi, K. H., Kwak, H. S., & Chang, Y. H. (2018). The preventive effects of nanopowdered red ginseng on collagen-induced arthritic mice. International Journal of Food Sciences and Nutrition, 69(3), 308–317. https://doi.org/10.1080/09637486.2017.1358359
- Leu, W. J., Chen, J. C., & Guh, J. H. (2019). Extract from Plectranthus amboinicus inhibit maturation and release of interleukin 1beta through inhibition of NF-kappaB nuclear translocation and NLRP3 inflammasome activation. Frontiers in Pharmacology, 10, 573. https://doi.org/10.3389/fphar.2019.00573
- Lin, Y.-C., Huang, D.-Y., Wang, J.-S., Lin, Y.-L., Hsieh, S.-L., Huang, K.-C., & Lin, W.-W. (2015). Syk is involved in NLRP3 inflammasome-mediated caspase-1 activation through adaptor ASC phosphorylation and enhanced oligomerization. Journal of Leukocyte Biology, 97(5), 825–835. https://doi.org/10.1189/jlb.3HI0814-371RR
- Liu, H., Liu, M., Jin, Z., Yaqoob, S., Zheng, M., Cai, D., Liu, J., Guo, S. (2019). Ginsenoside Rg2 inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat-diet-induced obese mice through the AMPK pathway. Food & Function, 10, 3603–3614. https://doi.org/10.1039/C9FO00027E
- Martinon, F., Petrilli, V., Mayor, A., Tardivel, A., & Tschopp, J. (2006). Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature, 440(7081), 237–241. https://doi.org/10.1038/nature04516
- McWherter, C., Choi, Y. J., Serrano, R. L., Mahata, S. K., Terkeltaub, R., & Liu-Bryan, R. (2018). Arhalofenate acid inhibits monosodium urate crystal-induced inflammatory responses through activation of AMP-activated protein kinase (AMPK) signaling. Arthritis Research & Therapy, 20(1), 204. https://doi.org/10.1186/s13075-018-1699-4
- Peng, S. L., & Guo, Z. A. (2010). Effect of total saponins of Panax notoginseng on urinary albumin in patients with chronic renal failure. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue, 22, 744–746.
- So, A. K., & Martinon, F. (2017). Inflammation in gout: Mechanisms and therapeutic targets. Nature Reviews Rheumatology, 13(11), 639–647. https://doi.org/10.1038/nrrheum.2017.155
- Stamp, L. K., & Dalbeth, N. (2019). Prevention and treatment of gout. Nature Reviews Rheumatology, 15(2), 68–70. https://doi.org/10.1038/s41584-018-0149-7
- Wang, Z., Xu, G., Gao, Y., Zhan, X., Qin, N., Fu, S., Li, R., Niu, M., Wang, J., Liu, Y., Xiao, X., & Bai, Z. (2019). Cardamonin from a medicinal herb protects against LPS-induced septic shock by suppressing NLRP3 inflammasome. Acta Pharmaceutica Sinica B, 9(4), 734–744. https://doi.org/10.1016/j.apsb.2019.02.003
- Xu, Y., Yang, C., Zhang, S., Li, J., Xiao, Q., & Huang, W. (2018). Ginsenoside Rg1 protects against non-alcoholic fatty liver disease by ameliorating lipid peroxidation, endoplasmic reticulum stress, and inflammasome activation. Biological and Pharmaceutical Bulletin, 41(11), 1638–1644. https://doi.org/10.1248/bpb.b18-00132
- Yi, Y. S. (2019). Roles of ginsenosides in inflammasome activation. Journal of Ginseng Research, 43(2), 172–178. https://doi.org/10.1016/j.jgr.2017.11.005
- Yoon, S.-J., Park, J.-Y., Choi, S., Lee, J.-B., Jung, H., Kim, T.-D., Yoon, S. R., Choi, I., Shim, S., & Park, Y.-J. (2015). Ginsenoside Rg3 regulates S-nitrosylation of the NLRP3 inflammasome via suppression of iNOS. Biochemical and Biophysical Research Communications, 463(4), 1184–1189. https://doi.org/10.1016/j.bbrc.2015.06.080
- Zaafan, M. A., Abdelhamid, A. M., & Ibrahim, S. M. (2019). The protective effect of Korean red ginseng against rotenone-induced Parkinson’s disease in rat model: Modulation of nuclear factor-kappabeta and caspase-3. Current Pharmaceutical Biotechnology, 20(7), 588–594. https://doi.org/10.2174/1389201020666190611122747