ABSTRACT
The objective was to characterize the effects of a supplemental Herbal Antibiotics Source (HAnS) on performance, blood chemistry, blood cells, and antibody counts of Holstein calves. Forty calves (initial BW 45.8 ± 7.2 kg) were randomly assigned to treatments: 0, 3, 4, and 5 g d−1 HAnS. The additive improved hip height and thoracic girth. The reduction of starter diet intake among individuals varied greatly, while milk replacer intake increased. HAnS at intermediate doses reduced the cases of pneumonia. It also linearly reduced the blood serum glucose and B-OH butyrate. Urea and monocytes showed a quadratic response, and basophils decreased. HAnS improves calf development at intermediate doses and reduces the incidence of diseases.
KEYWORDS:
Introduction
Rearing calves is among the most important and sensitive cattle management activities, as calves are a major source of profit for farms (Seifzadeh et al., Citation2017). During calf rearing, respiratory and digestive problems occur that can cause high morbidity and mortality (Beaver et al., Citation2019). Because of health issues, the use of antibiotics, anabolic products, and growth promoters has been limited. Legislation in many countries is restricting the use of antibiotics. Therefore, it is urgent to identify other sources of antimicrobial properties and immunostimulant effects. In recent years, research in the use of herbal supplements has reported beneficial effects during the suckling period in ruminants (Crosby et al., Citation2017; Roque-Jiménez et al., Citation2020). Assan (Citation2018) reported that supplementation with herbal formulas could lead to antibiotic-free feeds (Assan, Citation2018), and during the suckling period, they could maintain or improve calf health and productive indicators (Frankič et al., Citation2009; Wall et al., Citation2014).
In dairy and beef production systems, there is interest in using herbal formulas as alternatives to antibiotics during disease (Mendoza et al., Citation2019) and as enhancers of feed efficiency (Wall et al., Citation2014). Herbal formulas have mainly aimed to modify rumen fermentation and improve nutrient utilization in ruminant production (Frankič et al., Citation2009). Herbal feed additives or supplements contain different bioactive compounds, including alkaloids, polyphenols, isothiocyanates, tannins, saponins, and terpenoids, all of which can stimulate the immune system (Frankič et al., Citation2009). Standardized herbal antibiotic sources can contain Adhatoda vasica, Solanum xanthocarpum, Curcuma longa, Hedychium spicatum, Boerrhavia diffusa, Piper longum, and Albezia lebbek. These plants have been related to higher phytogenic contents that have been shown to have positive effects on ruminant production and health (Mendoza et al., Citation2019). Herbal formulas differ from extracts and essential oils in that they usually contain several molecules with one or more predominant active substances or nutrients responsible for positive biological effects that compromise diverse metabolites and mechanisms of action. In ruminant livestock production, herbal formulas have been reported to have positive effects: stimulating a non-specific immune response (Kumar & Pandey, Citation2014), epigenetic changes (Roque-Jiménez et al., Citation2020), antioxidant effects (Toghyani et al., Citation2015), inhibiting replication of specific pathogenic microorganisms (Rivera-Méndez et al., Citation2017), stimulating the immune response or providing nutrients with or without nutraceutical properties.
We hypothesized that the herbal compounds of Herbal Antibiotics Source (HAnS) have positive effects on the immune system, growth performance, blood biochemistry, and biometry of dairy calves. Therefore, the objective of this experiment was to characterize the natural compounds in HAnS and determine their effect on the immune system, growth performance, blood biochemistry, and biometry of dairy calves.
Material and methods
Ethics
The animal procedures were reviewed and approved by the Committee for Ethical Use of Animals in Experiments of Universidad Autónoma Metropolitana, in compliance with the regulations and standards issued by the Mexican government for the use of animals in diverse activities. Federal law on technical specifications for the care and use of laboratory animals and livestock on farms and production, reproduction, and breeding centres, and zoo and exhibition animals must meet the basic principles of animal welfare (NOM-062-ZOO-1995).
Location
The current experiment was conducted on a Mexican dairy farm in Torreon, Coahuila (25°39′14.4″ N 103°27′27.8″ W; altitude 1,139 m) in a semiarid climate (mean temperature 20.21°C).
Herbal Antibiotics Source characterization
The herbal formula used in the current experimental study (HAnS; Animunin Powder®, Nuproxa, LTD, Switzerland) is a labelled commercial standardized herbal formula that contains Adhatoda vasica, Solanum xanthocarpum, Curcuma longa, Hedychium spicatum, Boerrhavia diffusa, Piper longum and Albezia lebbek. Bioactive compounds were extracted using an ultrasonic processor (GEX130, 115 V 50/60 Hz) equipped with a 3 mm titanium tip and mechanical stirrers (Cole-Parmer, IL, USA). One gram of HAnS was mixed with 10 mL of hexane. Subsequently, the organic phase was separated, concentrated to 1 mL of extracted mixture, and evaporated (Zymark, Turbovap LV Concentration Evapotarot, NB, USA) for the final analysis.
HAnS was characterized by gas chromatography (GC-HP 6890) coupled with mass spectrophotometry (MSHP 5973), equipped with a capillary column 60 m long, 0.255 mm in diameter, and 0.25 µm film thickness (HP 5MS, Agilent). The temperature programme was 70°C for 2 min, which was then increased to 250°C at the rate of 20°C/min, then to 290°C at the rate of 5°C/min, then increased to 300°C at the rate of 1°C/min, then to 310°C at the rate of 5°C/min and held for 36 min. The injector temperature was 250°C in splitless mode. The helium flow rate was 1 mL/min. The mass spectrophotometry was programmed in SCAN mode (50–500 m/z) to identify compounds.
Animals, treatments, and sampling
Forty female Holstein calves (initial body weight (IBW) 45.8 ± 7.2 Kg), 20.4 ± 2.9 days of age were randomly assigned to one of four treatments. The treatments (10 calves per treatment) consisted of oral doses containing 0, 3, 4, or 5 gd−1 of HAnS. Treatments were top-dressed on the feed using gelatin as the medium. Gelatin was prepared following the protocol described by Sánchez-Hernández et al. (Citation2019). The calves were housed in individual pens equipped with steel buckets for feed and milk. All calves received colostrum after birth and the initial total serum protein was confirmed with a refractometer (Quigley, Citation2011).
Calves were fed twice a day (07:00–17.00) with milk (56%) and milk replacer (44% Milk replacer Nu-3 Group, México), reconstituted in hot water (65°C; 130 g/L). The milk mix was served at a temperature of 39°C in buckets. Milk replacer was offered twice per day (4 L/feeding) and reduced to once on day 25 as starter diet intake augmented. The starter diet concentrate was offered as of the third day of age and was gradually increased (Started Premium, Nuplen, México).
Experimental procedures
Calves were weighed on days 1, 20 and 90 of the experiment to calculate the average daily gain (ADG). Also, on day 1 and day 10, all the calves received a bovine rhinotracheitis vaccine (Pyramyd-5; Boehringer Ingelheim). Later, on day 50, all the calves were vaccinated with a clostridial bacterin-toxoid (Covexin 10; MSD). On day 90, hip, wither height, and thoracic girth were measured to evaluate growth and development (Maciel et al., Citation2016 Parish et al., Citation2012;). Feed intake (milk and starter diet concentrate) was recorded daily and based on the dry matter (DM) content of the starter diet and milk replacer. The feed efficiency ratio was estimated (feed efficiency = kg gain/kg feed) (Kazemi-Bonchenari et al., Citation2018) and the coefficient of variation of DM intake between days was calculated as a stability indicator (Britton et al., Citation1990). Calves were examined daily for disease and given veterinary treatment when needed (Pardon et al., Citation2013). On day 90, three blood samples (5 mL per sample) were collected from each of the calves from the jugular vein by puncture with blood collection tubes. The first sample without anticoagulant was centrifuged to obtain blood serum which was later used to determine contents of cholesterol, glucose, total protein, urea, uric acid, albumin, globulin, bilirubin, alkaline phosphatase (ALP), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), Ca, P and creatinine using an autoanalyzer (Kontrolab QS EasyVet, Italy). The second sample without anticoagulant was centrifuged at 1500 rpm for five minutes to obtain blood serum, which was collected in vials and stored at −80°C until ELISA analysis. Finally, the last blood sample collected in tubes with anticoagulant was used for complete blood count, haematocrit, and leukocyte differential count in a haematology analyser (Kontrolab QS EasyVet, Italy).
Quantification of immunoglobulins (IgG) by ELISA
An ELISA was performed using plates with antigen (Clostridium spp) obtained from Covexin 10. The antigen was incubated for 24 h at room temperature; then 200 µL was added to each well to decrease non-specific binding sites. Later, 100 µL of the serum sample was added to each well and incubated for 1 h at 37°C. Plate contents were decanted, and 100 µL of the bovine IgG specific secondary antibody was added and allowed to incubate for 1 h at 37°C. Subsequently, 100 µL of TMB (3,3’, 5,5'-liquid tetramethylbenzidine) was added to each well. Finally, 100 µL of buffer solution (H2SO4) was added to each well to stop the reaction. The plate was read at 540 nm.
Statistical analysis
Shapiro–Wilk tests were used to ascertain normal distribution, and data were analysed under a completely randomized design with R software, testing for linear and quadratic effects of HAnS supplementation dose by orthogonal polynomials (Mirman, Citation2014). The initial body was tested as a covariate. For main effects, a significant difference was established at p ≤ 0.05, trends were determined at p ≥ 0.05 and p ≤ 0.10.
Results
Herbal Antibiotics Source (HAnS) characterization
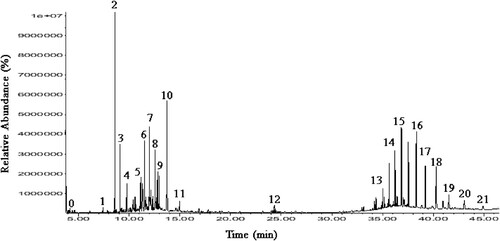
Gas chromatography coupled with mass spectrophotometry revealed various chemical compounds present in the HAnS formula (). The main components detected were a variety of alkanes such as hepacosane, heneicosane, hexacosane, eicosane, octacosane and octadecane. Alkaloids, such as piperine, as well as a type of allylbenzene called eugenol, terpenes such as thymol and vitamin E, and other components, were also detected.
Figure 1. Total ion chromatogram of the volatile components and chemical composition in HAnS. Chemical composition of Herbal Antibiotics Source (HAnS) by GC-MS with retention time(rt): 0. 3-Hexanol (standard mixture solvent); 1. Thymol; 2. Eugenol; 3. Benzene, 1-methyl-2-(1-methylethy); 4. Phenol, 2-methoxy-5-(1-propenyl)-(E)-; 5. Cyclobutane, 1,2-dipropenyl-; 6. 2-Cyclohexen-1-one, 5-methyl-2-(1 methylethyl); 7. Cyclohexane, bromo; 8. 3-Hexen-1-ol, 2,5-dimethyl-, formate,(Z)- 9. (-)-E-Pinane 10. s-Indacene, 1,2,3,5,6,7-hexahydro-1,1,7,7-tetramethyl; 11. 6-Methoxy-2,3-dihydro-1-benzofuran-3-acetic acid; 12. 9-Octadecenoic acid, (E)-; 13. Hexacosane; 14. Heptacosane; 15. Silicic acid, diethyl bis(trimethylsilyl) ester; 16. Piperine; 17. Vitamin E; 18. Heneicosane, 11-decyl-; 19. Eicosane; 20. Octacosane; 21. Octadecane.
Effects of an HAnS on calf productive performance and health
The productive performance and health of calves supplemented with HAnS was evaluated (). We observed no differences among the treatments in ADG and final BW, while final hip height tended to be greater (p = 0.08) for calves supplemented with HAnS. Also, there was an increase in final thoracic girth (quadratic, p = 0.02) with supplementation of 4 g d−1 of HAnS. HAnS supplementation to calves resulted in a lower starter intake g d−1 than the treatment without HAnS (p = 0.01). A similar response of calves without HAnS supplementation was observed for feed intake (p < 0.01) compared with the calves with different doses of HAnS. However, milk and milk replacer intake was higher in calves that received supplementation with HAnS. Calves that consumed 3 g d−1 of HAnS showed the highest consumption of milk and of milk replacer (linear p < 0.01; quadratic p < 0.01). Interestingly, when the health status of the calves was evaluated, a positive effect was observed during the experimental period (). Although no changes were observed in the incidence of diarrhoea, cases of pneumonia significantly decreased in calves that consumed HAnS (linear, p = 0.05), relative to the group without supplementation.
Table 1. Effect of an Herbal Antibiotics Source (HAnS) on calf productive performance and health.
Effects of an Herbal Antibiotics Source (HAnS) on metabolic parameters of calves
The evaluation of metabolic parameters () in serum samples from calves supplemented with HAnS showed considerable reduction in levels of glucose (linear, p < 0.01), B-OH butyrate (linear, p < 0.05) and urea (quadratic, p < 0.05). A decrease in serum albumin was also detected (linear, p = 0.05; quadratic, p < 0.05) in calves treated with 3–4 g d−1 of HAnS and, thus, there was also a reduction in the albumin/globulin ratio (quadratic, p < 0.05). Even though the serum bilirubin concentration increased slightly in calves that consumed HAnS (quadratic, p = 0.05), a decreasing trend was observed in aspartate aminotransferase levels (linear, p = 0.10). No changes were observed in the other evaluated parameters.
Table 2. Effect of an Herbal Antibiotics Source (HAnS) on blood serum biochemistry of calves.
Effects of an Herbal Antibiotics Source (HAnS) on blood cells of calves
The haematic biometry study () of blood cells from calves supplemented with HAnS showed no differences in the proportion of erythrocytes, platelets, lymphocytes, and eosinophils nor in the concentrations of hemoglobin and plasma proteins among the experimental groups. On the other hand, when other cells that participate in the immune response were measured in calves supplemented with HAnS, a quadratic increase in monocytes (p < 0.05) was detected with the dose of 5 g d−1 of HAnS and a linear increase in the number of basophils (p < 0.05) with the dose of 3 g d−1 of HAnS.
Table 3. Effect of an Herbal Antibiotics Source (HAnS) on blood cells of calves.
Serum titres of antibodies against Clostridium spp in calves supplemented with Herbal Antibiotics Source (HAnS)
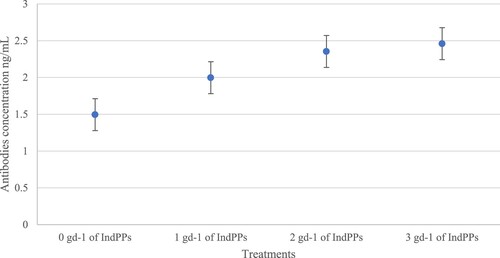
To further evaluate the immunological status of calves supplemented with HAnS, we performed antibody titres for antigens of Clostridium spp (), one of the pathogens that frequently affect claves during their growth. A linear increase in serum antibodies was detected in calves supplemented with 4 (p = 0.01) and 5 g d−1 (p < 0.05) of HAnS, relative to the control group.
Discussion
HAnS is an herbal extract composed of different medicinal plants native to India and East Asia (Kumar & Pandey, Citation2014). In the present study, twenty-one organic compounds were detected in HAnS, including thymol, eugenol, fatty acids, and vitamin E (). Previous studies (Silva-Júnior et al., Citation2020; Wall et al., Citation2014) provided solid evidence that several of the compounds in HAnS improve growth performance and feed intake and increase livestock immune response.
Results from in vitro (Castillejos et al., Citation2006; Fraser et al., Citation2007) and in vivo (Cardozo et al., Citation2006; Benchaar et al., Citation2006) studies on effects of the active component of thymol have been contradictory, and more research is needed to understand its effects on rumen fermentation and metabolism in dairy cattle. However, thymol has been found to have antimicrobial effects. Thymol has been attributed to disruption of the plasma membrane of bacteria and reduction in glucose uptake (Benchaar et al., Citation2008; Calsamiglia et al., Citation2007). It has been suggested that this occurs through the interaction of its carbonyl group with proteins in the periplasm, inactivating microbial enzymes (Vakili et al., Citation2013).
Based on in vitro rumen fermentation experiments, eugenol from herbal formulas improves the production of volatile fatty acids and nitrogen utilization by the rumen (Calsamiglia et al., Citation2007). Among the essential oils, eugenol has attracted much attention because of its potential antimicrobial activity against rumen microbes (Benchaar et al., Citation2012). Also, it has been reported in dairy cows that eugenol from herbal sources can increase dry matter intake and milk production (Wall et al., Citation2014). The vitamin E (α-tocopherol) status of dairy cattle is an important component of a well-functioning immune system of cows and young dairy calves because of its antioxidant effects (Kafilzadeh et al., Citation2014). Experimental reports of vitamin E from herbal formulas are few. Vitamin E content is high in fresh grass, but it decreases markedly during forage storage and conservation. For this reason, NRC (Citation2001) recommends that the entire vitamin E requirement be given via dietary supplements when conserved forages are fed. Extra supplementation may be useful during immune suppression periods, such as around calving (Kafilzadeh et al., Citation2014).
There was no significant effect of HAnS supplementation on ADG, final BW, or final wither height in the current study (). In contrast with our results, previous studies indicated that daily gain in feedlot lambs could be improved by 5% with dietary concentrations equivalent to 0.1 g kg−1 DM of HAnS (Hernández-Reyes et al., Citation2017). Other experimental tests showed that growth can increase by 11.1% with 1 g kg−1 DMI. However, 2 g kg−1 had reduced beneficial effects on different performance variables (Dorantes-Iturbide et al., Citation2019; Orzuna-Orzuna et al., Citation2019). This could indicate that the concentration of plant metabolites, such as terpenes, phenols, polyphenols, carotenoids, oligosaccharides, and vitamins, must be precise to obtain benefits from these phytogenic growth promoters (Cardinali et al., Citation2015; Dalle et al., Citation2016). Nevertheless, Jouany and Morgavi (Citation2007) concluded that these metabolites in greater amounts could cause negative or toxic effects in the organism (Jouany & Morgavi, Citation2007). Some of the plants that constitute HAnS have been evaluated individually with positive responses in lactating ewes (Jaguezeski et al., Citation2018) and growing lambs (Alade et al., Citation2010; Awohouedji et al., Citation2013).
There is no clear explanation of why HAnS decreased starter diet intake and increased milk consumption, as HAnS supplementation was offered through gelatins. In lambs, Hernández-Reyes et al. (Citation2017) did not observe changes in dry matter intake using 1.5 g d−1, while Orzuna-Orzuna et al. (Citation2019) reported an increment of 8% in dry matter intake in lambs supplemented with 1 or 2 g kg−1 DM. However, digestibility was not reported in either study. In non-ruminants, Shon et al. (Citation2004) observed an increase in feed digestibility using a herbal source similar to HAnS. The difference in results could be related to flavour and palatability, which could depend on the type and dose of the phytogenic source (Upadhaya & Kim, Citation2017). A few experimental studies have reported positive effects against bovine respiratory disease using herbal essential oils. In our experiment we observed fewer cases of pneumonia in the calves supplemented using HAnS. Amat et al. (Citation2019) observed that essential oils from an herbal formula (0.025%) reduced Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni in dairy calves. Spisni et al. (Citation2020) stated that oral administration of a herbal formula containing eugenol, thymol, eucalyptol, and menthol, may be related to an increase in antibacterial activity in the immune system of non-ruminants. However, not all bio-compounds from plants have been described, and data reported in the literature regarding antimicrobial activity of herbal formulas are minimal. Continued research with new studies focused on these interesting compounds is needed.
Several medicinal plants from India have shown hypoglycemic effects (Khosla et al., Citation2000) similar to those of other herbal products evaluated in calves (Sánchez-Hernández et al., Citation2019). El Barky et al. (Citation2017) reviewed the different hypoglycemic effects of different levels of plant saponins on insulin (modifying signalling, rejuvenation, release), glycogen synthesis, gluconeogenesis inhibition and reduction of mRNA gene expression of glycogen phosphorylase and glucose 6-phosphatase, and increased Glut4 expression. Other researchers interpret the lower glucose values in calves supplemented with phytogenics as an improved capacity to metabolize glucose (Lakhani et al., Citation2019). Like these experimental studies, our results showed lower serum glucose concentration in calves that received supplementation with HAnS, relative to calves without the supplement ().
The reduction in blood butyrate may indicate that phytogenic secondary metabolites could affect rumen microbiota development or have an indirect effect by reducing solid intake and causing slow rumen development. It has been recognized that VFA, particularly butyrate, affect papillary development (Mentschel et al., Citation2001). Differences in rumen development with milk only versus milk supplemented with forage or concentrates has been documented (Heinrichs & Lesmeister, Citation2004). Using a microarray approach, Connor et al. (Citation2013) have demonstrated that changes in feeding the growing calf can affect several gene networks involved in lipid metabolism, molecular transport, and cell proliferation, growth, and morphology of rumen epithelium (Connor et al., Citation2013). Additionally, secondary metabolites from the phytogenic could affect gene expression of proton-linked monocarboxylate transporters (MCT) (Nakamura et al., Citation2018; Wang et al., Citation2014). MCT 1 and 2 have been reported in rumen epithelial cells to transport short-chain fatty acids. Kirat et al. (Citation2006) stated that additives that can alter the gene expression of MCT1 in rumen might directly increase butyrate in the bloodstream.
The quadratic response in blood serum urea suggests that at intermediate doses protein efficiency and utilization were improved (Kohn et al., Citation2005), and there was better utilization of N in the rumen (Bruno et al., Citation2009) presumably as a result of greater ruminal microbial protein synthesis (Razavi et al., Citation2019). However, at the highest doses of phytogenics, blood urea nitrogen again increased, indicating negative effects associated with the lowest daily gain, even when the values were within the normal range (Issi et al., Citation2016; Nozad et al., Citation2012). Uric acid, a primary product of protein metabolism, was not affected (Eggum et al., Citation1982), and creatinine, a blood parameter of kidney function, remained unaffected (Karagul et al., Citation2000).
The changes observed in albumins and the A/G ratio cannot be related to liver damage or chronic or inflammatory response to HAnS because globulins were not elevated, calves were healthy, and HAnS reduced respiratory diseases (). Some secondary metabolites from herbal supplements containing polyphenolic compounds caused inhibitory effects on UGT1A9, the gene responsible for glucuronidation of bilirubin (Mohamed & Frye, Citation2011), which may alter the bilirubin concentrations, and other secondary metabolites could cause cross-reactions responsible for increasing bilirubin, rather than liver injury (Dufour et al., Citation2000).
The changes in monocytes and basophils were within the reference limits for calves (Roland et al., Citation2014) (). Therefore, our results do not indicate a pathologic process (Aslinia et al., Citation2006), bone marrow disorder, or autoimmune disease but rather suggest that some metabolites of the herbal mixture send stimuli to the immune system that are being interpreted as stress. This could be considered positive given that cattle have a small bone marrow reserve for granulocytes (Roland et al., Citation2014), and some leukocyte subpopulations may be stimulated. The AST activity we measured in calf blood serum tended to be greater in calves without HAnS. AST activity increased during the pathological processes associated with necrosis and subclinical acidosis in calves during solid feed intake. Following this increase in hepatic activity, AST is released into the bloodstream. AST concentration has been related to liver damage (Quezada-Tristán et al., Citation2014). An AST value between 5 and 40 U/L is considered normal (Huang et al., Citation2006). Our results fall within the normal range; however, it is interesting to observe a lower AST level in the calves with 3 g d−1 of HAnS.
In most of the little research on the effects of herbal sources on dairy calves, attention is focused on secondary metabolites (Frankič et al., Citation2009; Mendel et al., Citation2017), but herbal formulas also contain volatile compounds (Mendoza et al., Citation2019). In any case, herbal formulas are neutraceutical mixes that can reduce infections and diseases such as pneumonia. Another study reported a decrease in other diseases that most commonly occur during calving and calf growth (Gutiérrez et al., Citation2019). It may be that the herbal formulas act in multiple ways through nonspecific mechanisms of the phytogenic on immunomodulation, antioxidants, anti-inflammatories, antimicrobial compounds, intestinal microbiota modulation, and others that are being detected in gene expression modulation (Frankič et al., Citation2009; Upadhaya & Kim, Citation2017; Wang et al., Citation2014). Combining plants has additive effects since evaluations of individual plants do not show changes in haematological parameters (Bharti et al., Citation2004) or immune response (Chand et al., Citation2014).
Finally, the increase in antibodies by HAnS supplementation could be related to immune-stimulation through many pathways (). In contrast to antibiotics, herbal formulas and their essential oils contain different antibacterial agents that possibly employ several inhibitory mechanisms, making it difficult for pathogens to initiate resistance (Aljarallah, Citation2017). In non-ruminants, thymol has been reported to suppress pathogens in the small intestine without damaging beneficial commensal colonic bacteria (Aljarallah, Citation2017). Saleh et al. (Citation2018) reported that thymol negatively affected Salmonella typhimurium, Escherichia coli, Staphylococcus aureus, and Clostridium perfingers. These results suggest that the combination of antimicrobials contained in herb mixtures controls microflora in the gastrointestinal tract, which enhances feed digestibility and growth performance.
Regarding the plants included in the herbal formula HAnS, antihelmintic properties have been recognized in Adhatoda vasica, Piper longum, and Albizia lebbeck (D'Cruz et al., Citation1980; Khan et al., Citation2013; Ravikumar et al., Citation2009; Singh et al., Citation2008, Citation2009); Curcuma longa also has antihelmintic effects as well as anti-inflammatory and antioxidant properties (Cervantes-Valencia et al., Citation2016; Molosse et al., Citation2019). Boerhavia diffusa extracts have hypoglycemic and anti-inflammatory properties (Khan et al., Citation2013) and are hepatoprotective, nephroprotective, and immunomodulating (Khan et al., Citation2013; Kumar et al., Citation2018). Albizia lebbeck is analgesic, anti-infection, and anti-inflammatory (Duke & Bogenschutz, Citation1994). Solanum xanthocarpum has been reported to be antibacterial, antioxidant (Kumar & Pandey, Citation2014; Nithya et al., Citation2018), hypoglycemic, and hepatoprotective (Parmar et al., Citation2010). Hedychium spicatum is used as an analgesic, anti-inflammatory, antimicrobial, antioxidant, and antifungal, and has cytotoxic activity (Sravani & Paarakh, Citation2011).
Conclusion
We evaluated the effects of including HAnS in the early-life diet of calves on productive performance, health, blood serum biochemistry, blood cells, and antibodies. Significant changes in milk intake, cases of pneumonia, blood serum butyrate, blood serum albumin, and AST were attributed to the herbal formulas containing bioactive substances with phytogenic properties, which have a possible role in decreasing diseases. Supplementation with HAnS had no impact on growth, but it increased health parameters and antibody count and decreased disease incidence. Future research should focus on elucidating the effect of varying amounts of individual bio-compounds from herbal formulas on calving and calf early life, their metabolomic effect, and the subsequent health outcomes in adult life.
Acknowledgements
We are grateful to the owners and management of the dairy farms where we conducted this study. We are also grateful to Nuproxa for providing the Herbal Antibiotics Source.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alade, N. K., Dilala, M. A., & Abdulyekeen, A. O. (2010). Phenotypic and genetic parameter estimates of litter size and body weights in goats. International Journal of Science and Nature, 1(2), 262–266.
- Aljarallah, K. M. (2017). Conventional and alternative treatment approaches for Clostridium difficile infection. International Journal of Health Sciences, 11(1), 1–10.
- Amat, S., Baines, D., Timsit, E., Hallewell, J., & Alexander, T. (2019). Essential oils inhibit the bovine respiratory pathogens Mannheimia haemolytica, Pasteurella multocida and Histophilus somni and have limited effects on commensal bacteria and turbinate cells in vitro. Journal of Applied Microbiology, 126(6), 1668–1682. https://doi.org/10.1111/jam.14238
- Aslinia, F., Mazza, J. J., & Yale, S. H. (2006). Megaloblastic anemia and other causes of macrocytosis. Clinical Medicine & Research, 4(3), 236–241. https://doi.org/10.3121/cmr.4.3.236
- Assan, N. (2018). Plant based feed additives (phytogenic) as a primary solution to an antibiotic free nutritional program and feeding strategy in rabbit production. Scientific Journal of Animal Science, 7(3), 493–503. https://doi.org/10.14196/sjas.v7i3.2521
- Awohouedji, D. Y. G., Babatounde, S., Adounkpe, J. G., Houinato, M., & Hounzangbe-Adote, S. (2013). Supplementing panicum maximum with two medicinal forages in the diet of Djallonke sheep at the Benin national sheep center. Journal of Animal Science, 2(11), 284–295. https://doi.org/10.14196/sjas.v2i11.1025
- Beaver, A., Meagher, R. K., von Keyserlingk, M. A. G., & Weary, D. M. (2019). Invited review: A systematic review of the effects of early separation on dairy cow and calf health. Journal of Dairy Science, 102(7), 5784–5810. https://doi.org/10.3168/jds.2018-15603
- Benchaar, C., Calsamiglia, S., Chaves, A. V., Fraser, G. R., Colombatto, D., McAllister, T. A., & Beauchemin, K. A. (2008). A review of plant-derived essential oils in ruminant nutrition and production. Animal Feed Science and Technology, 145(1–4), 209–228. https://doi.org/10.1016/j.anifeedsci.2007.04.014
- Benchaar, C., Duynisveld, J. L., & Charmley, E. (2006). Effects of monensin and increasing dose levels of a mixture of essential oil compounds on intake, digestion, and growth performance of beef cattle. Journal of Animal Science, 86(1), 91–96. https://doi.org/10.4141/A05-027
- Benchaar, C., Lettat, A., Hassanat, F., Yang, W. Z., Forster, R. J., Petit, H. V., & Chouinard, P. Y. (2012). Eugenol for dairy cows fed low or high concentrate diets: Effects on digestion, ruminal fermentation characteristics, rumen microbial populations and milk fatty acid profile. Animal Feed Science and Technology, 178(3–4), 139–150. https://doi.org/10.1016/j.anifeedsci.2012.10.005
- Bharti, M. K., Dhuria, R. K., Sharma, T., & Garg, D. D. (2004). Haematobiochemical profile of magra sheep during feeding of sares (Albizia lebbeck) leaves. Veterinary Practice, 5(1), 48–50.
- Britton, R., Stock, R., Sindt, M., Oliveros, B., & Parrott, C. (1990). New feed additive and technique to evaluate acidosis in cattle. MP-University of Nebraska, Agricultural Experiment Station (USA).
- Bruno, R. G. S., Rutigliano, H. M., Cerri, R. L., Robinson, P. H., & Santos, J. E. P. (2009). Effect of feeding Saccharomyces Cerevisiae on performance of dairy cows during summer heat stress. Animal Feed Science and Technology, 150(3–4), 175–186. https://doi.org/10.1016/j.anifeedsci.2008.09.001
- Calsamiglia, S., Busquet, M., Cardozo, P. W., Castillejos, L., & Ferret, A. (2007). Invited review: Essential oils as modifiers of rumen microbial fermentation. Journal of Dairy Science, 90(6), 2580–2595. https://doi.org/10.3168/jds.2006-644
- Cardinali, R., Cullere, M., Dal Bosco, A., Mugnai, C., & Ruggeri, S. (2015). Oregano, rosemary, and vitamin E dietary supplementation in growing rabbits: Effect on growth performance, carcass traits, bone development and meat chemical composition. Livestock Science, 175, 83–89. https://doi.org/10.1016/j.livsci.2015.02.010
- Cardozo, P. W., Calsamiglia, S., Ferret, A., & Kamel, C. (2006). Effects of alfalfa extract, anise, capsicum and a mixture of cinnamaldehyde and eugenol on ruminal fermentation and protein degradation in beef heifers fed a high concentrate diet. Journal of Animal Science, 84(10), 2801–2808. https://doi.org/10.2527/jas.2005-593
- Castillejos, L., Calsamiglia, S., & Ferret, A. (2006). Effect of essential oil active compounds on rumen microbial fermentation and nutrient flow in in vitro systems. Journal of Dairy Science, 89(7), 2649–2658. https://doi.org/10.3168/jds.S0022-0302(06)72341-4
- Cervantes-Valencia, M. E., Alcalá-Canto, Y., Sumano-Lopez, H., Ducoing-Watty, A. M., & Gutierrez-Olvera, L. (2016). Effects of Curcuma longa dietary inclusion against Eimeria spp. in naturally infected lambs. Small Ruminant Research, 136, 27–35. https://doi.org/10.1016/j.smallrumres.2015.12.035
- Chand, N., Naz, S., Shah, Z., Khan, S., Ali, S. A., & Khan, R. U. (2014). Growth performance and immune status of broilers fed graded levels of Albizia lebbeck seeds. Pakistan Journal of Zoology, 46(2), 574–577.
- Connor, E. E., Baldwin, V. I. R. L., Li, C., Li, R. W., & Chung, H. (2013). Gene expression in bovine rumen epithelium during weaning identifies molecular regulators of rumen development and growth. Functional & Integrative Genomics, 13(1), 133–142. https://doi.org/10.1007/s10142-012-0308-x
- Crosby, M., Mendoza-Martínez, G. D., Relling, A., Vázquez, V. A., Lee-Rangel, H. A., Martínez, J. A., & Oviedo, M. (2017). Influence of supplemental choline on milk yield, fatty acid profile, and postpartum weight changes in suckling ewes. Journal of Dairy Science, 100(Suppl 2), 125.
- Dalle, Z. A., Celia, C., & Szendrő, Z. (2016). Herbs and spices inclusion as feedstuff or additive in growing rabbit diets and as additive in rabbit meat: A review. Livestock Science, 189, 82–90. https://doi.org/10.1016/j.livsci.2016.04.024
- D'Cruz, J. L., Nimbkar, A. Y., & Kokate, C. K. (1980). Evaluation of fruits of Piper longum and leaves of Adhatoda vasica nees for anthelmintic activity. Indian Drugs, 17(4), 99–101.
- Dorantes-Iturbide, G., Lara-Bueno, A., Orzuna-Orzuna, J. F., Mendoza-Martínez, G. D., Miranda-Romero, L. A., & Hernández-García, P. A. (2019). Efecto de la mezcla herbal Animunin en las características de la canal de corderos en finalización. Revista Acadêmica Ciência Animal, 17(Suppl 1), 183–185.
- Dufour, D. R., Lott, J. A., Nolte, F. S., Gretch, D. R., Koff, R. S., & Seeff, L. B. (2000). Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clinical Chemistry, 46(12), 2027–2049. https://doi.org/10.1093/clinchem/46.12.2027
- Duke, J., & Bogenschutz, M. J. (1994). Dr. Duke's phytochemical and ethnobotanical databases (pp. 1–8). USDA, Agricultural Research Service.
- Eggum, O. B., Juliano, O. B., & Maningat, C. C. (1982). Protein and energy utilization of rice milling fractions by rats. Plant Foods for Human Nutrition, 31(4), 371–376. https://doi.org/10.1007/BF01094048
- El Barky, A. R., Hussein, S. A., Alm-Eldeen, A. A., Hafez, A., & Mohamed, T. M. (2017). Saponins and their potential role in diabetes mellitus. Diabetes Management, 7(1), 148–158.
- Frankič, T., Voljč, M., Salobir, J., & Rezar, V. (2009). Use of herbs and spices and their extracts in animal nutrition. Acta Agriculturae Slovenica, 94(2), 95–102.
- Fraser, G. R., Chaves, A. V., Wang, Y., McAllister, T. A., Beauchemin, K. A., & Benchaar, C. (2007). Assessment of the effects of cinnamon leaf oil on rumen microbial fermentation using two continuous culture systems. Journal of Dairy Science, 90(5), 2315–2328. https://doi.org/10.3168/jds.2006-688
- Gutiérrez, A. R., Gutiérrez, A., Sánchez, C., & Mendoza, G. D. (2019). Effect of including herbal choline in the diet of a dairy herd; a multiyear evaluation. Emirates Journal of Food and Agriculture, 31, 477–481. https://doi.org/10.9755/ejfa.2019.v31.i6.1971
- Heinrichs, A. J., & Lesmeister, K. E. (2004, March 23–24). Rumen development in the dairy calf. Calf and heifer rearing: Principles of rearing the modern dairy heifer from calf to calving. 60th University of Nottingham Easter School in Agricultural Science, Nottingham, UK (pp. 53–65).
- Hernández-Reyes, J. C., Lara-Bueno, A., Miranda-Romero, L. A., Mendoza-Martínez, G. D., & Martínez-Gómez, D. (2017). Evaluación de productos herbales como aditivos en raciones de finalización de ovinos. Congreso Bienal AMENA.
- Huang, X. J., Choi, Y. K., Im, H. S., Yarimaga, O., Yoon, E., & Kim, H. S. (2006). Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors, 6(7), 756–782. https://doi.org/10.3390/s6070756
- Issi, M., Gül, Y., & Başbuğ, O. (2016). Evaluation of renal and hepatic functions in cattle with subclinical and clinical ketosis. Turkish Journal of Veterinary and Animal Sciences, 40, 47–52. https://doi.org/10.3906/vet-1505-16
- Jaguezeski, A. M., Perin, G., Bottari, N. B., Wagner, R., & Fagundes, M. B. (2018). Addition of curcumin to the diet of dairy sheep improves health, performance and milk quality. Animal Feed Science and Technology, 246, 144–157. https://doi.org/10.1016/j.anifeedsci.2018.10.010
- Jouany, J. P., & Morgavi, D. P. (2007). Use of “natural” products as alternatives to antibiotic feed additives in ruminant production. Animals, 1(10), 1443–1466. https://doi.org/10.1017/S1751731107000742
- Kafilzadeh, F., Kheirmanesh, H., Karami Shabankareh, H., Targhibi, M. R., Maleki, E., Ebrahimi, M., & Meng, G. Y. (2014). Comparing the effect of oral supplementation of vitamin E, injective vitamin E and selenium or both during late pregnancy on production and reproductive performance and immune function of dairy cows and calves. The Scientific World Journal, 2014, Article 165841. https://doi.org/10.1155/2014/165841
- Karagul, H., Altintas, A., Fidanci, U. R., & Sel, T. (2000). Klinik biyokimya (H. Karagül, ed.; pp. 161–164). Medisan Yayinlari.
- Kazemi-Bonchenari, M., Falahati, R., Poorhamdollah, M., Heidari, S. R., & Pezeshki, A. (2018). Essential oils improved weight gain, growth and feed efficiency of young dairy calves fed 18 or 20% crude protein starter diets. Journal of Animal Physiology and Animal Nutrition, 102(3), 652–661. https://doi.org/10.1111/jpn.12867
- Khan, M. S., Ansari, I. A., Ahmad, S., Akhter, F., Hashim, A., & Srivastava, A. K. (2013). Chemotherapeutic potential of Boerhavia diffusa Linn: A review. Journal of Applied Pharmaceutical Science, 3, 133–139. https://doi.org/10.7324/JAPS.2013.30126
- Khosla, P., Bhanwra, S., Singh, J., Seth, S., & Srivastava, R. K. (2000). A study of hypoglycaemic effects of Azadirachta indica (neem) in normal and alloxan diabetic rabbits. Indian Journal of Physiology and Pharmacology, 44(1), 69–74.
- Kirat, D., Masuoka, J., Hayashi, H., Iwano, H., Yokota, H., Taniyama, H., & Kato, S. (2006). Monocarboxylate transporter 1 (MCT1) plays a direct role in short-chain fatty acids absorption in caprine rumen. The Journal of Physiology, 576(2), 635–647. https://doi.org/10.1113/jphysiol.2006.115931
- Kohn, R. A., Dinneen, M. M., & Russek-Cohen, E. (2005). Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. Journal of Animal Science, 83(4), 879–889. https://doi.org/10.2527/2005.834879x
- Kumar, R., Gautam, S., Singh, K. D., Kumar, P., Haque, N., Yadav, V., Kumar, R., Diwakar, R. P., & Rishikant Kumar, S. B. (2018). Pharmacological properties of Boerhavia diffusa: A review. International Journal of Chemical Studies, SP4, 72–80.
- Kumar, S., & Pandey, A. K. (2014). Medicinal attributes of Solanum xanthocarpum fruit consumed by several tribal communities as food: An in vitro antioxidant, anticancer and anti-HIV perspective. BMC Complementary and Alternative Medicine, 14(1), 112. https://doi.org/10.1186/1472-6882-14-112
- Lakhani, N., Kamra, D. N., Lakhani, P., & Alhussien, M. N. (2019). Immune status and haemato-biochemical profile of buffalo calves supplemented with phytogenic feed additives rich in tannins, saponins and essential oils. Tropical Animal Health and Production, 51(3), 565–573. https://doi.org/10.1007/s11250-018-1727-z
- Maciel, P. R., Miranda, N. N. J., Restle, J., Oliveira, B. U., Chaves, M. R. F., João, F. A., Soares, F. C. M., & de Oliveira, A. R. (2016). Performance, rumen development, and carcass traits of male calves fed starter concentrate with crude glycerine. Revista Brasileira de Zootecnia, 45(6), 309–318. https://doi.org/10.1590/S1806-92902016000600005
- Mendel, M., Chłopecka, M., Dziekan, N., & Karlik, W. (2017). Phytogenic feed additives as potential gut contractility modifiers – a review. Animal Feed Science and Technology, 230, 30–46. https://doi.org/10.1016/j.anifeedsci.2017.05.008
- Mendoza, G. D., Oviedo, M. F., Pinos, J. M., Lee-Rangel, H. A., Vázquez, A., Flores, R., Pérez, F., Roque, A., & Cifuentes, O. (2019). Milk production in dairy cows supplemented with herbal choline and methionine. Revista de la Facultad de Ciencias Agrarias, 52(1), 332–343.
- Mentschel, J., Leiser, R., Műlling, C., Pfarrer, C., & Claus, R. (2001). Butyric acid stimulates rumen mucosa development in the calf mainly by a reduction of apoptosis. Archiv für Tierernaehrung, 55(2), 85–102. https://doi.org/10.1080/17450390109386185
- Mirman, D. (2014). Growth curve analysis and visualizationusing R. Chapman & Hall/CRC.
- Mohamed, M. E., & Frye, R. F. (2011). Inhibitory effects of commonly used herbal extracts on UDP-glucuronosyltransferase 1A4, 1A6, and 1A9 enzyme activities. Drug Metabolism and Disposition, 39(9), 1522–1528. https://doi.org/10.1124/dmd.111.039602
- Molosse, V., Souza, C. F., Baldissera, M. D., Glombowsky, P., & Campigotto, G. (2019). Diet supplemented with curcumin for nursing lambs improves animal growth, energetic metabolism, and performance of the antioxidant and immune systems. Small Ruminant Research, 170, 74–81. https://doi.org/10.1016/j.smallrumres.2018.11.014
- Nakamura, S., Haga, S., Kimura, K., & Matsuyama, S. (2018). Propionate and butyrate induce gene expression of monocarboxylate transporter 4 and cluster of differentiation 147 in cultured rumen epithelial cells derived from preweaning dairy calves. Journal of Animal Science, 96(11), 4902–4911. https://doi.org/10.1093/jas/sky334
- Nithya, M., Ragavendran, C., & Natarajan, D. (2018). Antibacterial and free radical scavenging activity of a medicinal plant Solanum xanthocarpum. International Journal of Food Properties, 21(1), 313–327. https://doi.org/10.1080/10942912.2017.1409236
- Nozad, S., Ramin, A. G., & Rezaie, S. A. (2012). Diurnal variations in milk macro-mineral concentrations in Holstein dairy cows in urmia, Iran. Veterinary Research Forum, 3(4), 281–285.
- NRC. (2001). Nutrient requirements of dairy cattle (7th rev ed.). National Research Council. Nat. Acad. Sci.
- Orzuna-Orzuna, J. F., Lara-Bueno, A., Dorantes-Iturbide, G., Mendoza-Martinez, G. D., Miranda-Romero, L. A., Hernández-García, P. A., & López-Ordaz, R. (2019). Efecto de la mezcla herbal Animunin en el comportamiento productivo de corderos en finalización. Revista Acadêmica: Ciência Animal, 17(Suppl 1), 180–182.
- Pardon, B., Hostens, M., Duchateau, L., Dewulf, J., De Bleecker, K., & Deprez, P. (2013). Impact of respiratory disease, diarrhea, otitis and arthritis on mortality and carcass traits in white veal calves. BMC Veterinary Research, 9(1), 79. https://doi.org/10.1186/1746-6148-9-79
- Parish, J. A., Bourg, B. M., Marks, M. L., Simmons, N. B., & Smith, T. (2012). Evaluation of different methods of cattle hip height data collection. The Professional Animal Scientist, 28(3), 292–299. https://doi.org/10.15232/S1080-7446(15)30358-2
- Parmar, S., Gangwal, A., & Sheth, N. (2010). Solanum xanthocarpum (yellow berried night shade): A review. Der Pharmacia Lettre, 2(4), 373–383.
- Quezada-Tristán, T., García-Flor, V. L., Ortiz-Martínez, R., Arredondo-Figueroa, J. L., Medina-Esparza, L. E., Valdivia-Flores, A. G., & Montoya-Navarrete, A. L. (2014). Biochemical parameters in the blood of Holstein calves given immunoglobulin Y-supplemented colostrums. BMC Veterinary Research, 10(1), 159. https://doi.org/10.1186/1746-6148-10-159
- Quigley, J. (2011). Direct fed microbials (probiotics) in calf diets (pp. 1–4). Bovine Alliance on Management and Nutrition.
- Ravikumar, B. R., Mohan, D., & Bhagwat, V. G. (2009). Efficacy study of styplon Vet bolus as supportive therapy in management of hemorrhagic conditions of ruminants. Veterinary World, 2(1), 470–471. https://doi.org/10.5455/vetworld.2009.470-471
- Razavi, S. A., Pourjafar, M., Hajimohammadi, A., Valizadeh, R., Naserian, A. A., Laven, R., & Mueller, K. R. (2019). Effects of dietary supplementation of bentonite and yeast cell wall on serum blood urea nitrogen, triglyceride, alkaline phosphatase, and calcium in high-producing dairy cattle during the transition period. Comparative Clinical Pathology, 28(2), 419–425. https://doi.org/10.1007/s00580-018-2849-4
- Rivera-Méndez, C., Plascencia, A., Torrentera, N., & Zinn, R. A. (2017). Effect of level and source of supplemental tannin on growth performance of steers during the late finishing phase. Journal of Applied Animal Research, 45(1), 199–203. https://doi.org/10.1080/09712119.2016.1141776
- Roland, L., Drillich, M., & Iwersen, M. (2014). Hematology as a diagnostic tool in bovine medicine. Journal of Veterinary Diagnostic Investigation, 26(5), 592–598. https://doi.org/10.1177/1040638714546490
- Roque-Jiménez, J. A., Mendoza-Martínez, G. D., Vázquez-Valladolid, A., Guerrero-González, M. D. L., Flores-Ramírez, R., Pinos-Rodriguez, J. M., Loor, J. J., Relling, A. E., & Lee-Rangel, H. A. (2020). Supplemental herbal choline increases 5-hmC DNA on whole blood from pregnant ewes and offspring. Animals, 10(8), 1277. https://doi.org/10.3390/ani10081277
- Saleh, A. A., Ebeid, T. A., & Abudabos, A. M. (2018). Effect of dietary phytogenics (herbal mixture) supplementation on growth performance, nutrient utilization, antioxidative properties, and immune response in broilers. Environmental Science and Pollution Research, 25(15), 14606–14613. https://doi.org/10.1007/s11356-018-1685-z
- Sánchez-Hernández, C., Castañeda-Gómez-del-Campo, J. A., Trejo-Castro, L., Mendoza-Martínez, G. D., & Gloria-Trujillo, A. (2019). Evaluation of a feed plant additive for coocidiosis control in broilers herbals for coccidiosis control. Brazilian Journal of Poultry Science, 21. https://doi.org/10.1590/1806-9061-2018-0846
- Seifzadeh, S., Aghjehgheshlagh, F. M., Hossein, A., Seifdavati, J., & Navidshad, B. (2017). The effects of a medical plant mix and probiotic on performance and health status of suckling Holstein calves. Ital. Journal of Animal Science, 16(1), 44–51. https://doi.org/10.1080/1828051X.2016.1249421
- Shon, K. S., Hong, J. W., Kwon, O. S., Min, B. J., Lee, W. B., Kim, J. H., Kim, I. H., & Kim, H. S. (2004). Effects of dietary Animunin powder® on growth performance and blood components in nursery and growing pigs. Journal of Animal Science and Technology, 46(3), 325–334. https://doi.org/10.5187/JAST.2004.46.3.325
- Silva-Júnior, C. D., Martins, C. C. S., Dias, F. T. F., Sitanaka, N. Y., Ferracioli, L. B., Moraes, J. E., Pizzolante, C. C., Budiño, F. E. L., Pereira, R., Tizioto, P., Paula, V. R. C., Coutinho, L. L., & Ruiz, U. S. (2020). The use of an alternative feed additive, containing benzoic acid, thymol, eugenol, and piperine, improved growth performance, nutrient and energy digestibility, and gut health in weaned piglets. Journal of Animal Science, 98(5), Article skaa119. https://doi.org/10.1093/jas/skaa119
- Singh, T. U., Kumar, D., & Tandan, S. K. (2008). Paralytic effect of alcoholic extract of Allium sativum and Piper longum on liver amphistome, gigantocotyle explanatum. Indian Journal of Pharmacology, 40(2), 64–68. https://doi.org/10.4103/0253-7613.41040
- Singh, T. U., Kumar, D., Tandan, S. K., & Mishra, S. K. (2009). Inhibitory effect of essential oils of Allium sativum and Piper longum on spontaneous muscular activity of liver fluke, Fasciola gigantica. Experimental Parasitology, 123(4), 302–308. https://doi.org/10.1016/j.exppara.2009.08.002
- Spisni, E., Petrocelli, G., Imbesi, V., Spigarelli, R., Azzinnari, D., Donati-Sarti, M., Campieri, M., & Valerii, M. C. (2020). Antioxidant, anti-inflammatory, and microbial-modulating activities of essential oils: Implications in colonic pathophysiology. International Journal of Molecular Sciences, 21(11), 4152. https://doi.org/10.3390/ijms21114152
- Sravani, T., & Paarakh, P. M. (2011). Hedychium spicatum buch. Ham. An overview. Pharmacologyonline Newsletter, 2, 633–642.
- Toghyani, M., Ghasemi, A., & Tabeidian, S. A. (2015). The effect of different levels of seed and extract of harmal (Peganum harmala L.) on immune responses of broiler chicks. International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering, 9(1), 51–54.
- Upadhaya, S., & Kim, I. H. (2017). Efficacy of phytogenic feed additive on performance, production and health status of monogastric animals – a review. Annals of Animal Science, 17(4), 929–948. https://doi.org/10.1515/aoas-2016-0079
- Vakili, A. R., Khorrami, B., Mesgaran, M. D., & Parand, E. (2013). The effects of thyme and cinnamon essential oils on performance, rumen fermentation and blood metabolites in Holstein calves consuming high concentrate diet. Asian-Australasian Journal of Animal Sciences, 26(7), 935–944. https://doi.org/10.5713/ajas.2012.12636
- Wall, E. H., Perry, H. D., Doane, S., Donkin, S., & Bravo, D. (2014). The effects of supplementation with a blend of cinnamaldehyde and eugenol on feed intake and milk production of dairy cows. Journal of Dairy Science, 97(9), 5709–5717. https://doi.org/10.3168/jds.2014-7896
- Wang, L., Waltenberger, B., Pferschy-Wenzig, E. M., Blunder, M., & Liu, X. (2014). Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review. Biochemical Pharmacology, 92(1), 73–89. https://doi.org/10.1016/j.bcp.2014.07.018