ABSTRACT
In this study, we evaluated the functionality of water extracts from the fruit of Actinidia polygama (APF) in macrophages. The APF is a medicinal plant belonging to Actinidiaceae, it has been reported to exert anti-inflammatory, analgesic and hypouricemic activities. However, the potential mechanism for the immune activation of the APF is still insufficient. So, we evaluated whether APF exerts immune activation activities and elucidated its potential mechanism in macrophages. The APF dose-dependently increased the production of immunomodulators in macrophages. The inhibition of Toll-like receptor 4 (TLR4) blocked APF-mediated production of immunomodulators in RAW264.7 cells. In addition, APF-mediated production of immunomodulators was attenuated by MAPKs and NF-κB inhibition in RAW264.7 cells. Also, we analyzed the sugars content of the APF. The contents of glucose, galactose and fructose were 3597, 904, 7582 mg/L, respectively. These results suggest that the APF may have great potential for the development of immunomodulatory drugs.
1. Introduction
In December 2019, in the Chinese Province of Wuhan the novel coronavirus has been identified in patients with atypical pneumonia characterized by fever, dry cough and progressive dyspnea. Worldwide, this coronavirus has rapidly led to severe lung inflammation, heart and kidney damage, and acute respiratory distress syndrome. Considering the current pandemic of coronavirus disease 2019 (COVID-19) where no effective preventive and curative medicine is available, a enhancing of immune system is one of the most important ways to prevention of COVID-19 (Jayawardena et al., Citation2020; Tufan et al., Citation2020).
The body’s immune response is mediated by internal and adaptive immune responses. The inherent immunity is an initial reaction to an external pathogen and is a non-specific response that occurs faster than adaptive immunity. In other words, the innate immune system is the body’s earliest barrier against foreign pathogens and tumour cells and plays protective and defensive roles. One of the innate immune cells, macrophages, generates cytotoxicity by producing effector molecules and exerting phagocytosis (Kim et al., Citation2009; Wang et al., Citation2020b). Therefore, immunity has emerged as an important strategy for improving the immune system in the body. Recently, attention has been focused on the development of natural products and dietary materials that can improve the immune potential in response to these demands (Chae, Citation2005; Geum et al., Citation2020).
Actinidia polygama (A. polygama) is a medicinal plant belonging to Actinidiaceae, it has been used to exert abdominal pain, rheumatoid arthritis and stroke (Kim et al., Citation2003; Park, Citation2016). The fruit of A. polygama (APF) is known to contain sugar, mucus, starch, protein, tannin, organic acid, vitamin C, vitamin A and vitamin P (Kang et al., Citation2003; Yu et al., Citation2016). In addition, the APF contains several monoterpenoids (aldehydes, alcohols, lactones and actinidine) and triterpenoids. These compounds have long been recognized as carriers of important physiological functions (Sashida et al., Citation1992; Shoyama et al., Citation1998). The APF has been reported to anti-inflammatory activity in induced-LPS macrophage cell line RAW264.7 (Kim et al., Citation2003). However, research on the action mechanism underlying the immuno-enhancing activity of APF is insufficient. Therefore, we investigated the immune-enhancing activity of f APF in RAW264.7 cells.
2. Materials and methods
2.1. Material
Thiazolyl blue tetrazolium bromide (MTT) was purchased from VWR life science (Radnor, PA, USA). Griess reagent, 2-(2-Amino-3-methoxyphenyl)-4H-1-benzopyran-4-one (PD98059, ERK1/2 inhibitor), 4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole (SB203580, p38 inhibitor), 1,9-Pyrazoloanthrone (SP600125, JNK inhibitor), (E)-3-(4-Methylphenylsulfonyl)-2-propenenitrile (BAY 11-7082, IKK inhibitor) and TAK-242 (Toll-like receptor 4 inhibitor, TLR4 inhibitor) were purchased from Sigma Aldrich (St. Louis, MO, USA). C29 (TLR2 inhibitor) was purchased from Bio Vision, Inc. (Milpitas, CA, USA).
2.2. Sample preparation
Actinidia polygama fruit was collected from Chungryeongsan, Sudong-myeon, Namyangju-si, Gyeonggi-do, Korea. 20 g of powdered APF was immersed in 400 ml of distilled water and then extracted for 48 h under stirring at 150 rpm at 4°C. After 48 h, the extracts were centrifuged for 10 min at 15,000 rpm, and then the supernatant was taken and lyophilized. The lyophilized water extracts from APF were stored at -80°C until use.
2.3. Cell culture and treatment
The mouse macrophage cell line, RAW264.7 cells were purchased American Type Culture Collection (ATCC, Virginia, USA) and grown at 37°C in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 1:1 Modified medium (Lonza, Walkersville, MD, USA) supplemented with 10% fetal bovine serum (FBS), penicillin (100 units/mL) and streptomycin (100 µg/mL) in a humidified atmosphere of 5% CO2. The sample was dissolved in dimethyl sulfide (DMSO) and treated in cells. DMSO was used as a vehicle and the final DMSO concentration did not exceed 0.1% (v/v).
2.4. Cell viability assay
Cell viability was performed by MTT assay. Briefly, RAW264.7 cells were seeded at a density of 1 × 106 cells/well in 12-well plate and incubated for 24 h. The RAW264.7 cells were treated with APF at the indicated concentrations for 24 h. Then, the RAW264.7 cells were incubated with 200 μl of MTT solution (1 mg/ml) for an additional 2 h. The resulting crystals were dissolved in DMSO. The formation of formazan was measured by reading absorbance at a wavelength of 570 nm using UV/Visible (Perkin Elmer, Norwalk, CT, USA).
2.5. Measurement of nitric oxide (NO) production
RAW264.7 cells were incubated 12-well plate for overnight. The RAW264.7 cells were pretreated with APF at the indicated concentrations for 24 h. NO level was evaluated by Griess assay. Briefly, 50 μl of the RAW264.7 cells culture supernatants were mixed with 50 μl of Griess reagent (Sigma Aldrich, St. Louis, MO, USA) and followed by reaction for 10 min at the room temperature. After 10 min, absorbance values were determined using a UV/Visible spectrophotometer (Perkin Elmer, Waltham, MA, USA) at 540 nm.
2.6. Reverse transcriptase-polymerase chain reaction (RT–PCR)
After treatment of APF, total RNA was extracted from RAW264.7 cells using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and total RNA (1 μg) was synthesized using a Verso cDNA Kit (Thermo Scientific, Pittsburgh, PA, USA) according to the manufacturer’s protocol. PCR was performed using PCR Master Mix Kit (Promega, Madison, WI, USA) and mouse primers for iNOS, COX-2, TNF-α, IL-6, IL-1β and GAPDH ().
Table 1. The sequence of oligonucleotide primers used for RT-PCR.
2.7. High performance liquid chromatography (HPLC) analysis
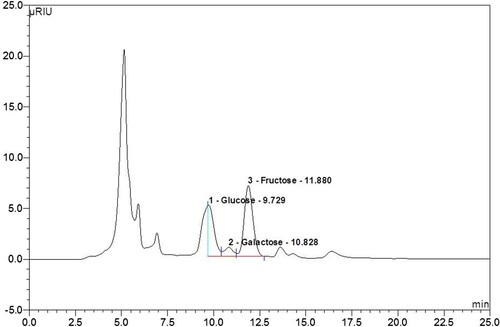
The free sugars contained in APF were analyzed by National Instrumentation Center for Environmental Management (NICEM) at Seoul National University (Seoul, Korea). Briefly, the analysis of the sugars contained in APF was performed using HPLC. In HPLC analysis, Dionex ultimate 3000 UHPLC system with Shodex RI-100 detector was used. The column was equipped with Sugar-pak (300 mm × 6.5 mm). The binary mobile phase consisted of distilled water. The flow rate was kept constant at 0.5 ml/min for a total runtime of 25 min. The injection volume of APF was 10 μl. The elution was monitored at 254 nm. The free sugars of APF were identified by the chromatogram of the analytical standards such as glucose, galactose and fructose.
2.8. Statistical analysis
All the data are shown as mean ± SD (standard deviation). Statistical analysis was determined by Student’s t-test. Differences with *P or #P < 0.05 were considered statistically significant.
3. Results and discussion
3.1. Effects of APF on the production of NO and cytokines in RAW264.7 cells
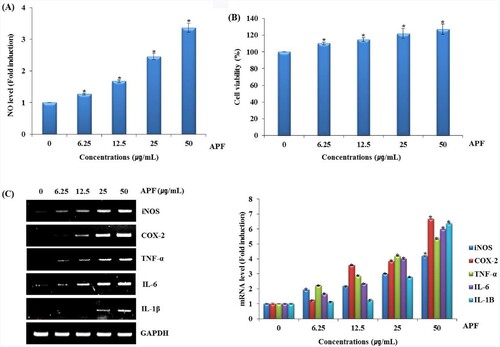
Immunity is the body’s defense against external factors, and the body’s immune system is sustained by interaction between various immune cells, such as monocyte, neutrophil, phagocyte and macrophage (Salminen et al., Citation2008). In the immune system, macrophage distinguished from monocytes plays an important role in host innate and adaptive immune responses as well as immunological homeostasis (Park et al., Citation2020). The nitric oxide (NO), produced by macrophages, is an important signalling agent that protects against tumour cells or infected microorganisms in the immune system (Duerksen-Hughes et al., Citation1992; Kim & Kang, Citation2008; Moncada & Higges, Citation1993; Vane et al., Citation1994). In the immune system, NO mediates host protection through either directly or indirectly regulating chemical modifications of important for the biological activity of innate and acquired immune cell lines (Gutierrez et al., Citation2009; Pavanelli & Silva, Citation2010). Typical cells involved in innate immunity include macrophages, dendritic cells, and natural killer cells, which produce cytokines to regulate immune cell activity and immune responses (Shin et al., Citation2020). Also, the production of NO and the secretion of cytokines such as cyclooxygenase-2 (COX-2), tumour necrosis factor (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) in macrophages such as RAW264.7 cells are indicators of immune function (Shen et al., Citation2017a). In particular, TNF-ɑ which is essential for innate immune response, can cause the release of other cytokines and activate and recruit immune cells (Li et al., Citation2017). To evaluate the effect of APF on the production of immunomodulators such as NO, iNOS, COX-2, TNF-α, IL-6 and IL-1β in RAW264.7 cells were treated with APF for 24 h. As shown in A, APF increased the production of NO in RAW264.7 cells. We investigated the cytotoxic effect of APF on RAW264.7 cells. As shown in B, APF enhanced the viability of RAW264.7 cells, which indicates that APF has no cytotoxic effect on RAW264.7 cells. Furthermore, APF dose-dependently activated mRNA expression of iNOS, COX-2, TNF-α, IL-6 and IL-1β in RAW264.7 cells (C). These results suggest that APF may induce macrophage activation.
3.2. The production of immunomodulators by APF is dependent on TLR4 in RAW264.7 cells
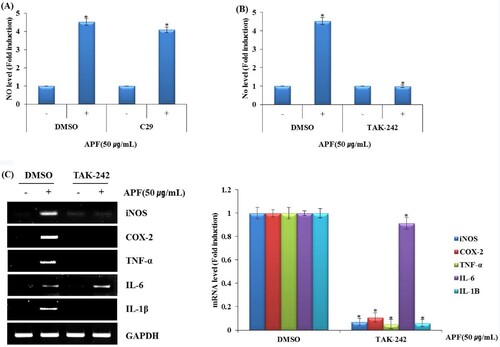
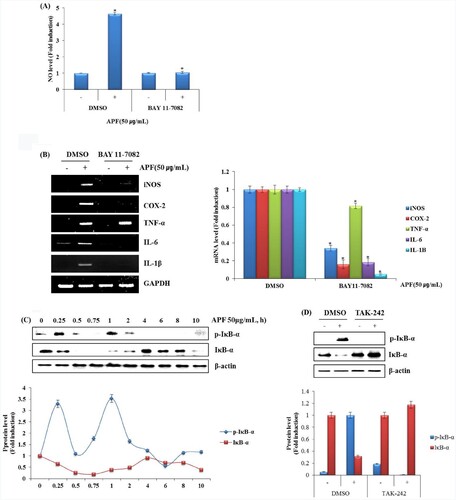
The Toll-like receptors (TLRs) have been reported to activate mitogen-activated protein kinases (MAPK) and nuclear factor-κB (NF-κB) signalling to induce the production of immunomodulators through the activation of macrophages (Shen et al., Citation2017b; Yang et al., Citation2017). The activation of MAPK and NF-κB is important for the subsequent development of innate immune responses as well as adaptive immune responses (Kawai & Akira, Citation2007). So, we investigated that the effects of TLR2 or TLR4 on the induction of immunomodulators by APF. As shown in A,B, the treatment of anti-TLR4 attenuated APF-induced NO production, but not the treatment of anti-TLR2 in RAW264.7 cells. In addition, the mRNA expressions of iNOS, COX-2, TNF-α, IL-6 and IL-1β induced by APF were blocked in RAW264.7 cells treated with anti-TLR4 (C). These results indicate that TLR4 may be a major receptor involved in the production of immunomodulators by APF. Thus, the effect of APF on the expression of TLR4 was investigated.
Figure 2. Effect of TLR2/TLR4 on APF-mediated production of immunomodulators in RAW264.7 cells. RAW264.7 cells were pretreated with C29 (TLR2 inhibitor, 10 μM) or TAK-242 (TLR4 inhibitor, 10 μM) for 2 h and co-treated with APF (50 μg/ml) for 24 h. (A) NO level (C29), (B) NO level (TAK-242), and (C) mRNA level (TAK-242) were measured by Griess assay (A and B) and RT-PCR (C), respectively. *p < 0.05 compared to the cells without the treatment.
3.3. MAPK signalling contributes to the production of immunomodulators by APF in RAW264.7 cells
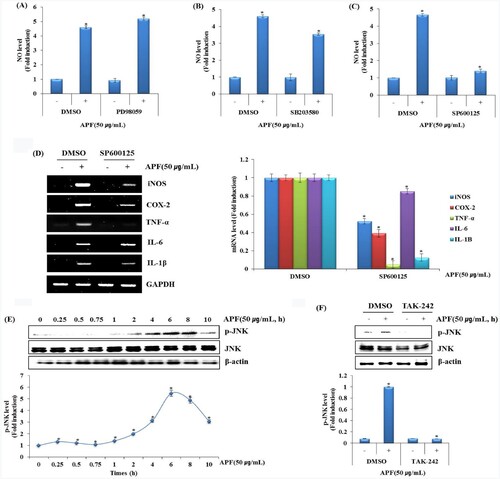
The immune system related pathways such as endocytosis, ubiquitin mediated proteolysis, MAPK signalling pathways, regulation of actin cytoskeleton, chemokine signalling pathways were included in the top 20 most significantly enriched signal pathways. Some other typical immune pathways such as TLR signal pathway and T cell receptor signalling pathway were also enhanced (Wang et al., Citation2020a). Especially, it is well known that the representative signalling related to the activation of macrophages is MAPK and NF-κB signalling pathways (Shin et al., Citation2019). MAPK signalling, mainly consisted of extracellular signal-regulated kinases 1/2 (ERK1/2), C-Jun N-terminal kinases (JNK) and p38-MAPK, which is known for their important role in switching on gene expression involved in TLR4-mediated immune response (Xu et al., Citation2020). Thus, the effects of MAPK signalling inhibition on APF-induced NO production were investigated in RAW264.7 cells. As shown in A,B, the inhibition of ERK1/2 by PD98059 or p38 by SB203580 did not affect NO production by APF. However, JNK inhibition by SP600125 dramatically inhibited NO production by APF (C). Because NO production by APF was dramatically suppressed by the inhibition of JNK, the mRNA expression of the immunomodulators such as iNOS, COX-2, TNF-α, IL-6 and IL-1β was investigated in RAW264.7 cells according to JNK inhibition (D). We confirmed that APF activated TLR4 and that JNK is a major upstream kinase involved in the production of immunomodulators in RAW264.7 cells. Thus, we first analyzed whether APF induces JNK activation. After treating APF for the indicated times in RAW264.7 cells, phosphorylation level of JNK was investigated by Western blot analysis. As a result, APF was found to phosphorylate JNK from 6 h after the treatment in RAW264.7 cells (E). And the effect of TLR4 on APF-induced JNK phosphorylation was investigated by Western blot analysis. As shown in F, inhibition of TLR4 by TAK-242 significantly attenuated APF-induced JNK phosphorylation. These results suggest that APF improves immunity by inducing activation of macrophages through activation of JNK.
Figure 3. Effect of MAPK signalling pathway on APF-mediated production of immunomodulators in RAW264.7 cells. RAW264.7 cells were pretreated with (A) PD98059 (ERK1/2 inhibitor, 40 μM), (B) SB203580 (p38 inhibitor, 40 μM) or (C) SP600125 (JNK inhibitor, 40 μM) for 2 h and then co-treated with APF (50 μg/ml) for 24 h. NO level was measured by the Griess assay. (D) RAW264.7 cells were pretreated with SP600125 (JNK inhibitor, 40 μM) for 2 h and then co-treated with APF (50 μg/ml) for 24 h. mRNA level was measured by the RT-PCR. (E) RAW264.7 cells were treated with APF (50 μg/ml) for the indicated times. Protein levels were measured by Western blot analysis. (F) RAW264.7 cells were pretreated with TAK-242 (TLR4 inhibitor, 10 μM) for 2 h and co-treated with APF (50 μg/ml) for 1 h. Protein levels were measured by the Western blot analysis. *p < 0.05 compared to the cells without the treatment.
3.4. NF-κB signalling contributes to the production of immunomodulators by APF in RAW264.7 cells
One of the primary physiological roles of NF-κB is in the immune system. Specifically, members of NF-κB family control the transcription of cytokines and antimicrobial effectors as well as genes that regulate cellular differentiation, survival and proliferation, thereby regulating various aspects of innate and adaptive immune responses. In addition, NF-κB contributes to the development and survival of the cells and tissues that performed immune responses in mammals (Chalmers et al., Citation2019; Hayden et al., Citation2006). NO production by APF was slightly attenuated in RAW264.7 cells pretreated with BAY 11-7082 (NF-κB inhibitor) (A). As shown in B, inhibition of NF-κB blocked the mRNA expression of iNOS, COX-2, IL-6, IL-1β and TNF-α by APF. We confirmed that APF activated TLR4 and that NF-κB is a major upstream kinase involved in the production of immunomodulators in RAW264.7 cells. Thus, we first analyzed whether APF induces NF-κB activation. After treating APF for the indicated times in RAW264.7 cells, phosphorylation level of NF-κB was investigated by Western blot analysis. As a result, APF was found to phosphorylate NF-κB from 1 h after the treatment in RAW264.7 cells (C). And the effect of TLR4 on APF-induced NF-κB phosphorylation was investigated by Western blot analysis. As shown in D, inhibition of TLR4 by TAK-242 significantly attenuated APF-induced NF-κB phosphorylation. These results suggest that APF promotes immunity by inducing activation of macrophages through activation of NF-κB.
Figure 4. Effect of NF-κB signalling pathway on APF-mediated production of immunomodulators in RAW264.7 cells. (A and B) RAW264.7 cells were pretreated with BAY 11–7082 (NF-κB inhibitor, 20 μM) for 2 h and then co-treated with APF (50 μg/ml) for 24 h. NO level was measured by the Griess assay and RT-PCR, respectively. (C) RAW264.7 cells were treated with APF (50 μg/ml) for the indicated times. Protein levels were measured by Western blot analysis. (D) RAW264.7 cells were pretreated with TAK-242 (TLR4 inhibitor, 10 μM) for 2 h and co-treated with APF (50 μg/ml) for 1 h. Protein levels were measured by the Western blot analysis. *p < 0.05 compared to the cells without the treatment.
3.5. The sugar contents analysis of APF using HPLC
The attention to polysaccharides in various biological activities of biochemical and nutritional researchers, particularly in immunostimulation and anti-tumour effects, resulted from the fault of traditional chemicals and hot research on functional foods. Therefore, to discover and evaluate polysaccharides as new safe chemicals or key compounds for functional foods has appeared as one of the important research fields in nutritional and biochemical nutritional sciences (Schepetkin et al., Citation2005; Zhao et al., Citation2005). A lot of polysaccharides were isolated from sea organisms, fungi, yeasts, mushrooms and plants. The plant-extracted polysaccharides are the best known and most potent immunomodulatory substances and have been shown to be clinically therapeutic (Schepetkin et al., Citation2005). So, we analyzed the sugars constituting the polysaccharides of APF using HPLC. As shown in , APF’s polysaccharides were 0.68% maltose, 46.43% glucose and 52.89% fructose. These results are thought to contribute to macrophage activation by measuring the content of polysaccharides contained in APF.
4. Conclusion
This study investigated the immune-enhancing effects and action mechanism of the APF extract in RAW 264.7 macrophages. Our results confirmed that the APF increased NO production and expression of the cytokines iNOS, COX-2, IL-1β, IL-6, and TNF-α in RAW264.7 cells. In conclusion, APF may potentially aid in the prevention of various immunoregulatory diseases via modulating the MAPK and NF-κB signalling pathways.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chae, S. W. (2015). Function and activation of NF-κB in immune system. Korean J Otolaryngol, 48, 284–288. https://www.kjorl.org/upload/pdf/0012005053
- Chalmers, S. A., Garcia, S. J., Reynolds, J. A., Herlitz, L., & Putterman, C. (2019). NF-κB signaling in myeloid cells mediates the pathogenesis of immunemediated nephritis. Journal of Autoimmunity, 98, 33–43. https://doi.org/10.1016/j.jaut.2018.11.00
- Duerksen-Hughes, P. J., Day, D., Laster, S. M., Zachariades, N. A., Aquino, L., & Gooding, L. R. (1992). Both tumornecrosis factor and nitric oxide participate in lysis of simian virus 40 transformed cells by activated macrophages. Journal of Immunology, 149(6), 2114–2122. https://www.jimmunol.org/content/149/6/2114.long
- Geum, N. G., Eo, H. J., Kim, H. J., Park, G. H., Son, H. J., & Jeong, J. B. (2020). Immune-enhancing activity of Hydrangea macrophylla subsp. serrata leaves through TLR4/ROS-dependent activation of JNK and NF-κB in RAW264.7 cells and immunosuppressed mice. Journal of Functional Foods, 73, 104139. https://doi.org/10.1016/j.jff.2020.104139
- Gutierrez, F. R. S., Mineo, T. W. P., Pavanelli, W. R., Guedes, P. M. M., & Silva, J. S. (2009). The effects of nitric oxide on the immune system during Trypanosoma cruzi infection. Memórias do Instituto Oswaldo Cruz, 104(Suppl. 1), 236–245. https://doi.org/10.1590/S0074-02762009000900030
- Hayden, M. S., West, A. P., & Ghosh, S. (2006). NF-κB and the immune response. Oncogene, 25(51), 6758–6780. https://doi.org/10.1038/sj.onc.1209943
- Jayawardena, R., Sooriyaarachchi, P., Chourdakis, M., Jeewandara, C., & Ranasinghe, P. (2020). Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 14(4), 367–382. https://doi.org/10.1016/j.dsx.2020.04.015
- Kang, H. J., Kim, U. K., Choi, G. J., & Chung, S. H. (2003). Hypouricemic activity of Actinidia polygama. Yakhak Hoeji, 47(5), 307–310. http://www.koreascience.or.kr/article/JAKO200311922266205
- Kawai, T., & Akira, S. (2007). Signaling to NF-κB by toll-like receptors. TRENDS in Molecular Medicine, 13(11), 460–469. https://doi.org/10.1016/j.molmed.2007.09.002
- Kim, H. S., & Kang, J. S. (2008). Preparation and characteristics of bread by medicinal herb composites with immunostimulating activity. Journal of the Korean Society of Food Science and Nutrition, 37(1), 109–116. https://doi.org/10.3746/jkfn.2008.37.1.109
- Kim, M. H., Byon, Y. Y., Ko, E. J., Song, J. Y., Yun, Y. S., Shin, T., & Joo, H. G. (2009). Immunomodulatory activity of ginsan, a polysaccharide of Panax ginseng, on dendritic cells. The Korean Journal of Physiology and Pharmacology, 13(3), 169–173. https://doi.org/10.4196/kjpp.2009.13.3.169
- Kim, Y. K., Kang, H. J., Lee, K. T., Choi, J. G., & Chung, S. H. (2013). Anti-Inflammation activity of Actinidia polygama. Culinary Science & Hospitality Research, 26, 1061–1066. https://doi.org/10.20878/cshr.2016.22.2.011011
- Li, Y., Meng, T., Hao, N., Tao, H., Zou, S., Li, M., Ming, P., Ding, H., Dong, J., Feng, S., Li, J., Wang, X., & Wu, J. (2017). Immune regulation mechanism of Astragaloside IV on RAW264.7 cells through activating the NF-κB/MAPK signaling pathway. International Immunopharmacology, 49, 38–49. https://doi.org/10.1016/j.intimp.2017.05.017
- Moncada, S., & Higges, A. (1993). The L-arginine-nitric oxide pathway. New England Journal of Medicine, 329(27), 2002–2012. https://doi.org/10.1056/nejm199312303292706
- Park, E. J. (2016). Quality characteristics of muffin added with Actinidia polygama powder. Culinary Science & Hospitality Research, 22(2), 125–135. https://doi.org/10.20878/cshr.2016.22.2.011011
- Park, H. E., Do, K. H., & Lee, W. K. (2020). The immune-modulating effects of viable weissella cibaria JW15 on RAW 264.7 macrophage cells. The Journal of Biomedical Research, 34(1), 36–43. https://doi.org/10.7555/jbr.33.20190095
- Pavanelli, W. R., & Silva, J. J. N. (2010). The role of nitric oxide in immune response against Trypanosoma cruzi infection. The Open Nitric Oxide Journal, 2(1), 1–6. https://doi.org/10.2174/1875042701002010001
- Salminen, A., Huuskonen, J., Ojala, J., Kauppinen, A., Kaarniranta, K., & Suuronen, T. (2008). Activation of innate immunity system during aging: NF-κB signaling is the molecular culprit of inflamm-aging. Ageing Research Reviews, 7(2), 83–105. https://doi.org/10.1016/j.arr.2007.09.002
- Sashida, Y., Ogawa, K., Mori, N., & Yamanouchi, T. (1992). Triterpenoids from the fruit galls of Actinidia polygama. Phytochemistry, 31(8), 2801–2804. https://doi.org/10.1016/0031-9422(92)83634-B
- Schepetkin, I. A., Faulkner, C. L., Nelson-Overton, L. K., Wiley, J. A., & Quinn, M. T. (2005). Macrophage immunomodulatory activity of polysaccharides isolated from Juniperus scopolorum. International Immunopharmacology, 5(13–14), 1783–1799. https://doi.org/10.1016/j.intimp.2005.05.009
- Shen, C. Y., Zhang, W. L., & Jiang, J. G. (2017a). Immune-enhancing activity of polysaccharides from Hibiscus sabdariffa Linn. via MAPK and NF-κB signaling pathways in RAW264.7 cells. Journal of Functional Foods, 34, 118–129. https://doi.org/10.1016/j.jff.2017.03.060
- Shen, T., Wang, G., You, L., Zhang, L., Ren, H., Hu, W., Qiang, Q., Wang, X., Ji, L., & Gu, Z. (2017b). Polysaccharide from wheat bran induces cytokine expression via the toll-like receptor 4-mediated p38 MAPK signaling pathway and prevents cyclophosphamideinduced immunosuppression in mice. Food & Nutrition Research, 61(1), 1344523. https://doi.org/10.1080/16546628.2017.1344523
- Shin, J. S., Han, H. S., Lee, S. B., Myung, D. B., Lee, K., Lee, S. H., & Lee, K. T. (2019). Chemical constituents from leaves of Hydrangea serrata and their anti-photoaging effects on UVB-irradiated human fibroblasts. Biological and Pharmaceutical Bulletin, 42(3), 424–431. https://doi.org/10.1248/bpb.b18-00742
- Shin, J. S., Park, J. J., & Jun, W. J. (2020). Effect of hot water extract of Salvia plebeia R. on the production of splenocyte cytokines in mouse. Journal of the Korean Society of Food Science and Nutrition, 49(7), 754–758. https://doi.org/10.3746/jkfn.2020.49.7.754
- Shoyama, Y., Chen, S., Tanaka, H., Sasaki, Y., & Sashida, Y. (1998). Actinidia polygama (Japanese name Matatabi): In vitro culture, micropropagation, and the production of monoterpenes and triterpenoids. Medicinal and Aromatic Plants X, 41(41), 1–13. https://doi.org/10.1007/978-3-642-58833-4_1
- Tufan, A., Avanoglu-Guler, A., & Matucci-Cerinic, M. (2020). COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turkish Journal of Medical Sciences, 50(SI-1), 620–632. https://doi.org/10.3906/sag-2004-168
- Vane, J. R., Mitchell, J. A., Appleton, I., Tomlinson, A., Bailey, D. B., Croxtal, J., & Willloughb, D. A. (1994). Inducible isoforms of cyclooxygenase and nitric oxide synthetase in inflammation. Proceedings of the National Academy of Sciences, 91(6), 2046–2050. https://doi.org/10.1073/pnas.91.6.2046
- Wang, W., Wang, L., Liu, Z., Song, X., Yi, Q., Yang, C., & Song, L. (2020a). The involvement of TLR signaling and anti-bacterial effectors in enhanced immune protection of oysters after Vibrio splendidus pre-exposure. Developmental and Comparative Immunology, 103, 103498. https://doi.org/10.1016/j.dci.2019.103498
- Wang, Y., Zhang, Q., Chen, Y., Liang, C. L., Liu, H., Qiu, F., & Dai, Z. (2020b). Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomedicine & Pharmacotherapy, 121, 109570. https://doi.org/10.1016/j.biopha.2019.109570
- Xu, Z., Lin, R., Hou, X., Wu, J., Zhao, W., Ma, H., Fan, Z., Li, S., Zhu, Y., & Zhang, D. (2020). Immunomodulatory mechanism of a purified polysaccharide isolated from Isaria cicadae Miquel on RAW264.7 cells via activating TLR4-MAPK-NF-κB signaling pathway. International Journal of Biological Macromolecules, 164, 4329–4338. https://doi.org/10.1016/j.ijbiomac.2020.09.035
- Yang, Y., Xing, R., Liu, S., Qin, Y., Li, K., Yu, H., & Li, P. (2017). Immunostimulatory effects of sulfated chitosans on RAW264.7 mouse macrophages via the activation of PI3K/Akt signaling pathway. International Journal of Biological Macromolecules, 108, 1310–1321. https://doi.org/10.1016/j.ijbiomac.2017.11.042
- Yu, M. H., Chae, I. G., Choi, J. H., Im, H. G., Choi, H. D., Yang, S. A., Lee, J. H., & Lee, I. S. (2016). Effects of supercritical fluid marc extracts from Actinidia polygama Max. on inflammation and atherosclerosis. Culinary Science and Hospitality Research, 22(2), 125–135. http://www.koreascience.or.kr/article/JAKO201611758626858
- Zhao, G., Kan, J., Li, Z., & Chen, Z. (2005). Characterization and immunostimulatory activity of an (1–>6)-a-D-glucan from the root of Ipomoea batatas. International Immunopharmacology, 5(9), 1436–1445. https://doi.org/10.1016/j.intimp.2005.03.012