ABSTRACT
Houttuynia cordata fermented drinks (HCFDs) are dietary liquid supplements widely marketed and consumed for immune enhancement. In this study, the acute oral toxicity and immunostimulatory activity of the selected lyophilized HCFD in healthy and cyclophosphamide-induced immunosuppressed rats were evaluated. The lyophilized selected commercialized HCFD was safe in animal model as there was no acute oral toxic death at up to 2000 mg/kg in male Wistar rats. After 14 days of treatment, in immunosuppressed rats, lyophilized HCFD enhanced rat body weight gain and significantly increased spleen index at a dose of 100 mg/kg. Moreover, B-cell proliferation and anti-sheep red blood cell antibody production were significantly increased at doses of 100 and 200 mg/kg, respectively. The delayed-type hypersensitivity response was significantly stimulated at a dose of 200 mg/kg. These findings exhibited the immunostimulatory activity of the lyophilized HCFD by enhancing the immune responses in both humoral and cell-mediated immunities in immunosuppressed rats.
1. Introduction
Immunodeficiency or immunocompromisation is a lack of ability to protect our body from viruses, bacteria and parasites. Immunodeficiency can be revealed to be the failure of one or more parts of the immune system. Secondary immunodeficiency disorders may be caused by several factors, such as a specific immune deficiency (AIDS), cancer, genetic disorders, medicine or treatments with anticancer drugs or chemotherapy (Fuller & Manford, Citation2010). An anticancer drug that is effective in the treatment of malignant cells may have side effect or immunosuppression. Cyclophosphamide (CTX) is one of an antineoplastic and immunosuppressive agent and is widely used for anticancer, the treatment of solid and hematological malignancies as well as severe autoimmune diseases. Long-term treatment with CTX in patients with cancer causes a major side effect, immunosuppression (Shirani et al., Citation2015). Both humoral and cellular immunities are suppressed by CTX. At cellular level, CTX leads to triggering of apoptosis and induces the cytotoxic effect on murine lymphocytes (Kumar & Venkatesh, Citation2016). Moreover, a high dose of CTX shows some changes in animals, such as decrease in body weight, relative weights of spleen and thymus, B-cell and T-cell proliferation, antibody production and delayed-type hypersensitivity (DTH) reaction (Hassan et al., Citation2016).
There is a rising interest in finding and advancement of nontoxic and effective chemotherapeutic substances and it has been reported that many medicinal plants, and their active ingredients can alleviate the toxic side effects of CTX as an alternative chemotherapy (Shirani et al., Citation2015). Furthermore, natural products are an alternative potential to chemotherapies for many illnesses, particularly when the host defense system requires to be triggered in immune response (Sharma et al., Citation2012). Herbal medicines and dietary therapies are regularly used as an alternative medicine for the treatment in conjunction with conventional medicine or after the conventional treatment (Poonthananiwatkul et al., Citation2015).
Advantages of fermented foods or drinks include the new desirable tastes and textures, which are quite different from the starting materials. Fermentation can also result in new compounds with health-modulating potentials (Marco et al., Citation2017). Specifically, Houttuynia cordata fermented drink (HCFD), is one of the commercially available dietary liquid supplements in Thailand. HCFD is made from Houttuynia cordata Thunb. (HC), an aromatic medicinal herb with a unique fishy smell, which is indigenous to Asia, including China, India, Korea, Japan and Thailand. HC is also rich in bioactive compounds, including volatile oils, organic acids, phenolic compounds and flavonoids (Yang & Jiang, Citation2009). HC has been described to have many biological activities such as antiviral (Cheng et al., Citation2019; Chiow et al., Citation2016; Hung et al., Citation2015; Meng et al., Citation2015), anticancer (Lai et al., Citation2010; Yanarojana et al., Citation2017), antiinflammation (Lu et al., Citation2006; Park et al., Citation2005; Shin et al., Citation2010) and immunostimulation (Lau et al., Citation2008; Lee et al., Citation2008; Satthakarn et al., Citation2015; Wigraiboon et al., Citation2016). Thus, liquid dietary supplements containing botanical ingredients from plant or fruit juice may also contain pharmacologically active constituents (Lobb, Citation2012).
Nowadays, there are a few scientific reports of safety and the HCFD biological activities of those commercially available HCFDs. The industrialized HCFD has been shown to possess anticancer activities against several cancer cell lines, which may be due to a function of their phenolic compound constituent (Senawong et al., Citation2015). Recently, the industrialized HCFD was reported to exhibit the anti-inflammation activities in both RAW 267.4 cell lines and Wistar rats (Woranam et al., Citation2020). Owing to the sour taste and strong smell of HCFD, it was transformed and developed into a lyophilized capsule for easier consumption. However, the biological activities of this lyophilized form of HCFD remains to be investigated. In this study, we aimed to study the acute oral toxicity and immunomodulatory activity of the HCFD lyophilized powder in both healthy and immunosuppressed Wistar rats. The adaptive immune responses, both humoral and cell-mediated immune responses, such as antibody production, lymphocyte activation and DTH reaction in immunosuppressed rats were investigated. Furthermore, the effects of HCFD on rat body weight, immune organ and hematological parameters were also demonstrated.
2. Materials and methods
2.1. Materials and reagents
Levamisole hydrochloride was purchased from AppliChem, Germany. Cyclophosphamide monohydrate used as an immunosuppressant was obtained from Enzo Life Sciences, Inc., New York, USA. Lipopolysaccharide (LPS), Concanavalin A (Con A) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich. The selected HCFD (Houttuynia fermented liquid supplement lot no. 14052015) was provided by the Prolac (Thailand) Co., Ltd., in Lamphun Province, Thailand, through the Research and Researcher for Industry (RRi) project, Thailand Research Fund (TRF). Primary cell culture materials including RPMI 1640 medium, fetal bovine serum (FBS), trypsin-EDTA and penicillin/streptomycin were obtained from Gibco/Invitrogen Crop. Fresh sheep blood in Alsever’s solution (1:1) was purchased from Salaya Pet Hospital, Nakhon Pathom, Thailand.
2.2. Sample preparation
The selected HCFD was prepared as lyophilized powder for testing acute oral toxicity and immunomodulatory activity in animal model. The HCFD was centrifuged at 3000 rpm for 15 min at 4°C and filtered using filter paper (Whatman no. 4). Aqueous solution of HCFD was lyophilized using a freeze-dryer. The yield of lyophilized HCFD was 23.00 ± 3.40 mg/mL. The lyophilized HCFD powder was freshly dissolved in normal saline for animal treatment.
2.3. Experimental animals and ethics statement
Male Wistar rats (250–300 g), 6–8 weeks old, were used in this study. Animals were obtained from the National Laboratory Animal Center, Mahidol University, Nakhon Pathom, Thailand and Nomura Siam International Co., Ltd., Bangkok, Thailand. Animals were housed and maintained under standard environmental conditions at temperature 23 ± 2°C under a 12-h light:12-h dark cycle at Northeast Laboratory Animal Center, Khon Kaen University, Khon Kaen, Thailand. Animals were provided standard pelleted diet and water ad libitum. Rats were deprived of food except water overnight prior the experiments. Animal study was registered with the Institutional Animal Care and Use Committee of Khon Kaen University, Khon Kaen, Thailand, on 29/05/2014 (AEKKU 17/2557), and were performed according to the guidelines established by the Ethical Principles and Guidelines for the Use of Animals for scientific purposes, National Research Council of Thailand. The study was carried out in compliance with the ARRIVE guidelines.
2.4. Acute oral toxicity
Acute oral toxicity of lyophilized HCFD was tested in animal model following the Organization of Economic Co-operation and Development (OECD) guideline for testing of chemicals 423, Acute Toxic Class Method (OECD, Citation2001). Three male Wistar rats were used per step in a stepwise procedure. The starting dose was 300 mg/kg body weight up to 2,000 mg/kg at a constant volume of 4 mL/kg of body weight. All rats were observed individually after dosing at least once during the first 30 min, occasionally during the first 24 h and daily thereafter, for a total of 14 days, and humanely killed for animal welfare reasons.
2.5. Antigen
Sheep red blood cells (SRBCs) were prepared from fresh sheep blood for immunization and challenge. Fresh sheep blood was washed five times with sterile phosphate buffer saine. SRBCs were adjusted to 10% (v/v) with normal saline for immunization and hemagglutination assay or 0.5 × 109 cells/mL in normal saline for DTH assay (Rasheed et al., Citation2016) by counting the cells using hematocytometer.
2.6. Immunization and treatment
The male Wistar rats were randomly divided to 10 groups (n = 6), five groups for healthy rats and five groups for immunosuppressed rats. For immunosuppressed rats, three days prior to immunization with antigen, the rats were injected intraperitoneally (i.p.) with cyclophosphamide (100 mg/kg). All rats were immunized i.p. with 0.2 mL of 10% SRBCs on days 0 and 7 (Bafna & Mishra, Citation2006). The treatment groups for both healthy and immunosuppressed rats were administered orally for 14 days with normal saline (4 mL/kg) for control group or vehicle control, levamisole 100 mg/kg for positive control (Rasheed et al., Citation2016) and the different doses (100, 200 and 400 mg/kg) of lyophilized HCFD for the test group. Rats were weighed daily. At 24 h after the last administration, all rats were humanely killed with overdosing Nembutal. Whole blood samples were collected from cardiac puncture and used for complete blood counting, analysis of biochemical parameters and hemagglutination assay. The treated rat spleens were aseptically removed and used for lymphocyte proliferation assay (ex vivo). For DTH assay, rats were divided as described above. All rats were immunized with SBRCs on day 0 and pretreated orally for seven days. At 24 h after last administration, all rats were used for determining DTH responses.
2.7. Complete blood count
A complete blood count (CBC) is a blood test used to measure several components and features of blood, including WBC, RBC, HGB, HCT and PCT. The whole blood was aliquoted 1 mL to microcentrifuge tube containing 20 µL of 0.1 M EDTA. The CBC was assessed by an automated hematological analyzer (Sysmex Xs-800i) and serviced by the Laboratory of Community Medical Laboratory, Faculty of Associated Medical Sciences, Khon Kaen University, Thailand.
2.8. Analysis of serum biochemical parameters
The biochemical evaluation was studied from serum samples to measure the side effect/toxicity on main organs, including kidney and liver. After 14 days of treatment, serum was separated from whole blood and biochemical parameters for liver and kidney functions were determined. Creatinine, BUN (blood urea nitrogen), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were assessed by a Chemistry Analyzer DxC 600 (Beckman Coulter Inc., Brea, CA, USA) serviced by the Laboratory of Community Medical Laboratory, Faculty of Associated Medical Sciences, Khon Kaen University, Thailand.
2.9. Lymphocyte proliferation assay (ex vivo)
Splenic lymphocytes were prepared from the spleen of experimental rats after 14 days of treatment, and the lymphocyte proliferation assay was performed using MTT assay. Briefly, the splenic lymphocytes were isolated from each treated rat spleen. The isolated splenic lymphocytes were plated at 3 × 105 cells/well in 96-wells plates in complete RPMI 1640 medium in the absence or presence of the Con A (5 μg/mL) or LPS (10 μg/mL). After 48 h, the cultures were incubated with MTT solution (5 mg/mL) for 4 h. DMSO was used to dissolve formazan and the absorbance was read at 550 nm using a microplate reader (EZ Read 2000; Cambridge, UK). The absorbance at 655 nm was used as a reference wavelength. The proliferative response was expressed as a stimulation index (SI): ratio of mean A550–A655 of triplicate cultures with stimulated-mitogen to triplicate cultures without stimulated-mitogen.
2.10. Hemagglutination assay
Blood samples were collected from individual animals and used for the determination of anti-SRBC antibody levels. Whole blood was collected on day 7 by tail vein puncture as primary antibody and on day 14 by cardiac puncture after humanely killed for secondary antibody. Serum was separated from blood, and the antibody level was determined by the hemagglutination technique. Briefly, two-fold dilutions of individual serum samples were made in 50 µL volume of normal saline in a microtitration plate (U-bottom) to which were added 50 µL volume of 2% suspension of SRBCs in normal saline. After mixing, the plates were incubated at 37°C for 1 h and examined for hemagglutination. The reciprocal of the highest dilution of the serum showing 50% agglutination was noted as the antibody titre. The data were shown as the mean ± standard deviation of the mean (SD) of log-2 titre.
2.11. DTH response
DTH response was determined by inducing immunogenic response using SRBCs in rat footpad with some modifications of previous protocol (Doherty, Citation1981). After pretreatment with lyophilized HCFD and immunization with SRBCs, all rats were induced paw edema by injecting 50 µL of SRBCs (0.5 × 109/mL) into the sub-plantar region of the right hind footpad. The volume of right hind footpad was measured using plethysmometer (Ugo Basile Model 7140, Italy) before injection as control and after 24, 48 and 72 h of challenge with SRBCs. The percentage of DTH response is the difference in footpad volume (mL) between before and after challenged with the antigen.
2.12. Data analysis
Data are expressed as mean ± standard deviation of the mean (SD). Statistical differences were calculated by one-way analysis of variance (ANOVA) followed by Dunnett’s test for comparison with the vehicle group. The data were compiled and statistically analyzed using GraphPad Prism 6.
3. Results
3.1. Acute toxicity of HCFD lyophilized powder
The acute oral toxicity of the supplement HCFD in animal was tested following OECD guideline 423. The starting dose was 300 mg/kg body weight. The single dose of lyophilized HCFD did not affect the mortality and morbidity of the animal throughout the study period (14 days) up to the dose of 2000 mg/kg. Our data demonstrated that lyophilized HCFD was safe for male Wistar rats. According to GSH (OECD) guideline 423, the toxicity of lyophilized HCFD was classified into category 5 (2000–5000 mg/kg), with an LD50 cut-off value of 2500 mg/kg, indicating that the lyophilized HCFD was of relatively low acute toxicity hazard. According to this result, three doses of the lyophilized HCFD (100, 200 and 400 mg/kg) were assigned for the investigation on its immunomodulatory activity in Wistar rats throughout the study.
3.2. Effect of lyophilized HCFD on SRBCs-induced antibody production in healthy and immunosuppressed rats
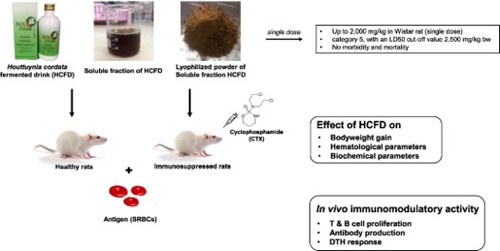
The effects of lyophilized HCFD on antibody production against SRBC antigen in healthy and immunosuppressed rats were presented as log-2 antibody titres for both primary and secondary antibodies in . In healthy rats, the levamisole (100 mg/kg; a positive control as an immunomodulator and immunoenhancer) and lyophilized HCFD at a dose of 400 mg/kg caused the increases in primary antibody production with no statistically significant differences when compared with the control, while lyophilized HCFD at doses of 100 and 200 mg/kg caused no statistically significant changes on SRBC-induced antibody production of both primary and secondary antibodies ((A)). CTX caused a significant reduction of only primary antibody production ((B)). The lyophilized HCFD at a dose of 200 mg/kg caused a significant increase in antibody titre when compared with CTX-Vehicle in both primary and secondary antibodies. This finding indicated that the lyophilized HCFD could enhance the antibody production against SRBC antigen in immunosuppressed rats.
Figure 1. Effect of lyophilized HCFD on antibody production as presented by antibody titre of treated rats for 14 days with different doses of lyophilized HCFD on healthy (A) and immunosuppressed rats (B). Control group received normal saline 4 mL/kg, while CTX-Vehicle group received cyclophosphamide (100 mg/kg) alone. CTX-Lev100 group received CTX (100 mg/kg) and levamisole (100 mg/kg), whereas CTX-HCFD100, CTX-HCFD200 and CTX-HCFD400 received CTX (100 mg/kg) and lyophilized HCFD of 100, 200 and 400 mg/kg, respectively. Results were expressed in log-2 antibody titre of primary and secondary antibodies as mean ± SD from six rats each group. No significant difference (ns) and ##p < .01 significant difference were expressed when compared between control group and CTX-Vehicle. **p < .01 significant difference was expressed when compared with CTX-Vehicle. Statistical significance was determined using one-way ANOVA followed by Dunnett’s multiple comparisons test.
3.3. Effect of lyophilized HCFD on body weight and immune organ in healthy and immunosuppressed rats
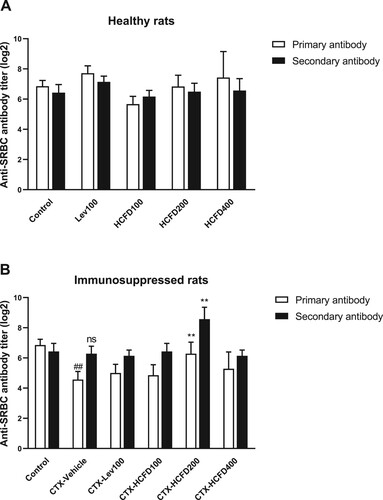
Body weight changes are responsive factors of adverse effects of drugs and chemicals (Santos et al., Citation2009; Wang et al., Citation2014). Spleen is a secondary lymphoid organ that is responsible for destroying pathogens and maintaining lymphocytes. The spleen weight is also recognized as an important index for nonspecific immunity (Chen et al., Citation2012). The body weight gain (g) and spleen index in healthy and immunosuppressed rats immunized with SRBCs were determined and the results are shown in . Treatments with levamisole and lyophilized HCFD (100–400 mg/kg) caused a reduction in body weight gains in healthy rats ((A)). However, no changes in the spleen index values were observed in healthy rats except for those fed with lyophilized HCFD at a dose of 400 mg/kg where spleen index was decreased significantly ((B)). In CTX-induced immunosuppressed rats (CTX-Vehicle), a significant decrease in body weight gain after the 14-day treatment was observed when compared with the control group (healthy rats) ((C)). In contrast, the body weight gains in immunosuppressed rats fed with lyophilized HCFD of 100 and 200 mg/kg were significantly increased when compared with CTX-Vehicle group ((C)). The rats treated with CTX alone (CTX-Vehicle group) did not show a significant change in spleen index when compared with the control group ((D)). The spleen index values of immunosuppressed rats fed with levamisole (100 mg/kg) and lyophilized HCFD (100, 200 and 400 mg/kg) were not significantly different when compared with that of CTX-Vehicle group ((D)). Nonetheless, the spleen index of immunosuppressed rats fed with 100 mg/kg lyophilized HCFD was increased significantly when compared with that of CTX-Vehicle group ((D)).
Figure 2. Effect of HCFD on body weight gain (g) and spleen index of treated rats for 14 days with different doses of lyophilized HCFD on healthy rats (A and B) and immunosuppressed rats (C and D). Control group received normal saline 4 mL/kg, while CTX-Vehicle group received cyclophosphamide (100 mg/kg) alone. CTX-Lev100 group received CTX (100 mg/kg) and levamisole (100 mg/kg), whereas CTX-HCFD100, CTX-HCFD200 and CTX-HCFD400 received CTX (100 mg/kg) and lyophilized HCFD of 100, 200 and 400 mg/kg, respectively. Results were expressed in body weight gain (g) and spleen index as mean ± SD from 6 rats each group. No significant difference (ns) and ####p < .0001 significant difference were expressed when compared between control group and CTX-Vehicle. *p < .05, **p < .01 and ****p < .0001 significant differences were expressed when compared with control group in healthy rats or CTX-Vehicle in immunosuppressed rats. Statistical significance was determined using one-way ANOVA followed by Dunnett’s multiple comparisons test.
3.4. Effect of lyophilized HCFD on hematological parameters in healthy and immunosuppressed rats
After administration of lyophilized HCFD for 14 days, blood samples were collected, and the hematological parameters were measured. The effects of lyophilized HCFD on hematological parameters in healthy and immunosuppressed rats immunized with SRBCs were presented in and , respectively. In healthy rats (), levamisole (100 mg/kg) caused a significant reduction in hematocrit (HCT), mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) but still in normal range (Clinical Laboratory Parameters for Crl:WI(Han) Rats - Charles River., Citation2008), while lyophilized HCFD at a dose of 400 mg/kg showed a significant reduction in both MCV and MCH. In immunosuppressed rats (), CTX caused decrease in white blood cells (WBC) and lymphocytes (LYM) as well as increase in basophils (BASO), red cell distribution width in standard deviation and coefficient of variation (RDW-SD, RDW-CV), platelet distribution width (PDW), mean platelet volume (MPV), platelet-large cell ratio (P-LCR) and plateletcrit (PCT). Levamisole (a positive control immunostimulant) caused a reduction in BASO and mean corpuscular hemoglobin concentration (MCHC) but increase in monocytes (MONO) (). Treatments with lyophilized HCFD generated some variations in hematological parameters of immunosuppressed rats. At a dose of 100 mg/kg, MCHC was significantly decreased, while MCV was significantly increased. At a dose of 400 mg/kg, the parameters including WBC, RDW-CV, PDW, MPV, P-LCR, PCT, LYM, MONO, neutrophils (NEU) and eosinophils (EO) were increased significantly, whereas MCV and MCH were decreased. Both BASO cell count and percentage were significantly decreased in all lyophilized HCFD-treated groups when compared with CTX-Vehicle group.
Table 1. Effect of lyophilized HCFD on hematological parameters in healthy rats after a 14-day treatment.
Table 2. Effect of lyophilized HCFD on hematological parameters in immunosuppressed rats after a 14-day treatment.
3.5. Effect of lyophilized HCFD on serum biochemical parameters in healthy and immunosuppressed rats
The serum biochemical parameters on liver and kidney functions in healthy and immunosuppressed rats treated with HCFD are shown in . There was no significant difference in creatinine level in both healthy and immunosuppressed rats. The BUN levels were significantly decreased in healthy rats (treatments with 100 mg/kg of levamisole and 400 mg/kg of HCFD) and in immunosuppressed rats (CTX-vehicle treatment) when compared with the control groups. Regarding the liver function parameters, the AST levels were significantly decreased at HCFD doses of 100 and 200 mg/kg in healthy rats. Additionally, the significant increases in ALP levels were observed in immunosuppressed rats at HCFD doses of 100 and 200 mg/kg. Moreover, the ALT level was significantly increased in a healthy rat group treated with the highest dose of HCFD (400 mg/kg) compared with the control group, and the ALT value was found above the normal range (Clinical Laboratory Parameters for Crl:WI(Han) Rats - Charles River., Citation2008).
Table 3. Effect of lyophilized HCFD on serum biochemical parameters in healthy and immunosuppressed rats after a 14-day treatment.
3.6. Effect of lyophilized HCFD on lymphocyte proliferation (ex vivo) in healthy and immunosuppressed rats
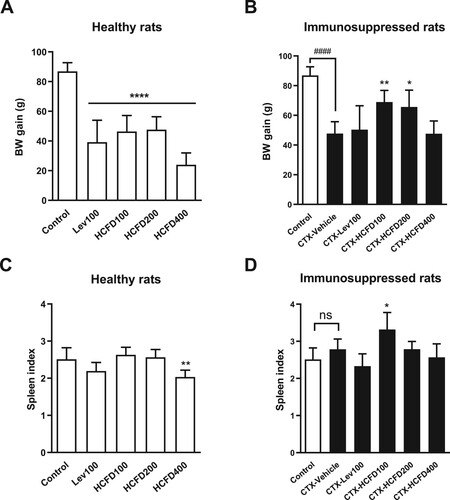
The lymphocyte proliferation is one of the parts in lymphocyte activation by mitogens or antigens on cell-mediated and humoral immune responses. The splenic lymphocytes were isolated from healthy and immunosuppressed rats. The effects of lyophilized HCFD on lymphocyte proliferation were shown as SI in . In healthy rats, levamisole (100 mg/kg) and lyophilized HCFD (100 and 400 mg/kg) caused a significant reduction in both T-cell and B-cell proliferations (A and B, respectively), while lyophilized HCFD (200 mg/kg) caused a non-significant reduction in both T-cell and B-cell proliferations ((A) and (B), respectively) when compared with the control group. In immunosuppressed rats, CTX caused a significant reduction in both T-cell and B-cell proliferations when compared with the control group. All groups treated with positive control (levamisole) and lyophilized HCFD did not show any changes in T-cell proliferations when compared with CTX-Vehicle ((C)). However, lyophilized HCFD at a dose of 100 mg/kg caused a significant increase in B-cell proliferation when compared with CTX-Vehicle ((D)).
Figure 3. Effect of lyophilized HCFD on proliferation (ex-vivo) of T cells (A and C) and B cells (B and D) isolated from healthy (A and B) and immunosuppressed (C and D) rats treated with different doses of lyophilized HCFD for 14 days. Control group received normal saline 4 mL/kg, while CTX-Vehicle group received cyclophosphamide (100 mg/kg) alone. CTX-Lev100 group received CTX (100 mg/kg) and levamisole (100 mg/kg), whereas CTX-HCFD100, CTX-HCFD200 and CTX-HCFD400 received CTX (100 mg/kg) and lyophilized HCFD of 100, 200 and 400 mg/kg, respectively. Results were expressed as SI (mean ± SD) from 6 rats each group. No significant difference (ns), *p < .05, **p < .01 and ***p < .001 indicated non-significant and significant differences when compared with control group (A and B). ns and ***p < 0.001 indicated non-significant and significant difference, respectively, when compared with CTX-Vehicle (C and D), whereas ####p < .0001 indicated significant difference when compared with control group (C and D). Statistical significances were determined using one-way ANOVA followed by Dunnett’s multiple comparisons test.
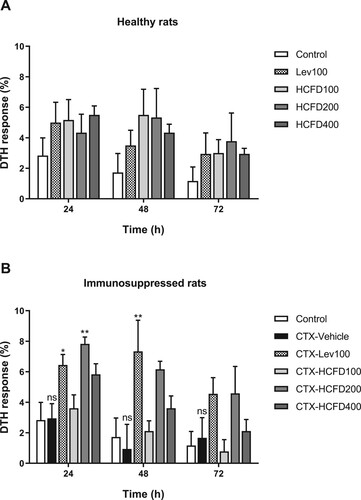
3.7. Lyophilized HCFD stimulated SRBC-induced DTH response in immunosuppressed rats
The activation of cell-mediated immune response by lyophilized HCFD was detected by DTH response (Rasheed et al., Citation2016). Rat paw edema was induced by injecting SRBCs into footpad and footpad volume was measured and calculated to be DTH response (%) after induction at each time. In healthy rats, levamisole (100 mg/kg) and lyophilized HCFD (100, 200 and 400 mg/kg) caused a non-significant increase in DTH responses ((A)). In immunosuppressed rats, no significant difference between control and CTX-Vehicle was observed at all times of detection ((B)). Levamisole caused a significant increase in DTH response at 24 and 48 h and caused a non-significant increase in SRBC-induced DTH response at 72 h when compared with CTX-Vehicle ((B)). Treatment with lyophilized HCFD of 100 mg/kg did not cause a significant change in SRBC-induced DTH response at all times of detection ((B)). However, treatment with lyophilized HCFD of 200 mg/kg caused a significant increase in SRBC-induced DTH response at 24 h and caused non-significant increases in SRBC-induced DTH response at both 48 and 72 h, indicating that cell-mediated immune response was stimulated ((B)). Moreover, treatment with lyophilized HCFD of 400 mg/kg caused a non-significant increase in SRBC-induced DTH response at all times of detection ((B)).
Figure 4. Effect of lyophilized HCFD on DTH response presented as percentage of DTH response in healthy (A) and immunosuppressed rats (B) treated with different doses of lyophilized HCFD for 7 days. Control group received normal saline 4 mL/kg, while CTX-Vehicle group received cyclophosphamide (100 mg/kg) alone. CTX-Lev100 group received CTX (100 mg/kg) and levamisole (100 mg/kg), whereas CTX-HCFD100, CTX-HCFD200 and CTX-HCFD400 received CTX (100 mg/kg) and lyophilized HCFD of 100, 200 and 400 mg/kg, respectively. Values are mean ± SEM of DTH response (%) at 24, 48 and 72 h., n = 6 (only CTX-HCFD 200, n = 4). ns indicated non-significant difference when compared between control and CTX-Vehicle. *p < .05 and **p < .01 indicated significant differences when compared with CTX-Vehicle. Statistical significances were determined using one-way ANOVA followed by Dunnett’s multiple comparisons test.
4. Discussion
In Thailand, some of the immunosuppressed patients are using the commercialized HCFDs as a dietary liquid supplement to cure their health and immunity along with the conventional medicine treatment. These products are believed to benefit the consumers in absorption of the bioactive ingredients digested by the microorganism during the fermentation process. The HPLC fingerprints of phenolic acids in aqueous solution of HCFD were determined for lot-to-lot quality control. A reference chromatographic fingerprint as a typical HPLC chromatogram of the HCFD samples (Lot no. 14052015) was partially identified based on the availability of phenolic acid standards and shown as Supplementary Figure S1. The predominant phenolic acid found in phenolic profiles was syringic acid which has been reported to possess the potential anti-inflammatory and anti-arthritic effects (Chanda & Juvekar, Citation2018) and hepatoprotective effect (Itoh et al., Citation2010). Fermentation can also increase the availability of bioactive ingredients such as secondary metabolites from both plants and probiotics. Fermentation of H. cordata (HC) by lactic acid bacteria caused an increase in the content of flavonoids (Kwon & Ha, Citation2012) and polyphenolic compounds (Wang et al., Citation2018). The fermentation process of HC with lactic acid bacteria enhanced phenolic acid content such as vanillic and caffeic acids (Lee et al., Citation2018). The extensive use of these products without scientific testing on their biological properties urged us to investigate the safety and immunomodulatory potential of the selected commercialized HCFD in animal models.
A previous animal study reported that the HC water extract of fresh plant was verified as safe with oral administration at 16 g/kg to the laboratory animals (Lau et al., Citation2008). Moreover, the fermented HC juice did not affect the behavioural character, internal organs, body mass, hematological and biochemical parameters of experimental rats, and the consumption of fermented HC juice was safe to rodent model system up to the concentration of 9 mL/kg/day (Chaiyasut et al., Citation2018). In this study, we examined the acute oral toxicity of the lyophilized form of a selected HCFD in male Wistar rats. The lyophilized HCFD was found safe at a dose of 2000 mg/kg which was classified into category 5 (2000-5000 mg/kg), with an LD50 cut-off value of 2500 mg/kg. The dose used in a Wistar rats could be calculated to a dose based on surface area for humans by multiplying 2500 mg/kg (male Wistar rat dose) by the Km factor (6) for a rat and then dividing by the Km factor (37) for a human (Reagan-Shaw et al., Citation2007). This calculation results in a human equivalent dose of 400 mg/kg HCFD lyophilized powder, which equates to a 24 g dose of HCFD lyophilized powder for a 60-kg adult. The 24 g of lyophilized HCFD was approximately 1043 mL of liquid HCFD. The supplements at this dose (24 g) or less may be available in pill or capsule form for more easily consumption.
In immunosuppressed rats immunized with SRBCs, CTX at a dose of 100 mg/kg caused a reduction in body weight gain. Lyophilized HCFD at doses of 100 and 200 mg/kg could improve rat body weight gain. This finding suggests that rich bioactive ingredients digested by microorganism may improve the absorption of foods by rats and thus increasing the rat’s weight. In immunosuppressed rats, CTX did not affect the spleen function. However, lyophilized HCFD could stimulate the spleen size as increased spleen index was observed at a dose 100 mg/kg. The increased spleen index represented improvement of spleen activity in rats (Aghili et al., Citation2014). In general, the best way to boost the immune system is to include foods naturally rich in nutrients and vitamins. However, the over supplementation could be detrimental such as toxicity, inhibition of phagocytes and obesity in relation to food excess. As observed in this study, treatment with lyophilized HCFD at a dose of 400 mg/kg did not show the beneficial effect on body weight and immune organ weight.
The side effect/toxicity of lyophilized HCFD on main organs of the experimental animals was investigated by analyzing the serum biochemical parameters to assess changes in the hepatic and renal profiles (). The key markers of kidney function are serum creatinine and BUN parameters. The incremental values of these parameters are symptomatic of kidney injury (Akindele et al., Citation2014). However, our results revealed that the highest dose (400 mg/kg) of HCFD caused a significant decrease in BUN level in the healthy rats with no significant change in creatinine level (). The decrease in only BUN level might be due to dietary and physiological conditions (Hosten, Citation1990). The most common parameters used to evaluate the liver function are ALP, AST (aspartate aminotransferase) and ALT (alanine aminotransferase). AST is mostly found in a variety of tissues, including the liver, brain, pancreas, heart, kidneys, lungs and skeletal muscles, whereas ALT is mainly found in the liver. The decreased level of AST may reflect an increased cardiovascular risk related to vitamin B6 deficiency, advanced chronic kidney or liver diseases, and inflammatory diseases (Ndrepepa, Citation2021). ALT is the most sensitive serum marker enzyme for liver damage. In this study, the increased ALT levels were observed in the healthy rats treated with the highest dose (400 mg/kg) of HCFD (), suggesting a significant indication of liver injury or damage. ALP is an enzyme produced by several tissues, including bones, liver, bile ducts and intestine. High ALP levels in the blood are usually due to a liver disorder or increased activity of the bone cells (Lab Tests Online, Citation2020). In this study, in immunosuppressed rats, the significant increases in ALP levels at doses of 100 and 200 mg/kg were observed but still within the normal limits (). However, the high level of ALP was also reported for its capability to reduce inflammation (Presbitero et al., Citation2018). In addition, the intraperitoneal immunization with SRBC caused no toxicity on both liver and kidney of the healthy rats (supplementary Table S1). The investigation on toxicity of HCFD by histopathological assessment of key organs, especially liver is still required in a future study.
The hematopoietic system is one of the targets for toxicity testing of the compounds or substances and plays a key index of physiological and pathological status in human and animal studies (Mukinda & Syce, Citation2007). In this study, SRBC was used as foreign antigen to immunize the rats and the immunomodulatory activity was tested after treatment with lyophilized HCFD. In SRBC-sensitized animals, SRBC antigen primarily develops and spreads to the extravascular space through lymphatic system to the lymph nodes (Solanki & Jain, Citation2010). Leucopenia refers to decrease in the number of WBC, LYM and platelet counts, which may be caused by CTX via alkylation of functional groups in cellular proteins and restraining medulla hematopoietic function (Kumar & Venkatesh, Citation2016). In this study, CTX was used to induce the immunosuppression, causing a decrease in the numbers of WBC and lymphocytes but with no significant changes in the platelet count (). After treatment with HCFD, the increasing numbers of neutrophils and EO were observed in immunosuppressed rats. Neutrophils are significant effector cells in the innate immune response (Mayadas et al., Citation2014). The increased numbers of neutrophils in blood circulation normally occur due to the infections and injuries, and the inflammatory reaction also causes an increase in neutrophil levels. Furthermore, the number of EO in blood is shown to enlarge in the specific immune responses such as allergic diseases (Huang et al., Citation2014). EO also play a role in sustenance of the increased numbers of peripheral B cells, and encouragement of B-cell survival, proliferation and immunoglobulin secretion by a contact-independent mechanism (Wong et al., Citation2014). Moreover, lyophilized HCFD caused a reduction in the number of BASO that was induced by CTX-immunosuppression (). Basophils are the least common granulocytes in the peripheral blood and have been shown to contribute to many human disease states, including allergic disease (hypersensitivity responses and asthma), autoimmunity, inflammatory disorders and cancer (acute and chronic myelogenous leukemia) (Siracusa et al., Citation2013). The reduction of high levels of basophils could reduce the allergy activation. Previous study demonstrated that the fresh HC extract caused a reduction in the release of histamine from the cells. This may apply its anti-allergic activity through down-regulation of FcepsilonRI expression (Shim et al., Citation2009). Further studies are needed to elucidate the mechanism and action of basophils associated with HCFD consumption.
Proliferation of T and B lymphocytes is a response to specific mitogen stimulation. Spleen lymphocyte proliferation induced by Con A was used as a method to evaluate T-lymphocyte activity, while that induced by LPS was used to examine B-lymphocyte activity (Han et al., Citation1998). In this study, the spleen cells of healthy and CTX-induced immunosuppressed rats were used to study the effect of lyophilized HCFD on lymphocyte proliferation (ex vivo). The lyophilized HCFD treatments caused no significant changes on T-cell proliferations. In contrast, lyophilized HCFD at a dose of 100 mg/kg caused an increase of SI in immunosuppressed rats ((D)), indicating that lyophilized HCFD could activate B-cell proliferation. B cells are one of the crucial components of adaptive immune response. Their differentiation into either specific memory B cells or antibody-secreting plasma cells is a consequence of activation steps that involve the processing and presentation of antigens (Yuseff et al., Citation2013). Further studies are needed to explain the stimulation of B cells by lyophilized HCFD.
Antibody production is a process to protect our body, specifically to antigen in humoral immune response. Antibody acts as the effector of the humoral immune response by binding to the antigens and neutralizing them or elimination by cross linking to form clusters which are digested by phagocytes (Nfambi & Sembajwe, Citation2015). Antibody production in response to a specific antigen involves several cellular actions, including antigen processing and presenting, recognition of the presented antigen, and activation and production of cytokines that enlarge the response of memory B cells. At a first or primary response after exposure to the antigen (SRBCs), IgM is initially secreted followed by IgG (Solanki & Jain, Citation2010). After second exposure to the same antigen, a secondary response is activated and characterized by a rapid and high level of antibody production (nearly all as IgG). According to our findings, the treatment with lyophilized HCFD improved the hemagglutination antibody titre, suggesting that lyophilized HCFD may support the enlargement of humoral response to SRBCs. The increased B-cell proliferation and hemagglutination titre observed in this study indicated that HCFD improved and recovered humoral immunity during immunosuppression.
DTH response is one of the important cell-mediated immune responses. DTH reactions are antigen-specific cell-mediated immune responses and play a role in many inflammatory disorders (Góngora et al., Citation2000). DTH reaction to SRBCs in rats is exquisitely sensitive to a wide variety of environmental suppressors. Thus, increased DTH reactions in rats are connected with the T-cell-dependent antigen recognition and elimination (Rasheed et al., Citation2016). The increased DTH response in immunosuppressed rats ((B)) suggests that lyophilized HCFD at a dose of 200 mg/kg may possess a stimulatory effect on lymphocytes and on other necessary cell types required for the expression of the reaction.
In summary, the selected commercialized HCFD was safe in animal model as there was no acute oral toxic death at up to 2000 mg/kg in male Wistar rats. In the CTX-induced immunosuppression model, lyophilized HCFD could improve body weight gain and spleen index. Lyophilized HCFD improved and recovered humoral immunity during immunosuppression due to cell-mediated and humoral antibody−mediated activation of T and B cells. Therefore, the lyophilized form of HCFD may be utilized as a potential immunotherapeutic supplement for enhancing the immune response in both humoral and cell-mediated immunity in immunosuppressed diseases.
Declaration of competing interest
The authors declare that they have no relation with the Prolac (Thailand) Co., Ltd., who is a co-founder with Thailand Research Fund (TRF) on the Research and Researcher for Industry (RRi) project. The company provided 60,000 baht in cash and the HCFD samples (product Lot no. 14052015), while the TRF provided a Ph.D. scholarship and major funding source for the project. This does not alter our adherence to the journal policies on sharing data and materials.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
Acknowledgements
The authors thank all staffs from the Northeast Laboratory Animal Center, Khon Kaen University, for their support in animal experiments. The authors also thank Mr Wirote Namsorn for his technical assistance in DTH assay.
Data availability
The datasets generated and/or analysed during the study are available from the corresponding author on reasonable request.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/09540105.2023.2279392)
Additional information
Funding
References
- Aghili, T., Arshami, J., Tahmasbi, A. M., & Haghparast, A. R. (2014). Effects of hypericum perforatum extract on IgG titer, leukocytes subset and spleen index in rats. Avicenna Journal of Phytomedicine, 4(6), 413–420. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4224955/pdf/AJP-4-413.pdf.
- Akindele, A. J., Adeneye, A. A., Salau, O. S., Sofidiya, M. O., & Benebo, A. S. (2014). Dose and time-dependent sub-chronic toxicity study of hydroethanolic leaf extract of Flabellaria paniculata Cav. (malpighiaceae) in rodents. Frontiers in Pharmacology, 5, APR. https://doi.org/10.3389/fphar.2014.00078.
- Bafna, A. R., & Mishra, S. H. (2006). Immunostimulatory effect of methanol extract of Curculigo orchioides on immunosuppressed mice. Journal of Ethnopharmacology, 104(1–2), 1–4. https://doi.org/10.1016/j.jep.2005.06.048
- Chaiyasut, C., Sivamaruthi, B. S., Duangjitcharoen, Y., Kesika, P., Sirilun, S., Chaiyasut, K., & Peerajan, S. (2018). Assessment of subchronic toxicity of fermented Houttuynia Cordata Thunb. using rodent model system. Asian Journal of Pharmaceutical and Clinical Research, 11(8), 307–311. https://doi.org/10.22159/ajpcr.2018.v11i8.26633.
- Chanda, S., & Juvekar, A. R. (2019). In vitro anti-inflammatory activity of syringic acid. International Journal of Pharmacy and Pharmaceutical Sciences, 11(2), 71–73. https://doi.org/10.22159/ijpps.2019v11i2.30387.
- Chen, Y., Tang, J., Wang, X., Sun, F., & Liang, S. (2012). An immunostimulatory polysaccharide (SCP-IIa) from the fruit of Schisandra chinensis (Turcz.) Baill. International Journal of Biological Macromolecules, 50(3), 844–848. https://doi.org/10.1016/j.ijbiomac.2011.11.015.
- Cheng, D., Sun, L., Zou, S., Chen, J., Mao, H., Zhang, Y., Liao, N., Zhang, R., Cheng, D., Sun, L., Zou, S., Chen, J., Mao, H., Zhang, Y., Liao, N., & Zhang, R. (2019). Antiviral effects of Houttuynia cordata polysaccharide extract on murine norovirus-1 (MNV-1): A human norovirus surrogate. Molecules, 24(9), 1835. https://doi.org/10.3390/molecules24091835
- Chiow, K. H., Phoon, M. C., Putti, T., Tan, B. K. H., & Chow, V. T. (2016). Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pacific Journal of Tropical Medicine, 9(1), 1–7. https://doi.org/10.1016/j.apjtm.2015.12.002.
- Clinical Laboratory Parameters for Crl:WI(Han) Rats - Charles River. (2008). https://www.criver.com/sites/default/files/resources/rm_rm_r_Wistar_Han_clin_lab_parameters_08.pdf.
- Doherty, N. S. (1981). Selective effects of immunosuppressive agents against the delayed hypersensitivity response and humoral response to sheep red blood cells in mice. Agents and Actions, 11(3), 237–242. https://doi.org/10.1007/BF01967620
- Fuller, G., & Manford, M. (2010). Infections of the nervous system II. In Neurology (pp. 100–101). Elsevier. https://doi.org/10.1016/b978-0-7020-3224-0.00051-3.
- Góngora, L., Máñez, S., Giner, R. M., Recio, M. C., & Ŕios, J. L. (2000). On the activity of trifluoperazine and palmitoylcarnitine in mice: Delayed hypersensitivity models. Life Sciences, 66(14), PL183–PL188. https://doi.org/10.1016/S0024-3205(00)00447-1
- Han, S. B., Kim, Y. H., Lee, C. W., Park, S. M., Lee, H. Y., Ahn, K. S., Kim, I.-H., & Kim, H. M. (1998). Characteristic immunostimulation by angelan isolated from Angelica gigas Nakai. Immunopharmacology, 40(1), 39–48. https://doi.org/10.1016/S0162-3109(98)00026-5
- Hassan, E. M., Matloub, A. A., Aboutabl, M. E., Ibrahim, N. A., & Mohamed, S. M. (2016). Assessment of anti-inflammatory, antinociceptive, immunomodulatory, and antioxidant activities of Cajanus cajan L. seeds cultivated in Egypt and its phytochemical composition. Pharmaceutical Biology, 54(8), 1380–1391. https://doi.org/10.3109/13880209.2015.1078383
- Hosten, A. O. (1990). BUN and Creatinine. Clinical Methods: The History, Physical, and Laboratory Examinations. https://www.ncbi.nlm.nih.gov/books/NBK305/.
- Huang, L., Gebreselassie, N. G., Gagliardo, L. F., Ruyechan, M. C., Lee, N. A., Lee, J. J., & Appleton, J. A. (2014). Eosinophil-derived IL-10 supports chronic nematode infection. Journal of Immunology (Baltimore, Md. : 1950), 193(8), 4178–4187. https://doi.org/10.4049/jimmunol.1400852
- Hung, P. Y., Ho, B. C., Lee, S. Y., Chang, S. Y., Kao, C. L., Lee, S. S., & Lee, C. N. (2015). Houttuynia cordata targets the beginning stage of herpes simplex virus infection. PLoS ONE, 10(2). https://doi.org/10.1371/journal.pone.0115475.
- Itoh, A., Isoda, K., Kondoh, M., Kawase, M., Watari, A., Kobayashi, M., Tamesada, M., & Yagi, K. (2010). Hepatoprotective effect of syringic acid and vanillic acid on CCl4-induced liver injury. Biological & Pharmaceutical Bulletin, 33(6), 983–987. https://doi.org/10.1248/bpb.33.983
- Kumar, V. P., & Venkatesh, Y. P. (2016). Alleviation of cyclophosphamide-induced immunosuppression in Wistar rats by onion lectin (Allium cepa agglutinin). Journal of Ethnopharmacology, 186, 280–288. https://doi.org/10.1016/j.jep.2016.04.006
- Kwon, R. H., & Ha, B. J. (2012). Increased flavonoid compounds from fermented Houttuynia cordata using isolated six of bacillus from traditionally fermented Houttuynia cordata. Toxicological Research, 28(2), 117–122. https://doi.org/10.5487/TR.2012.28.2.117
- Lab Tests Online. (2020). Alkaline Phosphatase (ALP) | Lab Tests Online. https://labtestsonline.org/tests/alkaline-phosphatase-alp.
- Lai, K. C., Chiu, Y. J., Tang, Y. J., Lin, K. L., Chiang, J. H., Jiang, Y. L., Jen, H. F., Kuo, Y. H., Agamaya, S., Chung, J. G., & Yang, J. S. (2010). Houttuynia cordata Thunb: Extract inhibits cell growth and induces apoptosis in human primary colorectal cancer cells. Anticancer Research, 30(9), 3549–3556. http://www.ncbi.nlm.nih.gov/pubmed/20944136.
- Lau, K. M., Lee, K. M., Koon, C. M., Cheung, C. S. F., Lau, C. P., Ho, H. M., Lee, M. Y. H., Au, S. W. N., Cheng, C. H. K., Lau, C. B. S., Tsui, S. K. W., Wan, D. C. C., Waye, M. M. Y., Wong, K. B., Wong, C. K., Lam, C. W. K., Leung, P. C., & Fung, K. P. (2008). Immunomodulatory and anti-SARS activities of Houttuynia cordata. Journal of Ethnopharmacology, 118(1), 79–85. https://doi.org/10.1016/j.jep.2008.03.018
- Lee, J.-S., Kim, I. S., Kim, J.-H., Kim, J. S., Kim, D.-H., & Yun, C.-Y. (2008). Suppressive effects of Houttuynia cordata Thunb. (Saururaceae) extract on Th2 immune response. Journal of Ethnopharmacology, 117(1), 34–40. https://doi.org/10.1016/j.jep.2008.01.013
- Lee, S. J., Hu, W., Lee, E. J., Choi, J. Y., & Koo, O. K. (2018). Polyphenolic profile of fermented Houttuynia cordata Thunb andnd overall contribution to antioxidant and lipolytic activities. Food Engineering Progress, 22(4), 295–303. https://doi.org/10.13050/foodengprog.2018.22.4.295
- Lobb, A. L. (2012). Science in liquid dietary supplement promotion: The misleading case of mangosteen juice. Hawai’i Journal of Medicine & Public Health : A Journal of Asia Pacific Medicine & Public Health, 71(2), 46–48. http://www.fda.
- Lu, H. M., Liang, Y. Z., Yi, L. Z., & Wu, X. J. (2006). Anti-inflammatory effect of Houttuynia cordata injection. Journal of Ethnopharmacology, 104(1–2), 245–249. https://doi.org/10.1016/j.jep.2005.09.012
- Marco, M. L., Heeney, D., Binda, S., Cifelli, C. J., Cotter, P. D., Foligné, B., Gänzle, M., Kort, R., Pasin, G., Pihlanto, A., Smid, E. J., & Hutkins, R. (2017). Health benefits of fermented foods: Microbiota and beyond. Current Opinion in Biotechnology, 44, 94–102. https://doi.org/10.1016/j.copbio.2016.11.010
- Mayadas, T. N., Cullere, X., & Lowell, C. A. (2014). The multifaceted functions of neutrophils. Annual Review of Pathology: Mechanisms of Disease, 9(1), 181–218. https://doi.org/10.1146/annurev-pathol-020712-164023
- Meng, Z., Wen, T., Kang, J., Lei, B., & Hyde, K. D. (2015). Efficacy of Houttuynia cordata Lour extracts against herpes simplex virus infection. Chiang Mai Journal of Science, 42(2), 317–330. http://epg.science.cmu.ac.th/ejournal/.
- Mukinda, J. T., & Syce, J. A. (2007). Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. Journal of Ethnopharmacology, 112(1), 138–144. https://doi.org/10.1016/j.jep.2007.02.011
- Ndrepepa, G. (2021). Aspartate aminotransferase and cardiovascular disease – a narrative review. Journal of Laboratory and Precision Medicine, 6(0), 6–6. https://doi.org/10.21037/jlpm-20-93
- Nfambi, J., & Sembajwe, L. F. (2015). Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in Wistar albino rats. Journal of Basic Clinic Physiology Pharmacol, 26(6), 603–611. https://doi.org/10.1515/jbcpp-2014-0104.Immunomodulatory.
- OECD. (2001). Acute Oral Toxicity – Acute Toxic Class Method. Oecd Guideline for Testing of Chemicals, December, 1–14. https://doi.org/10.1787/9789264071001-en.
- Park, E., Kum, S., Wang, C., Park, S. Y., Kim, B. S., & Schuller-Levis, G. (2005). Anti-inflammatory activity of herbal medicines: Inhibition of nitric oxide production and tumor necrosis factor-α secretion in an activated macrophage-like cell line. The American Journal of Chinese Medicine, 33(3), 415–424. https://doi.org/10.1142/S0192415X05003028
- Poonthananiwatkul, B., Lim, R. H. M., Howard, R. L., Pibanpaknitee, P., & Williamson, E. M. (2015). Traditional medicine use by cancer patients in Thailand. Journal of Ethnopharmacology, 168, 100–107. https://doi.org/10.1016/j.jep.2015.03.057
- Presbitero, A., Mancini, E., Brands, R., Krzhizhanovskaya, V. V., & Sloot, P. M. A. (2018). Supplemented alkaline phosphatase supports the immune response in patients undergoing cardiac surgery: Clinical and computational evidence. Frontiers in Immunology, 9(OCT), 2342. https://doi.org/10.3389/fimmu.2018.02342
- Rasheed, H. M. F., Rasheed, F., Qureshi, A. W., & Jabeen, Q. (2016). Immunostimulant activities of the aqueous methanolic extract of Leptadenia pyrotechnica, a plant from Cholistan desert. Journal of Ethnopharmacology, 186, 244–250. https://doi.org/10.1016/j.jep.2016.03.039
- Reagan-Shaw, S., Nihal, M., & Ahmad, N. (2008). Dose translation from animal to human studies revisited. The FASEB Journal, 22(3), 659–661. https://doi.org/10.1096/fj.07-9574LSF
- Santos, S. R., Rangel, E. T., Lima, J. C. S., Silva, R. M., Lopes, L., Noldin, V. F., Cechinel Filho, V., Delle Monache, F., & Martins, D. T. D. O. (2009). Toxicological and phytochemical studies of Aspidosperma subincanum Mart. stem bark (Guatambu). Pharmazie, 64(12), 836–839. https://doi.org/10.1691/ph.2009.9639.
- Satthakarn, S., Chung, W., Promsong, A., & Nittayananta, W. (2015). Houttuynia cordata modulates oral innate immune mediators: Potential role of herbal plant on oral health. Oral Diseases, 21(4), 512–518. https://doi.org/10.1111/odi.12313
- Senawong, T., Khaopha, S., Misuna, S., Komaikul, J., Senawong, G., Wongphakham, P., & Yunchalard, S. (2014). Phenolic acid composition and anticancer activity against human cancer cell lines of the commercially available fermentation products of Houttuynia cordata. ScienceAsia, 40(6), 420–427. https://doi.org/10.2306/scienceasia1513-1874.2014.40.420
- Sharma, U., Bala, M., Kumar, N., Singh, B., Munshi, R. K., & Bhalerao, S. (2012). Immunomodulatory active compounds from Tinospora cordifolia. Journal of Ethnopharmacology, 141(3), 918–926. https://doi.org/10.1016/j.jep.2012.03.027
- Shim, S. Y., Seo, Y. K., & Park, J. R. (2009). Down-regulation of FcepsilonRI expression by Houttuynia cordata Thunb. extract in human basophilic KU812F cells. Journal of Medicinal Food, 12(2), 383–388. https://doi.org/10.1089/jmf.2007.0684
- Shin, S., Soo Joo, S., Jeon, J. H., Park, D., Jang, M.-J., Kim, T.-O., Kim, H.-K., Hwang, Y., Kim, K.-Y., & Kim, Y.-B. (2010). Anti-inflammatory effects of a Houttuynia cordata supercritical extract. Journal of Veterinary Science, 11(3), 273–275. https://doi.org/10.4142/jvs.2010.11.3.273
- Shirani, K., Hassani, F. V., Razavi-Azarkhiavi, K., Heidari, S., Zanjani, B. R., & Karimi, G. (2015). Phytothrapy of cyclophosphamide-induced immunosuppression. Environmental Toxicology and Pharmacology, 39(3), 1262–1275. https://doi.org/10.1016/j.etap.2015.04.012
- Siracusa, M. C., Kim, B. S., Spergel, J. M., & Artis, D. (2013). Basophils and allergic inflammation. Journal of Allergy and Clinical Immunology, 132(4), 789–801. https://doi.org/10.1016/j.jaci.2013.07.046
- Solanki, Y., & Jain, S. (2010). Immunostimolatory activities of Vigna mungo L. extract in male Sprague–Dawley rats. Journal of Immunotoxicology, 6901. https://doi.org/10.3109/15476911003792278.
- Wang, L., Li, Z., Li, L., Li, Y., Yu, M., Zhou, Y., Lv, X., Arai, H., & Xu, Y. (2014). Acute and sub-chronic oral toxicity profiles of the aqueous extract of Cortex Dictamni in mice and rats. Journal of Ethnopharmacology, 158(PART A), 207–215. https://doi.org/10.1016/j.jep.2014.10.027
- Wang, L. C., Pan, T. M., & Tsai, T. Y. (2018). Lactic acid bacteria-fermented product of green tea and Houttuynia cordata leaves exerts anti-adipogenic and anti-obesity effects. Journal of Food and Drug Analysis, 26(3), 973–984. https://doi.org/10.1016/j.jfda.2017.11.009
- Wigraiboon, S., Nomura, N. P., & Whangchai, N. (2016). Effect of essential oils from Houttuynia cordata Thunb. supplemented diets on growth performance and immune response of hybrid red tilapia. International Journal of Fisheries and Aquatic Studies, 4(3), 677–684.
- Wong, T. W., Doyle, A. D., Lee, J. J., & Jelinek, D. F. (2014). Eosinophils regulate peripheral B cell numbers in both mice and humans. Journal of Immunology (Baltimore, Md. : 1950), 192(8), 3548–3558. https://doi.org/10.4049/jimmunol.1302241
- Woranam, K., Senawong, G., Utaiwat, S., Yunchalard, S., Sattayasai, J., & Senawong, T. (2020). Anti-inflammatory activity of the dietary supplement Houttuynia cordata fermentation product in RAW264.7 cells and Wistar rats. PLoS ONE, 15(3), e0230645. https://doi.org/10.1371/journal.pone.0230645
- Yanarojana, M., Nararatwanchai, T., Thairat, S., & Tancharoen, S. (2017). Antiproliferative activity and induction of apoptosis in human melanoma cells by Houttuynia cordata Thunb. extract. Anticancer Research, 37(12), 6619–6628. https://doi.org/10.21873/anticanres.12119.
- Yang, L., & Jiang, J.-G. (2009). Bioactive components and functional properties of Hottuynia cordata and its applications. Pharmaceutical Biology, 47(12), 1154–1161. https://doi.org/10.3109/13880200903019200
- Yuseff, M. I., Pierobon, P., Reversat, A., & Lennon-Duménil, A. M. (2013). How B cells capture, process and present antigens: A crucial role for cell polarity. Nature Reviews Immunology, 13(7), 475–486. https://doi.org/10.1038/nri3469