ABSTRACT
Naturally fermented filed water-dropwort (Oenanthe javanica) extracts by steeping with oligosaccharides (FDE) show a hepatoprotective activity against the liver injury induced by carbon tetrachloride (CCl4) and ethanol. However, the role of FDE in non-alcoholic steatohepatitis (NASH) has not yet been reported. In the present study, we investigated the effect of FDE on NASH using a mouse model with a methionine/choline-deficient diet (MCD). C57BL/6 male mice (9 weeks old) were fed on an MCD diet for 6 weeks with parallel water or FDE orally administration each day. FDE administered mice showed decreasing MCD diet-induced triglyceride (TG) levels, oxidative stress, infiltrating macrophages and elevating inflammatory cytokines in the liver. Our results suggest that FDE suppressed MCD diet-induced liver injury by inhibiting TG synthesis, the blocking of oxidative stress and hepatic inflammation, highlighting FDE as a potential therapeutic agent for the prevention and treatment of NASH.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the hepatic symptom of metabolic dysfunction and is histologically characterized by hepatic triglyceride accumulation, resulting in steatosis and hepatic inflammation (Tarantino et al., Citation2010). NAFLD encompasses a broad spectrum of liver damages ranging from mild hepatic lipid accumulation (steatosis) to non-alcoholic steatohepatitis (NASH), which include hepatic inflammation and apoptosis (Popescu et al., Citation2016). In NAFLD, hepatosteatosis develops into NASH by lipotoxicity, oxidative stress and inflammation (Vernon et al., Citation2011). In the USA, approximately 2%–5% of the population has NASH (Vernon et al., Citation2011). NASH patients have a risk of the more aggressive liver diseases, such as liver fibrosis, cirrhosis and hepatocellular carcinoma (Heidelbaugh & Sherbondy, Citation2006; Matteoni et al., Citation1999).
The methionine/choline-deficient (MCD) diet in mice is a frequently used and reproducible nutritional model of NAFLD (Stephenson et al., Citation2018). Several studies reported that feeding wild-type mice the MCD diet resulted in non-alcoholic fatty liver (Kim et al., Citation2016; Sutti et al., Citation2014; Wehr et al., Citation2014). In the MCD diet, choline deficiency leads to hepatic steatosis, and the absence of methionine leads to hepatic injury and inflammation (Caballero et al., Citation2010). Matsunaga et al. reported that C57BL/6J mice fed the MCD diet for 8 weeks developed increased hepatic inflammation, fat droplets in the liver, and liver cell injury (Matsunaga et al., Citation2015). Also, similar findings by Wang et al. showed the development of inflammation in mice model fed an MCD diet for only 2 weeks (Wang et al., Citation2014). Thus, the MCD diet leads to hepatic steatosis and NASH in the mice model.
Some studies demonstrated that the necroinflammatory process is initiated by metabolic dysregulation, which increases lipid accumulation, followed by inflammation, oxidative stress and lipid peroxidation (Bettaieb et al., Citation2015; Wang et al., Citation2018). Lipid peroxidation, which is considered one of the hits involved in steatohepatitis, is attributed to oxidative stress and the accumulation of reactive oxygen species (ROS) that acts on the accumulated hepatic lipids. Thus, the development of steatohepatitis is associated with increased lipid peroxidation, as indicated by the increased levels of lipid peroxidation products such as malondialdehyde (MDA) (Jorgacevic et al., Citation2014). The oxidized lipids and proteins in patients with NASH and animals fed the MCD diet indicate an increased oxidative state under these conditions (Casoinic et al., Citation2016; Ore & Akinloye, Citation2019). Based on these and other observations, it has been proposed that ROS-mediated lipid peroxidation could be an initiating event in NASH pathogenesis that might precede steatosis.
Besides oxidative stress, inflammation also plays an important role in NASH. NASH is distinguished from non-alcoholic fatty liver (NAFL) by inflammation and liver cell injury. Histopathological analysis of liver tissue and measurements of inflammatory cytokines, including tumour necrosis factor-a (TNF-a), interleukin-1b (IL-1b) and interleukin-6 (IL-6), are important markers of hepatic inflammation. Matsunaga et al. reported that C57BL/6J mice were fed an MCD diet for 8 weeks’ developed increased hepatic inflammation in liver sections increasing fat droplets and increased inflammatory cell infiltration (Matsunaga et al., Citation2015). Similar findings by Tosello-Trampont et al. were also shown that after only 10 days on the MCD diet, WT mice developed increased expression of TNF-a (Tosello-Trampont et al., Citation2012), indicating that NASH may develop considerably early in this model.
Dropwort (Oenanthe javanica) is a medicinal plants widely used to treat diseases, including jaundice, hypertension, and polydipsia, and their therapeutic benefits have been recognized for centuries in Korea, Japan, and China. Several studies reported that dropwort has functional activities against various liver diseases induced by bromobenzene (Park et al., Citation1996), hepatitis B virus (Han et al., Citation2008) and carbon tetrachloride (CCl4) (Yang et al., Citation2014). Recently, we have shown that field water-dropwort extracts naturally fermented by steeping with oligosaccharides (FDE) attenuate ethanol-induced liver injury (Lee et al., Citation2020). Therefore, we investigated whether FDE has a hepatoprotective role in the mouse model of the MCD diet to confirm the potency as a medication for NASH.
Materials and methods
Plant materials and preparation of FDE
FDE was kindly provided by Dr. Lee (Keimyung University, South Korea) (Yang et al., Citation2014). Briefly described obtaining FDE, filed water dropwort (Oenanthe javanica) was cultivated in the dryland region of Bissel Chunglog Farm (Daegu, South Korea) and naturally fermented by steeping with oligosaccharide (1:1, v/v) at room temperature for one year. The fermented extract was then stored at 4 °C for two years for maturation. For the animal experiment, the extract was diluted in water (1:5, v/v). In the case of cell treatment, the extract was concentrated, and froze dried (< 5% of water content).
Animals experiment and housing condition
Male C57BL/6 mice (9week age) were purchased from DBL Co., Ltd (South Korea). The experiment was performed following the guidelines proscribed by the Chungbuk National University Animal Care Committee (CBNUA-1272-19-01). The mice were acclimatized to the laboratory environment, maintained at 22 ± 1 °C and relative humidity of 55 ± 10%, with 12 h light–dark cycles throughout the experiment. All mice were fed with an MCD diet for 6 weeks to induce NASH. Following acclimation, mice were randomly divided into four groups (n = 10/group): (1) standard diet and water administration (Con-Water); (2) standard diet with FDE administration (2 mL/kg) (Con-FDE); (3) MCD diet and water administration (MCD-Water) (4) MCD diet and FDE administration (2 mL/kg) (MCD-FDE). During standard or MCD diets for 6 weeks, FDE or water was administrated every day.
Measurements of serum aspartate transaminase and alanine transaminase
Blood was collected at 9 h after ethanol administration. Serum was separated by centrifugation at 3000 rpm for 8 min at 21 °C. Serum aspartate transaminase (AST) and alanine transaminase (ALT) were measured using a biochemical analyzer (AU480, Beckman Coulter, CA, USA).
Histopathological analysis
For histological processing, liver tissues were fixed in a 4% formalin solution. Then, liver tissues were embedded in paraffin. Specimens were sectioned 4 μm and stained with haematoxylin and eosin stain (H&E), then observed with a light microscope (Nikon, Tokyo, Japan).
Mouse primary hepatocyte isolation and cell culture
The primary mouse hepatic cells were obtained from the liver tissue of 9-week-old male C57BL/6 mice, as described previously.(Severgnini et al., Citation2012) Briefly, we anaesthetized the mice by intraperitoneal injecting a mixture of ketamine (80 mg/kg) and xylazine (5 mg/kg) in 200 μL of saline. After the perfusion with Hank’s Balanced Salt Solution (HBSS)-EGTA solution (0.5 mM EGTA in HBSS, Gibco, Grand Island, NY, USA) pH = 8), we collected the liver and then dissected it to release hepatocytes. The resulting cells were gently pressed through a 100 μm cell strainer (BD, Franklin Lakes, New Jersey, USA). The filtered cells were washed with a Dulbecco’s modified Eagle (DMEM) medium and plated into 100 mm2 dishes. The cells were grown at 37°C in 5% CO2-humidified air in a DMEM medium that contained 10% foetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin. DMEM, penicillin, streptomycin, and FBS were purchased from Gibco Life Technologies (Grand Island, NY, USA). Stock solutions of 5 mM palmitic acid (PA)/5% BSA were heated for 15 min at 55 °C and cooled to room temperature to obtain the PA-BSA complex. To confirm the role of FDE in PA-treated mouse primary hepatic cells, the PA-BSA mixture was added to a serum-containing cell culture medium to a final concentration of 250 μM. Cells were starved in serum-free DMEM for 12 h followed by PA induction for an additional 24 h in the absence or presence of FDE (5, 10, 20 µg/mL). Lactate dehydrogenase (LDH) was measured using an LDH release assay kit following the manufacture’s instructions (Abcam, Cambridge, MA, USA)
Oil Red O staining
Liver tissues were fixed in 10% formalin in PBS and cut by a frozen section at 10 mm. Next, sections were rinsed with propylene glycol and stained with a 0.2% Oil Red O solution in propylene glycol for 30 min at room temperature and subsequently washed with tap water. Primary mouse hepatic cells were washed twice with phosphate-buffered saline (PBS), fixed with 0.5% glutaraldehyde for 3 h at room temperature, washed again with PBS, and allowed to dry completely. Next, fixed cells were stained with a 0.2% Oil Red O solution in isopropanol diluted in distilled water (6:4) for 1 h at room temperature and subsequently washed twice with PBS. Stained lipid droplets were observed with a light microscope (Nikon, Tokyo, Japan).
Western blot analysis
Homogenized liver tissues were lysed by protein extraction solution (PRO-PREP, iNtRON, Sungnam, Korea), and the total protein concentration was determined using the Bradford reagent (Bio-Rad, Hercules, CA, USA). 100 ㎍ extracted proteins were separated by SDS/PAGE and transferred to ImmobilonⓇ PVDF membranes (Millipore, Bedford, MA, USA). The membrane was blocked with 5% dried skimmed milk for 1 h at room temperature, followed by incubation with specific primary antibodies overnight at 4 °C. The membranes were washed with Tris-buffered saline containing 0.05% Tween-20 (TBST) and incubated with diluted horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. After washes, antibodies binding to the PVDF membrane were detected using the Immobilon Western Chemilum HRP substrate (Millipore, Bedford, MA, USA). The band intensities were measured using the Fusion FX 7 image acquisition system (Vilber Lourmat, Eberhardzell, Germany). Specific primary antibodies were purchased from Abcam (Caspase-3, Cleaved caspase-3 and 4-HNE; Cambridge, MA, USA). Secondary antibodies were purchased from Santo Cruz Biotechnology (anti-mouse and anti-rabbit; Dallas, TX, USA).
RNA isolation and quantitative real-time RT–PCR
Total RNA from liver tissues was extracted by RiboEx™ Total RNA isolation solution (GeneAll Biotechnology, Seoul, Korea) and cDNA were synthesized using a High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA, USA). Quantitative real-time RT–PCR was performed on a 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA) for custom-designed primers, and β-actin was used for house-keeping control using HiPi Real-Time PCR 2X Master Mix (ELPIS, Daejeon, Korea). Cycling conditions consisted of denaturation of 15 s at 94 °C, combined annealing of 30 s at 55 °C, and an extension of 60 s at 72 °C by 40 cycles. The values obtained for the target gene expression were normalized to β-actin and quantified relative to the expression in control samples.
Measurement of triglycerides
The hepatic level of triglycerides (TG) was measured as described in the manufacturer’s protocol using a triglycerides assay kit (Abcam, Cambridge, MA, USA)
Oxidative stress assay
Hydrogen peroxides assay was performed following the manufacturer’s protocol (Cell biolabs, San Diego, CA, USA). Hepatic levels of reduced glutathione (GSH) and oxidized glutathione (GSSG) were measured using a GSH/GSSG Ratio Detection Assay Kit (Abcam, Cambridge, MA, USA). Lipid peroxidation was measured by determining the generation of malondialdehyde (MDA; TBARS Assay kit, Cayman, Ann Arbor, MI, USA).
Statistical analysis
The data were analysed using the GraphPad Prism 4 version 4.03 software (Graph-Pad Software, La Jolla, CA). Data are presented as mean ± SEM. The differences in all data were assessed by a one-way analysis of variance. When the P-value in the analysis of variance test indicated statistical significance, the differences were assessed by Tukey’s test. p ≤ 0.05 was considered to be statistically significant.
Results
FDE ameliorated hepatic steatosis and liver injury induced by MCD in mice
To investigate the role of FDE in NASH, mice were induced by an MCD diet for 4 weeks. Male age-matched (age 9 weeks) mice fed a control (chow) diet were included as a reference group in most assessments. Histological analysis revealed that the liver of mice with an MCD diet exhibited several large lipid droplets, but the size of lipid droplets was significantly smaller in the liver of FDE-treated mice ((a)). We next studied the protective role of FDE in the hepatic injury induced by MCD. The evidence suggests that increased hepatocyte apoptosis is a critical mechanism, contributing to oxidative stress and inflammation in NASH (Hatting et al., Citation2013). The elevated serum levels of AST and ALT by MCD feeding were decreased in mice with FDE administration compared to water administration ((b)). To further study hepatic lipogenesis because hepatic steatosis is the most remarkable pathological feature of NAFL and NASH (Popescu et al., Citation2016), we measured TG level in the liver. The level of TG in the liver was increased by the MCD diet, and its increased level was reduced by FDE administration ((c)). Moreover, the mRNA level of SREBP1 and its target gene, such as FAS involved in TG synthesis in the liver, were increased by the MCD diet, but these increased levels were lowered in the liver of MCD-fed mice with FDE administration ((d)).
Figure 1. Liver steatosis and injury were attenuated by FDE in mice fed with the MCD diet. Mice were fed with the MCD or standard diet for 6 weeks. Mice with the MCD diet were orally injected with water (MCD-water) or FDE (MCD-FDE), and mice with the standard diet were also orally injected with water (Con-Water) or FDE (Con-FDE) daily in the period of the experiment. (a) Representative histology of haematoxylin and eosin (H&E) or oil red O (scale bars, 100μm). (b) Serum aspartate transaminase (AST) and alanine transaminase (ALT) levels. n = 10 per group; means ± SEM of the mean. **p < .01 (c) The triglyceride level in the liver of Con-Water, Con-FDE, MCD-water and MCD-FDE mice. n = 10 per group; means ± SEM of the mean. **p < .01 (d) The mRNA levels of FAS and SREBP-1 were measured by qPCR in the liver of Con-Water, Con-FDE, MCD-water and MCD-FDE mice. n = 10 per group; means ± SEM of the mean. **p < .01.
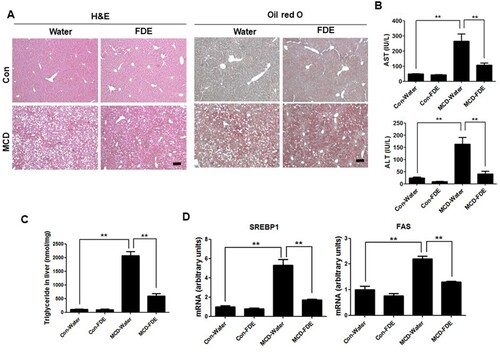
MCD-induced oxidative stress was reduced by FDE administration in the liver of mice
Human NASH and MCDdiet-induced experimental steatohepatitis in rodents are associated with increased oxidative stress (Sutti et al., Citation2014). We found that 4-HNE, which is a key enzyme of ROS production, was significantly increased in the MCD diet-fed mice. Conversely, 4-HNE was markedly decreased in the liver of MCD-fed mice treated with FDE ((a and b)). When the level of oxidative stress increases, GSSG, which is oxidized GSH accumulated; thus, GSH/GSSG ratio decreased. As shown in (c), the GSH/GSSG ratio in the liver of MCD-fed mice with water administration was lower than those in the liver of MCD-fed mice with FDE administration. The level of hydrogen peroxide was elevated in the liver of MCD-fed mice, whereas it was reduced in the liver of MCD-fed mice with FDE administration ((c)). Accordingly, the level of thiobarbituric acid (TBARS), a marker of lipid peroxidation, was also increased in the liver of MCD-fed mice. However, the increase in TBARS caused by MCD was suppressed in the liver of MCD-fed mice with FDE administration ((c)).
Figure 2. Effect of FDE on ethanol-induced oxidative stress. (a) Representative immunofluorescent staining of 4-HNE in the liver of Con-Water, Con-FDE, MCD-water and MCD-FDE mice (scale bars, 100μm). (b) The expression of 4-HNE was determined in the total protein extracts of mice liver tissues by Western blot analysis. (c) The hydrogen peroxide level, the ratio of GSH/GSSG and TBARS levels were measured in the liver of Con-Water, Con-FDE, MCD-water and MCD-FDE mice. n = 10 per group; means ± SEM of the mean. **p < .01 and ***p < .001.
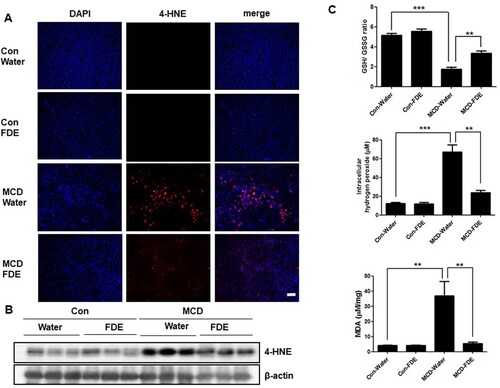
FDE relieves hepatocyte apoptosis in mice induced by the MCD diet
To discover the mechanism of FDE alleviating liver injury, apoptotic cells of the liver as determined by the method of TdT-mediated dUTP nick end labelling (TUNEL) and immunochemical staining of cleaved caspase-3 were significantly decreased in mice treated by FDE ((a and b)). The cleaved caspase-3 expression and caspase-3 activity were also markedly increased in MCD-induced mice but significantly decreased in mice treated by FDE ((c)).
Figure 3. FDE attenuated liver injury and hepatocyte apoptosis in mice induced by MCD. (a) Apoptotic cells of the liver were detected using a TUNEL assay (scale bars, 100μm). (b) Representative immunofluorescent staining of cleaved-caspase3 (scale bars, 100μm). (c) The protein expressions of cleaved caspase-3 and caspase-3 were determined by Western blot analysis.
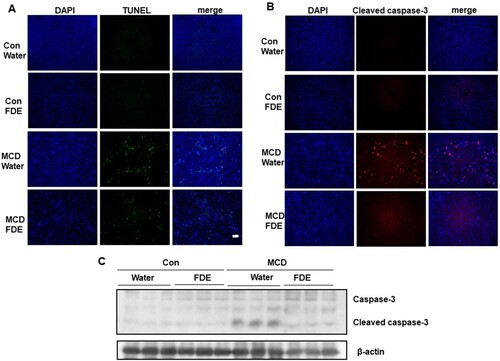
FDE blocks inflammation in mice induced by the MCD diet
The analysis of immunohistochemistry of liver sections revealed that FDE ingestion protected from hepatic inflammation by MCD diet in mice. Hepatic macrophages, the key mediators in the progress of NASH, can arise from circulating monocytes or from tissue-resident macrophages, named Kupffer cells (Tacke & Zimmermann, Citation2014). As shown in , the expression of F4/80 (macrophage marker), was markedly increased by the MCD diet. However, the increased expression of F4/80 by the MCD diet was significantly decreased in FDE treatment. In agreement with the change of F4/80, the hepatic mRNA levels of pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6, were increased in mice fed by the MCD diet, but decreased by FDE treatment ((b)).
Figure 4. FDE inhibits liver inflammation in mice induced by MCD. (a) Representative immunofluorescent staining of F4/80 (scale bars, 100μm) (b) The mRNA levels of TNF-α, IL-1b and IL-6 were measured by qPCR in the liver of Con-Water, Con-FDE, MCD-water and MCD-FDE mice. n = 10 per group; means ± SEM of the mean. **p < .01.
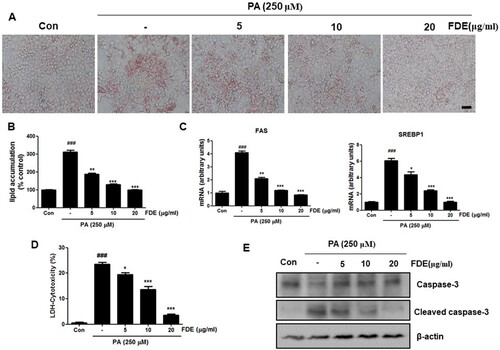
FDE suppressed lipid accumulation and apoptosis in hepatocytes
To investigate the protective roles of FDE in hepatocytes, we next examined the effects of FDE on cellular damage and steatosis by PA in mouse primary hepatocytes. This replicates the early features of NASH in vivo. PA exposure is frequently used in vitro model of the NASH model (Soret et al. Citation2020). Oil red O staining showed that accumulated lipid droplets by PA in hepatocytes. As shown in (a and b), FDE treatment significantly reduced the lipid droplet accumulation by PA. Furthermore, the q-PCR assay showed that hepatic mRNA levels of lipid synthesis genes (FAS and SREBP-1) were markedly increased in PA-induced mouse primary hepatocytes, whereas significantly decreased by FDE treatment ((c)). As expected, LDH release into the medium was markedly increased in PA-cultured mouse primary hepatocytes. In contrast, the level of LDH was significantly decreased in FDE-treated cells ((d)). In hepatic apoptosis, the cleaved caspase-3 expression and caspase-3 activity were also markedly increased in PA-induced mouse primary hepatocytes but significantly decreased by FDE treatment ((c)).
Figure 5. FDE suppressed hepatocellular lipid accumulation and hepatocyte apoptosis by PA. The mouse primary hepatocytes were induced by 0.25 mM palmitic acid (PA) in the absence or presence of FDE (5, 10, 20 μg/mL) for 24 h. (a) Lipid droplets in mouse hepatocytes were determined by oil red O staining assay and (b) measured by dissolving stained oil red O. Values are expressed as the mean ± SEM of three different experiments conducted in triplicates. *p < .05, **p < .01 and ***p < .001. (scale bars, 100μm). (c) The mRNA levels of FAS and SREBP-1c were measured by q-PCR. (d) The proportion of LDH release from mouse hepatocytes. (e) The protein expression of cleaved caspase-3, caspase-3 was determined by Western blot analysis.
Discussion
Feeding mice with an MCD diet is a well-established nutritional model of NASH and similar to human NASH in liver histological changes, including hepatic steatosis, inflammation and fibrosis (Wang et al., Citation2018). In the MCD model, the impaired mitochondrial β-oxidation, which leads to hepatosteatosis, firstly came up, and then increased oxidative stress and lipid peroxidation (Anstee & Goldin, Citation2006), which induce pro-inflammatory gene expression, occurred (George et al., Citation2003). The accumulating hepatic lipids can trigger the pathogenesis of the chronic liver disease (Osna et al., Citation2017), and contributes to liver inflammation leading to hepatocellular apoptosis (Hatting et al., Citation2013). In the present study, we found that FDE prevented hepatocellular apoptosis and liver injury by the MCD diet in the mice, suggesting FDE is a promising hepatoprotective agent for NASH therapy.
Several studies reported that dropwort extracts have hepatoprotective effects. Ethanol extracts of dropwort have several anti-oxidants and anti-oxidant activities through DPPH (1,1-diphenyl-2-picrylhydrazyl) and ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) radical scavenging assay (Hwang et al., Citation2011). Kim et al. reported that hot-water extracts of dropwort eliminate ingested ethanol by accelerating ethanol metabolism in rabbits and mice (Kim et al., Citation2009). Recently a study reported that FDE also showed hepatoprotective effects against CCl4-induced liver injury (Yang et al., Citation2014). Furthermore, we previously demonstrated that FDE attenuates ethanol-induced liver injury (Lee et al., Citation2020). Several studies reported that various plant extracts had been suggested as protectants against NASH (Jadeja et al., Citation2014). Thus, we examined whether FDE prevents NASH-like ethanol-induced fatty liver in mice. In the current study, we found that FDE ingestion ameliorates integral features of steatohepatitis by the MCD diet in mice, including repressed hepatic lipid accumulation and hepatocellular damage, decreased plasma ALT and AST, suppression of hepatic oxidative stress and inflammation. Moreover, NASH pathological features by PA were blocked by FDE treatment in mouse liver cells.
Excessive hepatic lipid accumulation. which can be caused by elevated synthesis of fatty acids, is considered a main risk factor for the development of NASH (Tilg & Moschen, Citation2010). Our current study showed that FDE regulates hepatic fatty acids synthesis by repressing fatty acid synthesis regulators such as FAS and SREBP-1. Notably, SREBP-1 is a transcription factor for TG synthesis by activating genes required for fatty acid synthesis and storage of TG such as FAS (Zhang et al., Citation2016).
Although the intensity of hepatic steatosis is associated with the severity of the liver injury, many obese humans and mice with diet-induced obesity did not always exhibit liver injury or hepatic inflammation even they readily develop hepatic steatosis (Begriche et al., Citation2013). Thus, only regulation of the liver lipid accumulation may not be sufficient to induce progression towards steatohepatitis. Some studies suggest that oxidative stress is one of these cofactors because hepatic oxidative stress correlates with the intensity of hepatic inflammation (Seki et al., Citation2002; Sutti et al., Citation2014). Among the ROS, we focused the hydrogen peroxide because hydrogen peroxide may play an important role in tissue injury and inflammation due to being more stable than other ROS (van der Vliet and Janssen-Heininger Citation2014). In the present study, hydrogen peroxide levels were significantly elevated in the liver of MCD-fed mice with water administration but not in the liver of MCD-fed mice with FDE administration. Oxidative stress depends on the balance between oxidant and anti-oxidant particles (Cichoz-Lach & Michalak, Citation2014). For example, a level of hydrogen peroxide is significantly elevated in glutathione peroxidase knock-out mice compared to wild-type mice (Harrison-Findik & Lu, Citation2015). Moreover, the activity of anti-oxidant enzymes, such as GSH levels, decreases in patients with NASH and animal models of diet-induced steatohepatitis (Varela-Rey et al., Citation2009). GSH is the most abundant redox buffer in cells and is reversibly oxidized to GSSG by glutathione peroxidase in hydrogen peroxide. Therefore, we examined the ratio of GSH and GSSG (oxidized glutathione). The GSH/GSSG ratio was increased in the liver of MCD-fed mice, but it was inhibited by FDE administration. Moreover, increased expression of 4-HNE and level of TBARS, a marker of lipid peroxidation, were suppressed by FDE administration in the liver of MCD-fed mice. 4-HNE is also a hepatic injury marker related to chronic oxidative stress in the NASH model (Ore & Akinloye, Citation2019). Consistently, our present data showed that FDE attenuates TG synthesis and oxidative stress in mouse liver induced by the MCD diet.
Regarding hepatic inflammation, the number of F4/80 positive cells, which reflect the macrophages, and the IL-1β, IL-6 and TNF-α mRNA levels were obviously increased by MCD diet induction. Several studies showed that dropwort extracts showed significant anti-inflammatory activities (Ahn & Lee, Citation2017; Kim et al., Citation2012; Kim et al., Citation2013; Yang et al., Citation2013). In the current study, FDE can suppress the inflammatory response in the MCD-fed mouse with NASH features. FDE counteracted the MCD-induced increase in hepatic inflammation score and the number of macrophages. Our data showed that FDE alleviates the expression of pro-inflammatory factors in livers from the MCD diet-fed mice and prevents PA-induced hepatic apoptosis and lipid accumulation in mouse primary hepatocytes.
In sum, the present study presents compelling evidence that FDE attenuates lipid accumulation, oxidative stress and inflammation in NASH, suggesting that FDE may be useful for treating NASH.
| List of abbreviation | ||
| NASH | = | non-alcoholic steatohepatitis |
| NAFLD | = | non-alcoholic fatty liver disease |
| FDE | = | naturally fermented filed water-dropwort (Oenanthe javanica (Blume) DC.) extracts by steeping with oligosaccharides |
| CCl4 | = | carbon tetrachloride |
| MCD diet | = | methionine/choline-deficient diet |
| PA | = | palmitic acid |
| TG | = | triglyceride |
| SREBP1 | = | sterol regulatory element-binding protein 1 |
| FAS | = | fatty acid synthase |
| ROS | = | reactive oxygen species |
| GSH | = | glutathione |
| GSSG | = | oxidized GSH |
| MDA | = | malondialdehyde |
| TNF-a | = | tumour necrosis factor-a |
| IL-1b | = | interleukin-1b |
| IL-6 | = | interleukin-6 |
| 4-HNE | = | 4-hydrocynoneal |
| AST | = | aspartate transaminase |
| ALT | = | alanine transaminase |
| H&E | = | haematoxylin and eosin stain |
| PBS | = | phosphate-buffered saline |
| DPPH | = | 1,1-diphenyl-2-picrylhydrazyl |
| ABTS | = | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Ahn, H., & Lee, G. S. (2017). Isorhamnetin and hyperoside derived from water dropwort inhibits inflammasome activation. Phytomedicine, 24, 77–86. https://doi.org/10.1016/j.phymed.2016.11.019
- Anstee, Q. M., & Goldin, R. D. (2006). Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. International Journal of Experimental Pathology, 87(1), 1–16. https://doi.org/10.1111/j.0959-9673.2006.00465.x
- Begriche, K., Massart, J., Robin, M. A., Bonnet, F., & Fromenty, B. (2013). Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology, 58(4), 1497–1507. https://doi.org/10.1002/hep.26226
- Bettaieb, A., Jiang, J. X., Sasaki, Y., Chao, T. I., Kiss, Z., Chen, X., Tian, J., Katsuyama, M., Yabe-Nishimura, C., Xi, Y., Szyndralewiez, C., Schröder, K., Shah, A., Brandes, R. P., Haj, F. G., & Natalie J. T. (2015). Hepatocyte nicotinamide adenine dinucleotide phosphate reduced oxidase 4 regulates stress signaling, fibrosis, and insulin sensitivity during development of steatohepatitis in mice. Gastroenterology, 149(2), 468–480.e10. https://doi.org/10.1053/j.gastro.2015.04.009
- Caballero, F., Fernandez, A., Matias, N., Martinez, L., Fucho, R., Elena, M., Caballeria, J., Morales, A., Fernandez-Checa, J. C., & Garcia-Ruiz, C. (2010). Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: Impact on mitochondrial S-adenosyl-L-methionine and glutathione. Journal of Biological Chemistry, 285(24), 18528–18536. https://doi.org/10.1074/jbc.M109.099333
- Casoinic, F., Sampelean, D., Buzoianu, A. D., Hancu, N., & Baston, D. (2016). Serum levels of oxidative stress markers in patients with type 2 diabetes mellitus and Non-alcoholic steatohepatitis. Romanian Journal of Internal Medicine, 54(4), 228–236. https://doi.org/10.1515/rjim-2016-0035
- Cichoz-Lach, H., & Michalak, A. (2014). Oxidative stress as a crucial factor in liver diseases. World Journal of Gastroenterology, 20(25), 8082–8091. https://doi.org/10.3748/wjg.v20.i25.8082
- George, J., Pera, N., Phung, N., Leclercq, I., Yun Hou, J., & Farrell, G. (2003). Lipid peroxidation, stellate cell activation and hepatic fibrogenesis in a rat model of chronic steatohepatitis. Journal of Hepatology, 39(5), 756–764. https://doi.org/10.1016/S0168-8278(03)00376-3
- Han, Y. Q., Huang, Z. M., Yang, X. B., Liu, H. Z., & Wu, G. X. (2008). In vivo and in vitro anti-hepatitis B virus activity of total phenolics from Oenanthe javanica. Journal of Ethnopharmacology, 118(1), 148–153. https://doi.org/10.1016/j.jep.2008.03.024
- Harrison-Findik, D. D., & Lu, S. (2015). The effect of alcohol and hydrogen peroxide on liver hepcidin gene expression in mice lacking antioxidant enzymes, glutathione peroxidase-1 or catalase. Biomolecules, 5(2), 793–807. https://doi.org/10.3390/biom5020793
- Hatting, M., Zhao, G., Schumacher, F., Sellge, G., Al Masaoudi, M., Gabetaler, N., Boekschoten, M., Muller, M., Liedtke, C., Cubero, F. J., & Trautwein C. (2013). Hepatocyte caspase-8 is an essential modulator of steatohepatitis in rodents. Hepatology, 57(6), 2189–2201. https://doi.org/10.1002/hep.26271
- Heidelbaugh, J. J., & Sherbondy, M. (2006). Cirrhosis and chronic liver failure: Part II. Complications and Treatment. American Academy of Family Physicians, 74(5), 767–776.
- Hwang, C. R., Hwang, I. G., Kim, H. Y., Kang, T. S., Kim, Y. B., Joo, S. S., Lee, I. S., & Jeong, H. S. (2011). Antioxidant component and activity of dropwort (Oenanthe javanica) ethanol extracts. Journal of the Korean Society of Food Science and Nutrition, 40(2), 316–320. https://doi.org/10.3746/jkfn.2011.40.2.316
- Jadeja, R., Devkar, R. V., & Nammi, S. (2014). Herbal medicines for the treatment of nonalcoholic steatohepatitis: Current scenario and future prospects. Evidence-Based Complementary and Alternative Medicine, 2014, 1–18. https://doi.org/10.1155/2014/648308
- Jorgacevic, B., Mladenovic, D., Ninkovic, M., Prokic, V., Stankovic, M. N., Aleksic, V., Cerovic, I., Vukicevic, R. J., Vucevic, D., Stankovic, M., & Radosavljević T. (2014). Dynamics of oxidative/nitrosative stress in mice with methionine-choline-deficient diet-induced nonalcoholic fatty liver disease. Human & Experimental Toxicology, 33(7), 701–709. https://doi.org/10.1177/0960327113506723
- Kim, D. W., Cho, H. I., Kim, K. M., Kim, S. J., Choi, J. S., Kim, Y. S., & Lee, S. M. (2012). Isorhamnetin-3-O-galactoside protects against CCl4-induced hepatic injury in mice. Biomolecules & Therapeutics (Seoul), 20(4), 406–412. https://doi.org/10.4062/biomolther.2012.20.4.406
- Kim, J. Y., Kim, K. H., Lee, Y. J., Lee, S. H., Park, J. C., & Nam, D. H. (2009). Oenanthe javanica extract accelerates ethanol metabolism in ethanol-treated animals. BMB Reports, 42(8), 482–485. https://doi.org/10.5483/BMBRep.2009.42.8.482
- Kim, S. B., Kang, O. H., Lee, Y. S., Han, S. H., Ahn, Y. S., Cha, S. W., Seo, Y. S., Kong, R., & Kwon, D. Y. (2016). Hepatoprotective effect and synergism of bisdemethoycurcumin against MCD diet-induced nonalcoholic fatty liver disease in mice. PLoS One, 11(2), e0147745.
- Kim, T. H., Ku, S. K., & Bae, J. S. (2013). Anti-inflammatory activities of isorhamnetin-3-O-galactoside against HMGB1-induced inflammatory responses in both HUVECs and CLP-induced septic mice. Journal of Cellular Biochemistry, 114(2), 336–345. https://doi.org/10.1002/jcb.24361
- Lee, D. H., Lee, J. S., Lee, I. H., & Hong, J. T. (2020). Therapeutic potency of fermented field water-dropwort (Oenanthe javanica (Blume) DC.) in ethanol-induced liver injury. RSC Advances, 10(3), 1544–1551. https://doi.org/10.1039/C9RA08976D
- Matsunaga, Y., Nakatsu, Y., Fukushima, T., Okubo, H., Iwashita, M., Sakoda, H., Fujishiro, M., Yamamotoya, T., Kushiyama, A., Takahashi, S., Tsuchiya, Y., Kamata, H., Tokunaga, F., Iwai, K., & Asano, T. (2015). LUBAC formation Is impaired in the livers of mice with MCD-dependent nonalcoholic steatohepatitis. Mediators of Inflammation, 2015, 1–10. https://doi.org/10.1155/2015/125380
- Matteoni, C. A., Younossi, Z. M., Gramlich, T., Boparai, N., Liu, Y. C., & McCullough, A. J. (1999). Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology, 116(6), 1413–1419. https://doi.org/10.1016/S0016-5085(99)70506-8
- Ore, A., & Akinloye, O. A. (2019). Oxidative stress and antioxidant biomarkers in clinical and experimental models of Non-alcoholic fatty liver disease. Medicina (Kaunas), 55(2). https://doi.org/10.3390/medicina55020026
- Osna, N. A., Donohue, T. M., Jr., & Kharbanda, K. K. (2017). Alcoholic liver disease: Pathogenesis and current management. Alcohol Research: Current Reviews, 38(2), 147–161.
- Park, J. C., Yu, Y. B., Lee, J. H., Hattori, M., Lee, C. K., & Choi, J. W. (1996). Protective effect of Oenanthe javanica on the hepatic lipid peroxidation in bromobenzene-treated rats and its bioactive component. Planta Medica, 62(6), 488–490. https://doi.org/10.1055/s-2006-957954
- Popescu, M., Popescu, I. A., Stanciu, M., Cazacu, S. M., Ianosi, N. G., Comanescu, M. V., Singer, C. E., & Neagoe, C. D. (2016). Non-alcoholic fatty liver disease - clinical and histopathological aspects. Romanian Journal of Morphology and Embryology, 57(4), 1295–1302.
- Seki, S., Kitada, T., Yamada, T., Sakaguchi, H., Nakatani, K., & Wakasa, K. (2002). In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. Journal of Hepatology, 37(1), 56–62. https://doi.org/10.1016/S0168-8278(02)00073-9
- Severgnini, M., Sherman, J., Sehgal, A., Jayaprakash, N. K., Aubin, J., Wang, G., Zhang, L., Peng, C. G., Yucius, K., Butler, J., & Fitzgerald K. (2012). A rapid two-step method for isolation of functional primary mouse hepatocytes: Cell characterization and asialoglycoprotein receptor based assay development. Cytotechnology, 64(2), 187–195. https://doi.org/10.1007/s10616-011-9407-0
- Soret, P. A., Magusto J., Housset C., & Gautheron J. 2020 In vitro and In vivo models of non-alcoholic fatty liver disease: A critical appraisal. Journal of Clinical Medicine. 10(1), 36. https://doi.org/10.3390/jcm10010036
- Stephenson, K., Kennedy, L., Hargrove, L., Demieville, J., Thomson, J., Alpini, G., & Francis, H. (2018). Updates on dietary models of nonalcoholic fatty liver disease: Current studies and insights. Gene Expression Patterns, 18(1), 5–17. https://doi.org/10.3727/105221617X15093707969658
- Sutti, S., Jindal, A., Locatelli, I., Vacchiano, M., Gigliotti, L., Bozzola, C., & Albano, E. (2014). Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology, 59(3), 886–897. https://doi.org/10.1002/hep.26749
- Tacke, F., & Zimmermann, H. W. (2014). Macrophage heterogeneity in liver injury and fibrosis. Journal of Hepatology, 60(5), 1090–1096. https://doi.org/10.1016/j.jhep.2013.12.025
- Tarantino, G., Savastano, S., & Colao, A. (2010). Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. World Journal of Gastroenterology, 16(38), 4773–4783. https://doi.org/10.3748/wjg.v16.i38.4773
- Tilg, H., & Moschen, A. R. (2010). Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology, 52(5), 1836–1846. https://doi.org/10.1002/hep.24001
- Tosello-Trampont, A. C., Landes, S. G., Nguyen, V., Novobrantseva, T. I., & Hahn, Y. S. (2012). Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. Journal of Biological Chemistry, 287(48), 40161–40172. https://doi.org/10.1074/jbc.M112.417014
- van der Vliet, A., Janssen-Heininger Y. M. 2014 Hydrogen peroxide as a damage signal in tissue injury and inflammation: Murderer, mediator, or messenger? Journal of Cellular Biochemistry. 115(3):427-435. https://doi.org/10.1002/jcb.24683
- Varela-Rey, M., Embade, N., Ariz, U., Lu, S. C., Mato, J. M., & Martinez-Chantar, M. L. (2009). Non-alcoholic steatohepatitis and animal models: Understanding the human disease. International Journal of Biochemistry and Cell Biology, 41(5), 969–976. https://doi.org/10.1016/j.biocel.2008.10.027
- Vernon, G., Baranova, A., & Younossi, Z. M. (2011). Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Alimentary Pharmacology & Therapeutics, 34(3), 274–285. https://doi.org/10.1111/j.1365-2036.2011.04724.x
- Wang, X., Hausding, M., Weng, S. Y., Kim, Y. O., Steven, S., Klein, T., Daiber, A., & Schuppan, D. (2018). Gliptins suppress inflammatory macrophage activation to mitigate inflammation, fibrosis, oxidative stress, and vascular dysfunction in models of nonalcoholic steatohepatitis and liver fibrosis. Antioxidants & Redox Signaling, 28(2), 87–109. https://doi.org/10.1089/ars.2016.6953
- Wang, Y., Li, J., Zhuge, L., Su, D., Yang, M., Tao, S., & Li, J. (2014). Comparison between the efficacies of curcumin and puerarin in C57BL/6 mice with steatohepatitis induced by a methionine- and choline-deficient diet. Experimental and Therapeutic Medicine, 7(3), 663–668. https://doi.org/10.3892/etm.2013.1461
- Wehr, A., Baeck, C., Ulmer, F., Gassler, N., Hittatiya, K., Luedde, T., Neumann, U. P., Trautwein, C., Tacke, F., & Alisi A. (2014). Pharmacological inhibition of the chemokine CXCL16 diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. PLoS One, 9(11), e112327. https://doi.org/10.1371/journal.pone.0112327
- Yang, J. H., Kim, S. C., Shin, B. Y., Jin, S. H., Jo, M. J., Jegal, K. H., Kim, Y. W., Lee, J. R., Ku, S. K., Cho, I. J., & Ki, S. H. (2013). O-Methylated flavonol isorhamnetin prevents acute inflammation through blocking of NF-kappaB activation. Food and Chemical Toxicology, 59, 362–372. https://doi.org/10.1016/j.fct.2013.05.049
- Yang, S. A., Jung, Y. S., Lee, S. J., Park, S. C., Kim, M. J., Lee, E. J., Byun, H. J., Jhee, K. H., & Lee, S. P. (2014). Hepatoprotective effects of fermented field water-dropwort (Oenanthe javanica) extract and its major constituents. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 67, 154–160. https://doi.org/10.1016/j.fct.2014.02.010
- Zhang, X., Han, J., Man, K., Li, X., Du, J., Chu, E. S., Go, M. Y., Sung, J. J., & Yu, J. (2016). CXC chemokine receptor 3 promotes steatohepatitis in mice through mediating inflammatory cytokines, macrophages and autophagy. Journal of Hepatology, 64(1), 160–170. https://doi.org/10.1016/j.jhep.2015.09.005
