ABSTRACT
It has been proved that polyphenols have positive effects against inflammatory response and oxidative stress. We investigated whether dietary supplementation of the holly polyphenols (HP) might relieve a liver injury induced by lipopolysaccharide (LPS) in weaned pigs. Twenty-four weaned pigs were fed diets containing 0 or HP and saline or LPS challenge in 2×2 factorial arrangement of treatments. On d 17, pigs were intraperitoneally injected with an LPS or saline. Dietary HP supplementation reduced plasma AST and GGT activities and liver MDA levels, increased plasma ALB and TP and liver GSH concentrations, and ameliorated LPS-induced liver histopathological damage. HP supplementation reduced plasma and /or liver TNF-α, IL-6 and IL-1β levels, and liver mRNA expression of IL-6, TNF-α and COX2. Moreover, HP decreased the mRNA abundance of liver MyD88, TRAF6 and NOD1. These results suggest that HP supplementation attenuates liver injury induced by LPS in weaned pigs, and inhibits inflammatory response and oxidative stress.
1. Introduction
The liver is the metabolism centre of macronutrient and xenobiotic compounds. Moreover, the liver is also an important microbial defense line and a target for dysregulated inflammation (Strnad et al., Citation2017). Research has shown that inflammation and oxidative stress could induce hepatic injury (Reyes-Gordillo et al., Citation2017).
Lipopolysaccharide (LPS), endotoxin of Gram-negative bacteria, can induce host inflammatory response and overproduction of pro-inflammatory cytokines, which causes acute liver injury (Yang et al., Citation1997). Moreover, LPS-induced inflammation also leads to oxidative damage by producing oxidants in the liver (Su et al., Citation2014). As we all know, oxidative damage plays a remarkable role in hepatic injury induced by poisons and drugs (Li et al., Citation2019). Therefore, antioxidants and anti-inflammatories are considered to be effective against liver damage and diseases (Mohebbati et al., Citation2017).
Natural polyphenols in plants are micronutrients that are beneficial to health (Manach et al., Citation2004). Interestingly, polyphenols have various pharmacological effects in the pathological processes of liver diseases, such as inflammation, oxidative stress and insulin resistance (Yahfoufi et al., Citation2018). Therefore, polyphenols have potential protective effects on liver injury and have been shown to prevent LPS-induced liver inflammation in mice (Wang, Zhang et al., Citation2019). However, the protective effect of polyphenols on LPS-induced hepatic injury in weaned pigs is still unclear.
To this purpose, we hypothesized that the dietary supplementation of holly polyphenol extracts (HP) could ameliorate LPS-induced liver damage in pigs, and inhibit the inflammatory response and oxidative stress. We established the hepatic injury model of weaned piglets through injecting Escherichia coli LPS. Our aim was to evaluate the protective effect of HP on hepatic injury caused by LPS in weaned pigs.
2. Materials and methods
2.1. Animals and experimental design
The Animal Care and Use Committee of Wuhan Polytechnic University approved experimental animal protocols and procedures (EM20170521, 21 May 2017). Twenty-four weaned pigs (Duroc × Large White × Landrace, initial body weight (BW) of 8.09 ± 0.23 kg) were randomly allotted to four treatments based on initial BW and the litter of origin. Each treatment included six pigs (replicates) that were kept individually in pens (1.8 × 1.10 m). The pigs were fed with a basal diet supplemented with or without a commercial HP product. The HP product contained 65.5% total polyphenols, mainly phenolic acids and tannins. The ingredient composition of the basal diet has been reported previously (Xu et al., Citation2020). Water and experimental diets were freely available.
The experiment was designed as a 2×2 factorial arrangement. The factors consisted of HP supplementation (0 vs. 250 mg/kg HP) and LPS challenge (saline vs. 100 μg/kg BW LPS). On d 17 of the trial, the challenged group was given an intraperitoneal injection of LPS (E. coli serotype 055: B5, Sigma Chemical), and the unchallenged group was given an injection of the same amount of saline. The LPS was dissolved in sterile saline.
2.2. Sample collection
Blood samples were collected at 4 h after the LPS or saline injection. Plasma was harvested from blood samples and stored at −80°C until further analysis. All pigs were anesthetized and slaughtered after blood collection, and the livers were exposed. The left lobe of the liver was removed. One part of the liver sample (∼1 g) was excised, snap-frozen and preserved at −80°C for further analysis. The liver tissue was homogenized at 4°C, centrifuged, and the supernatant was used to determine liver antioxidant status, and tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-10 (IL-10) concentrations. The other part of the liver samples (3 × 4 × 5 mm3) were fixed in 4% paraformaldehyde in the PBS for histological observation.
2.3. Biochemical analysis of plasma
Activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamyl transpeptidase (GGT), and alkaline phosphatase (AKP), as well as the concentrations of albumin (ALB), total bilirubin (T.BIL) and total protein (TP) in plasma were determined with the commercial kits according to the manufacturer’s protocol (Nanjing).
2.4. Determination of antioxidant status in liver
Catalase (CAT), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) activities and glutathione (GSH) and malondialdehyde (MDA) concentrations in the liver were detected with the commercial kits according to the instructions (Nanjing).
2.5. Histological analysis
The fixed hepatic tissues were embedded in paraffin and sliced into a 4μm section. The slides were deparaffinized, and stained with haematoxylin and eosin, according to the standard protocols. Hepatic pathological changes were evaluated by a blinded pathologist under light microscopy.
2.6. TNF-α and IL-6 concentrations in plasma, and TNF-α, IL-6, IL-1β and IL-10 concentrations in liver
Pig ELISA kits (R&D Systems, Minneapolis, MN, USA) were used for analyzing the concentrations of TNF-α and IL-6 in plasma and TNF-α, IL-6, IL-1β and IL-10 in the liver.
2.7. Real-time quantitative PCR
Total RNA extraction from the liver tissue, cDNA synthesis, and quantitative real-time PCR was performed, as described by Liu et al. (Citation2012). Supplemental Table 1 showed primer sequences. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene. mRNA expression of the target gene related to GAPDH was calculated using the 2−ΔΔCT method (Livak & Schmittgen, Citation2001).
2.8. Statistical analysis
The experimental data were presented as means and SEMs, and analyzed using SAS (SAS Inst. Inc., Cary, NC). The general linear model procedure (GLM) was conducted to evaluate the main effects of LPS challenge, diet type and its interactions. If there was a significant interaction or a trend for interaction between the LPS and diet type, post hoc testing was performed using Duncan’s multiple comparison tests. P < 0.05 was defined as significant and 0.05 < P ≤ 0.10 as trends.
3. Results
3.1. Plasma biochemical parameters
LPS challenge increased plasma AST activity (P = 0.009) and tended to increase plasma GGT activity (P = 0.052) (). In comparison with pigs fed control diet, pigs fed HP had lower activity of GGT in plasma (P = 0.016). A LPS challenge × diet type interaction for plasma AST activity (P = 0.039) was found. HP supplementation decreased plasma AST activity in LPS-challenged pigs, but there was no difference among pigs injected with saline. Both the HP supplementation and LPS challenge had no effect on plasma ALT and AKP activities. LPS challenge increased plasma concentration of T.BIL. There was an interaction between the LPS challenge and diet type for ALB (P = 0.007) and TP (P = 0.041) concentrations. HP supplementation increased plasma ALB and TP concentrations in LPS-challenged pigs. However, no difference among saline-treated pigs was observed.
Table 1. Effects of HP supplementation on plasma biochemical parameters after Escherichia coli LPS challenge in weaned pigs.Table Footnote1
3.2. Histology of liver
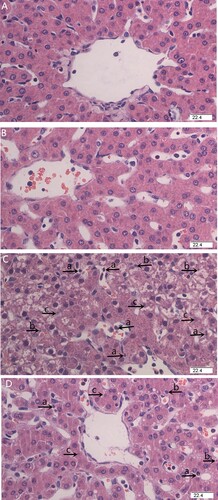
Histological examination was used to assess the degree of liver tissue damage. The liver lobular architecture and cell structure were normal in pigs injected with saline ((a, b)). LPS challenge resulted in evident liver pathologic changes, which exhibited hepatic plate structure disorders, inflammatory cell infiltration, hepatocyte karyopyknosis and karyolysis ((c)). In contrast, LPS-induced liver pathological changes were ameliorated by dietary HP supplementation ((d)).
Figure 1. Effects of HP supplementation on liver histopathological changes in weaned pigs challenged by Escherichia coli lipopolysaccharide (LPS) (H&E staining, 400×). (A) Pigs fed with control diet and injected with sterile saline. The liver tissues showed normal morphology. (B) Pigs fed with HP dietary and injected with sterile saline. The liver tissues showed normal morphology. (C) Pigs fed with control diet and injected with LPS. The liver displayed obvious pathologic changes, including hepatic plate structure disorders, inflammatory cell infiltration (a), hepatocyte karyopycnosis (b), and karyolysis (c). (D) Pigs fed with HP and injected with LPS. Liver pathological changes were significantly ameliorated by dietary HP supplementation.
3.3. TNF-α and IL-6 concentrations in plasma and TNF-α, IL-6, IL-1β and IL-10 concentrations in liver
LPS challenge increased plasma and liver TNF-α and IL-6 (P < 0.001) concentrations, and liver IL-1β (P = 0.001) and IL-10 (P = 0.008) concentrations (). HP supplementation decreased the concentrations of TNF-α (plasma, P = 0.043; liver, P < 0.001), IL-6 (plasma, P = 0.043; liver, P < 0.001) and IL-1β (liver, P = 0.031) compared to pigs fed control diet. There was an interaction between the LPS challenge and diet type for plasma and liver TNF-α (plasma, P < 0.001; liver, P = 0.008) and liver IL-6 (P < 0.001) concentrations, and a trend for a LPS challenge × diet type interaction observed for plasma IL-6 (P = 0.085) concentrations. HP supplementation decreased plasma and liver TNF-α and IL-6 concentrations in LPS-challenged pigs. However, no difference among saline-treated pigs was observed.
Table 2. Effects of HP supplementation on plasma and liver TNF-α and IL-6 concentrations after Escherichia coli LPS challenge in weaned pigs.Table Footnote1
3.4. Liver inflammatory mediator mRNA expression
LPS-challenged pigs had higher mRNA expression of liver toll-like receptor 4 (TLR4) (P < 0.001), myeloid differentiation factor 88 (MyD88) (P < 0.001), TNF-α receptor-associated factor 6 (TRAF6) (P = 0.029), IL-1 receptor-associated kinase 1(IRAK1) (P = 0.002), nucleotide-binding oligomerization domain protein 1 (NOD1) (P < 0.001), nucleotide-binding oligomerization domain protein 2 (NOD2) (P < 0.001), receptor-interacting serine/threonine-protein kinase 2 (RIPK2) (P < 0.001), cyclooxygenase 2 (COX2) (P < 0.001), heat shock protein 70 (HSP70) (P = 0.002), TNF-α (P < 0.001) and IL-6 (P = 0.001) than saline-injected pigs (). HP supplementation decreased the mRNA expression of liver NOD1 (P = 0.006), COX2 (P = 0.01), TNF-α (P = 0.002) and IL-6 (P = 0.015) and tended to decrease liver TRAF6 mRNA abundance (P = 0.083) in comparison with those fed with the control diet. There was a LPS challenge × diet type interaction for mRNA abundance of MyD88 (P = 0.025), NOD1 (P = 0.001), COX2 (P = 0.004), TNF-α (P = 0.001) and IL-6 (P = 0.011) and a trend for interaction between the LPS challenge and diet type for mRNA abundance of TRAF6 (P = 0.051). HP supplementation decreased mRNA expression of MyD88, TRAF6, NOD1, COX2, TNF-α and IL-6 in LPS-challenged pigs. However, no difference among saline-treated pigs was found.
Table 3. Effect of HP supplementation on the hepatic mRNA expression of TLR4 and NOD and their downstream signals after Escherichia coli LPS challenge in weaned pigs.Table Footnote1
3.5. Antioxidant status in liver
There was no LPS challenge × diet interaction for the concentrations of MDA and GSH, and the activities of SOD, CAT and GSH-Px () in the liver. However, pigs challenged with LPS had higher liver MDA levels (P = 0.037) and tended to have lower GSH levels (P = 0.073), CAT (P = 0.056) and GSH-Px (P = 0.097) activities than those treated with saline. HP supplementation decreased the level of MDA (P = 0.030) and increased GSH concentration (P = 0.038) compared with pigs fed control diet. Moreover, neither LPS treatment nor HP supplementation affected liver SOD activity.
Table 4. Effects of HP supplementation on the concentrations of hepatic MDA and GSH, as well as the enzymatic activities of SOD, CAT and GSH-Px after Escherichia coli LPS challenge in weaned pigs.Table Footnote1
4. Discussion
As secondary metabolites of plants, polyphenols have been shown to have multiple physiological functions, including anti-inflammatory, antioxidant and antiviral effects, and reduction in cholesterol (Domitrovic & Potocnjak, Citation2016; Li et al., Citation2014). In our study, the protective effects of dietary HP on LPS-induced liver damage in weaned pigs were evaluated.
The plasma liver-derived enzymes such as ALT, AST and GGT are regarded as indicators of acute liver damage (Wan et al., Citation2008). In the present study, we measured the activities of plasma ALT, AST, AKP, and GGT, and the levels of ALB, T.BIL and TP to assess the liver injury in pigs challenged with LPS. Our results indicated that the LPS challenge led to an increase in AST and GGT activities and T.BIL concentration. The increase of AST and GGT activities often suggests the severity of liver injury (Sai, Citation2006). Moreover, when the liver is damaged, the concentration of T.BIL increases, while ALB and TP concentrations decrease (Olga et al., Citation2019). In this study, dietary HP supplementation reduced LPS-induced release of AST and GGT and increased plasma ALB and TP concentrations. It suggested that HP mitigated the LPS-induced liver damage and restored the hepatic structure. Furthermore, histopathological findings further confirmed that HP mitigated the LPS-induced liver injury.
Accumulating evidence indicates that the LPS-induced liver injury is involved with inflammatory responses (Cho et al., Citation2014). In fact, the release of pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α boosts inflammatory response, leading to liver injury (Eltzschig & Eckle, Citation2011; Klune & Tsung, Citation2010). In addition, a previous study showed that COX2 was also a crucial inflammatory mediator that played a role in liver injury (Xia et al., Citation2014). In the present study, the LPS challenge caused an increase of plasma and /or liver IL-6 IL-1β and TNF-α concentrations and liver IL-6, TNF-α and COX2 mRNA expression. HP supplementation attenuated the LPS-induced increase of plasma and / or liver IL-6, IL-1β and TNF-α concentrations, and liver IL-6, TNF-α and COX2 mRNA expression. It has been extensively demonstrated that the polyphenols exerted protective effects against liver injury through anti-inflammatory and antioxidant activities (Lama et al., Citation2017; Li et al., Citation2018). Wang, Zhang et al. (Citation2019) reported that green tea polyphenols decreased hepatic mRNA expression of TNF-α and IL-6 as well as plasma TNF-α and IL-6 concentrations, attenuating LPS-induced hepatic inflammation in mice. Therefore, our results indicated that HP ameliorated liver injury was caused by LPS via suppressing the release of inflammatory mediators.
To investigate the anti-inflammatory mechanisms of HP, the TLR4 and NOD signalling pathways were evaluated. TLRs, significant components of innate immunity, are used to identify signature pathogen-associated microbial compounds (Kawai & Akira, Citation2006; Maynard et al., Citation2012). TLR4 is a membrane receptor and transmembrane signalling molecule in the TLR family (Wang, Han et al., Citation2019). LPS is a TLR4 specific ligand. After sensing and binding to extracellular LPS, TLR4 then activates the MyD88, which further activates downstream molecules such as TRAF6 and IRAK1. Thus, it ultimately activates the nuclear factor kappa-B (NF-κB) (Kawai & Akira, Citation2006). In contrast to TLR4, NODs (NOD1 and NOD2) are cytoplasmic pathogen recognition receptors. NODs activate mitigen-activated protein kinase (MAPKs) and NF-κB signals (Liu et al., Citation2014). The activated NF-κB induces the transcription and translation of cytokines, leading to the overproduction of inflammatory mediators (Covert et al., Citation2005). Our results indicated that the LPS challenge increased the mRNA expression of TLR4, MyD88, IRAK1, TRAF6 as well as NOD1, NOD2, and RIPK2. HP supplementation decreased MyD88, TRAF6, and NOD1 mRNA expression. We also found that the LPS challenge induced IL-1β, IL-6 and TNF-α concentrations to increase in plasma and the liver. A previous study showed that the degradation product of polyphenols suppressed the activation of NF-κB in the liver of mice challenged with LPS (Radnai et al., Citation2009). Ranneh et al. (Citation2017) indicated that polyphenol-rich stingless bee honey attenuated inflammation through the suppression of NF-κB and p38 MAPK signalling in LPS-induced rats. In our study, the protective effect of HP against the LPS-induced inflammatory liver damage was associated with inhibiting inflammatory pathways.
Except for inflammation, LPS also induces excessive reactive oxygen species (ROS) production, which leads to oxidative stress (Su et al., Citation2014). In turn, oxidative stress boosts the inflammatory process (Ranneh et al., Citation2017). Oxidative stress is also an important factor that causes hepatic injury (Bailey & Cunningham, Citation2002). Antioxidant enzymes, such as SOD, CAT, GSH-Px and GSH, eliminate ROS in the liver. Thus, they can effectively prevent tissue damage caused by oxidative stress (Xu et al., Citation2017). MDA is the last product of lipoperoxidation and is also considered as a key indicator of oxidative stress (Del Rio et al., Citation2005). Our results showed that LPS increased MDA level, tended to reduce GSH content, and also inhibited antioxidant enzyme activity (CAT and GSH-Px) in the liver. It suggested that LPS might cause the imbalance of redox, and increase ROS and lipid peroxidation in the liver. However, HP supplementation decreased MDA level and increased GSH concentration. Various polyphenols have great antioxidative effects (Yahfoufi et al., Citation2018). Dietary apple polyphenols supplementation increased hepatic CAT activity and the mRNA expression of CAT, glutathione S-transferase (GST) and SOD in weaned piglets (Xu et al., Citation2019). Green tea polyphenols attenuated the LPS-induced liver injury and oxidative stress by the reduction of SOD activity and GSH content (Wang, Zhang et al., Citation2019). Our results suggested that dietary HP supplementation could reduce oxidative damage induced by LPS through enhancing antioxidant capacity.
5. Conclusion
In conclusion, dietary HP supplementation has a protective effect on the LPS-induced liver injury in weaned pigs. These functions of HP might be associated with the inhibition of LPS-induced inflammatory responses and oxidative stress.
Supplemental Material
Download MS Word (15 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bailey, S. M., & Cunningham, C. C. (2002). Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radical Biology and Medicine, 32(1), 11–16. https://doi.org/10.1016/S0891-5849(01)00769-9
- Cho, H. I., Park, J. H., Choi, H. S., Kwak, J. H., Lee, D. U., Lee, S. K., & Lee, S. M. (2014). Protective mechanisms of acacetin against D-glactosamine and lipopolysaccharide-induced fulminant hepatic failure in mice. Journal of Natural Products, 77(11), 2497–2503. https://doi.org/10.1021/np500537x
- Covert, M. W., Leung, T. H., Gaston, J. E., & Baltimore, D. (2005). Achieving stability of lipopolysaccharide-induced NF-kappa B activation. Science, 309(5742), 1854–1857. https://doi.org/10.1126/science.1112304
- Del Rio, D., Stewart, A. J., & Pellegrini, N. (2005). A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutrition, Metabolism and Cardiovascular Diseases, 15(4), 316–328. https://doi.org/10.1016/j.numecd.2005.05.003
- Domitrovic, R., & Potocnjak, I. (2016). A comprehensive overview of hepatoprotective natural compounds: Mechanism of action and clinical perspectives. Archives of Toxicology, 90(1), 39–79. https://doi.org/10.1007/s00204-015-1580-z
- Eltzschig, H. K., & Eckle, T. (2011). Ischemia and reperfusion-from mechanism to translation. Nature Medicine, 17(11), 1391–1401. https://doi.org/10.1038/nm.2507
- Kawai, T., & Akira, S. (2006). TLR signaling. Cell Death and Differentiation, 13(5), 816–825. https://doi.org/10.1038/sj.cdd.4401850
- Klune, J. R., & Tsung, A. (2010). Molecular biology of liver ischemia/reperfusion injury: Established mechanisms and recent advancements. Surgical Clinics of North America, 90(4), 665–677. https://doi.org/10.1016/j.suc.2010.04.003
- Lama, A., Pirozzi, C., Mollica, M. P., Trinchese, G., Guida, F. D., Cavaliere, G., Calignano, A., Raso, G. M., Canani, R. B., & Meli, R. (2017). Polyphenol-rich virgin olive oil reduces insulin resistance and liver inflammation and improves mitochondrial dysfunction in high-fat diet fed rats. Molecular Nutrition & Food Research, 61(3), 3. https://doi.org/10.1002/mnfr.201600418
- Li, A. N., Li, S., Zhang, Y. J., Xu, X. R., Chen, Y. M., & Li, H. B. (2014). Resources and biological activities of natural polyphenols. Nutrients, 6(12), 6020–6047. https://doi.org/10.3390/nu6126020
- Li, F. F., Huang, D. F., Nie, S. P., & Xie, M. Y. (2019). Polysaccharide from the seed of plantago asiatica L. protect against lipopolysaccharide-induced liver injury. Journal of Medicinal Food, 22(10), 1058–1066. https://doi.org/10.1089/jmf.2018.4394
- Li, S., Tan, H. Y., Wang, N., Cheung, F., Hong, M., & Feng, Y. (2018). The potential and action mechanism of polyphenols in the treatment of liver diseases. Oxidative Medicine and Cellular Longevity, 2018. https://doi.org/10.1155/2018/8394818
- Liu, J., Wang, Y., & Ouyang, X. (2014). Beyond toll-like receptors: Porphyromonas gingivalis induces IL-6, IL-8, and VCAM-1 expression through NOD-mediated NF-kB and ERK signaling pathways in periodontal fibroblasts. Inflammation, 37(2), 522–533. https://doi.org/10.1007/s10753-013-9766-0
- Liu, Y. L., Chen, F., Odle, J., Lin, X., Jacobi, S. K., Zhu, H. L., Wu, Z. F., & Hou, Y. Q. (2012). Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathway in weaned pigs after LPS challenge. The Journal of Nutrition, 142(11), 2017–2024. https://doi.org/10.3945/jn.112.164947
- Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and 2-ΔΔCT method. Methods, 25(4), 402–408. https://doi.org/10.1006/meth.2001.1262
- Manach, C., Scalbert, A., Morand, C., Remesy, C., & Jimenez, L. (2004). Polyphenols: Food sources and bioavailability. The American Journal of Clinical Nutrition, 79(5), 727–747. https://doi.org/10.1093/ajcn/79.5.727
- Maynard, C. L., Elson, C. O., Hatton, R. D., & Weaver, C. T. (2012). Reciprocal interactions of the intestinal microbiota and immune system. Nature, 489(7415), 231–241. https://doi.org/10.1038/nature11551
- Mohebbati, R., Anaeigoudari, A., & Khazdair, M. R. (2017). The effects of Curcuma longa and curcumin on reproductive systems. Endocrine Regulations, 51(4), 220–228. https://doi.org/10.1515/enr-2017-0024
- Olga, U., Olga, V., Jarmila, K., Pavol, J., & Iveta, W. (2019). Rooibos tea (Aspalathus linearis) ameliorates the CCl4-induced injury to mitochondrial respiratory function and energy production in rat liver. General Physiology and Biophysics, 38(1), 15–25. https://doi.org/10.4149/gpb_2018037
- Radnai, B., Tucsek, Z., Bognar, Z., Antus, C., Mark, L., Berente, Z., GallyasJr.F., Sumegi, B., & Veres, B. (2009). Ferulaldehyde, a water-soluble degradation product of polyphenols, inhibits the lipopolysaccharide-induced inflammatory response in mice. The Journal of Nutrition, 139(2), 291–297. https://doi.org/10.3945/jn.108.097386
- Ranneh, Y., Ali, F., Akim, A. M., Hamid, H. A., Khazaai, H., & Fadel, A. (2017). Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases a review. Applied Biological Chemistry, 60(3), 327–338. https://doi.org/10.1007/s13765-017-0285-9
- Reyes-Gordillo, K., Shah, R., & Muriel, P. (2017). Oxidative stress and inflammation in hepatic diseases: Current and future therapy. Oxidative Medicine and Cellular Longevity, 2017, Article 3140673. https://doi.org/10.1155/2017/3140673
- Sai, G. U. (2006). Establishment of experimental model of chronic alcoholic fatty liver in rats. Journal of Chongqing Medical University, 31, 80–84. https://doi.org/10.1038/0253-3626(2006)01-0081-04.
- Strnad, P., Tacke, F., Koch, A., & Trautwein, C. (2017). Liver-guardiam, modifier and target of sepsis. Nature Review Gastroenterology & Hepatology, 14(1), 55–66. https://doi.org/10.1038/nrgastro.2016.168
- Su, Z. Q., Mo, Z. A., Liao, J. B., Feng, X. X., Liang, Y. Z., Zhang, X., Liu, Y. H., Chen, X. Y., Chen, Z. W., Su, Z. R., & Lai, X. P. (2014). Usnic acid protects LPS-induced acute lung injury in mice through attenuating inflammatory responses and oxidative stress. International Immunopharmacology, 22(2), 371–378. https://doi.org/10.1016/j.intimp.2014.06.043
- Wan, J. Y., Gong, X., Zhang, L., Li, H. Z., Zhou, Y. F., & Zhou, Q. X. (2008). Protective effect of baicalin against lipopolysaccharide/D-galactosamine-induced liver injury in mice by up-regulation of heme oxygenase-1. European Journal of Pharmacology, 587(1-3), 302–308. https://doi.org/10.1016/j.ejphar.2008.02.081
- Wang, D. X., Zhang, M., Wang, T. T., Cai, M., Qian, F., Sun, Y., & Wang, Y. J. (2019). Green tea polyphenols prevent lipopolysaccharide-induced inflammatory liver injury in mice by inhibiting NLRP3 inflammasome activation. Food & Function, 10(7), 3898–3908. https://doi.org/10.1039/C9FO00572B
- Wang, X., Han, C., Qin, J. J., Wei, Y. Y., Qian, X. F., Bao, Y. Z., & Shi, W. Y. (2019). Pretreatment with Salvia miltiorrhiza polysaccharides protects from lipopolysaccharides/D-galactosamine-induced liver injury in mice through inhibiting TLR4/MyD88 signaling pathway. Journal of Interferon and Cytokine Research, 39(8), 495–505. https://doi.org/10.1089/jir.2018.0137
- Xia, X. M., Su, C. Y., Fu, J. L., Zhang, P., Jiang, X. J., Xu, D. M., Hu, L. H., & Song, Y. (2014). Role of α-lipoic acid in LPS/D-GalN induced fulminant hepatic failure in mice: Studies on oxidative stress, inflammation and apoptosis. International Immunopharmacology, 22, 93–302. https://doi.org/10.1016/j.intimp.2014.07.008.
- Xu, M. C., Rui, D. S., Yan, Y. Z., Xu, S. Z., Niu, Q., Feng, G. L., Wang, Y., Li, S. G., & Jing, M. X. (2017). Oxidative damage induced by arsenic in mice or rats: A systematic review and meta-analysis. Biological Trace Element Research, 176(1), 154–175. https://doi.org/10.1007/s12011-016-0810-4
- Xu, X., Hua, H. W., Wang, L. M., He, P. W., Zhang, L., Qin, Q., Yu, C., Wang, X. Y., Zhang, G. L., & Liu, Y. L. (2020). Holly polyphenols alleviate intestinal inflammation and alter microbiota composition in lipopolysaccharide-challenged pigs. British Journal of Nutrition, 123(8), 881–891. https://doi.org/10.1017/S0007114520000082
- Xu, X. J., Chen, X. L., Huang, Z. Q., Chen, D. W., Yu, B., Chen, H., He, J., Luo, Y. H., Zheng, J., & Luo, J. Q. (2019). Dietary apple polyphenols supplementation enhances antioxidant capacity and improves lipid metabolism in weaned piglets. Journal of Animal Physiology and Animal Nutrition, 103(5), 1512–1520. https://doi.org/10.1111/jpn.13152
- Yahfoufi, N., Alsadi, N., Jambi, M., & Matar, C. (2018). The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients, 10(11), 1618. https://doi.org/10.3390/nu10111618
- Yang, S. Q., Lin, H. Z., Lane, M. D., Clemens, M., & Diehl, A. M. (1997). Obesity increases sensitivity to endotoxin liver injury: Implications for the pathogenesis of steatohepatitis. Proceedings of the National Academy of Sciences of the United States of America, 94(6), 2557–2562. https://doi.org/10.1073/pnas.94.6.2557
