ABSTRACT
This study was conducted to investigate the effects of solid-state fermented wheat bran on immune performance and inflammatory response in laying hens. A total of 225 18-week-old Hy-Line brown-egg laying hens were randomly divided into 3 groups with 5 replicates per group and 15 hens per replicate. Laying hens were fed a basal diet (corn-soybean meal diet) supplemented with 0 (control group), 10% wheat bran and 10% fermented wheat bran, respectively. The results showed: (1) Compared to wheat bran group, the contents of crude protein, trichloroacetic acid-soluble protein, dietary fibre (DF), mannan and total polyphenols in wheat bran were increased by solid state fermentation. (2) Compared to the control group, fermented wheat bran increased the levels of serum biochemical parameters, reproductive hormones, immunoglobulins and anti-inflammatory factors. Therefore, long-term dietary supplementation of 10% fermented wheat bran plays an important role in improving the immune performance of laying hens.
Introduction
With the growth of global population, competition between grain and animal feed has become increasingly serious, which will threaten grain security (Kim et al., Citation2019). Along with the intensive feeding mode and the prohibition of antibiotics, some physiological health problems may occur during laying stage, such as decreased immune performance, which further reduces the resistance of laying hens to diseases, and ultimately leads to decreased laying performance and egg quality. Soybean meal is the main protein feedstuff in animal feed because of its high feeding value (Banaszkiewicz, Citation2011). However, the contradiction between supply and demand of soybeans is greatly severe in China, so it is critical to expand the feed resources to substitute soybean meal. Wheat bran, a by-product of wheat starch process, is a rich feedstuff. The global annual output is 112 million tons, and China accounts for 20 million tons (Hell et al., Citation2014; Prückler et al., Citation2014). However, wheat bran contains 10%∼26% arabinoxylan, 11% cellulose, and 5.6% lignin (Apprich et al., Citation2014). These non-starch polysaccharides (NSP) limit the application proportion of wheat bran in animal dites (Balandrán-Quintana et al., Citation2015; Onipe et al., Citation2015).
Biological fermentation can improve the nutritional properties of wheat bran. Microbial species play a key role in fermentation process. Zhao et al. (Citation2017) discovered that when wheat bran was fermented using commercial baker's yeast and lactic acid bacteria, the levels of phytic acid and arabinoxylan were reduced, cell wall components were degraded, and more taste compounds were produced. The total phenols content of wheat bran increased during yeast fermentation, and the taste compounds were primarily 4-ethyl-2-methoxyphenol (Tu et al., Citation2020). Furthermore, wheat bran fermented with Trichoderma might boost xylanase and cellulase activity. Lin and Lee (Citation2020a) and Chu et al. (Citation2016) reported that dietary supplementation with 5% fermented wheat bran could effectively improve the growth performance and intestinal health of broilers. Teng et al. (Citation2017) also reported that wheat bran fermented with Bacillus amylolytic increased the content of lactobacillus and lactic acid in the ileum of broilers. Although the fermentation process increased the level of bioactive substances in wheat bran, the effect of fermented wheat bran on laying hens needs to be further studied. Therefore, this study was conducted to investigate the effects of long-term dietary supplementation of fermented wheat bran on laying performance, egg quality, serum biochemical indexes, reproductive hormones, immune performance and Inflammatory response in laying hens.
Materials and methods
Preparation of solid-state fermented wheat bran
The basal solid-state fermentation substrates containing 98% wheat bran and 2% glucose were mixed with water at a ratio of 1:0.8 (w:w), Lactobacillus plantarum, Bacillus subtilis, Saccharomyces cerevisiae and Aspergillus fragrans were mixed at a ratio of 3:2:4:1 with an inoculation concentration of 0.5% (v/v). Saccharomyces cerevisiae was purchased from the Angel Yeast Co., Ltd (Yichang, China), and the other strains were preserved by our laboratory. The mixture was fermented in stainless steel fermentation apparatus (, diameter 1360 mm, height 1360 mm) at 30°C for 3 days and stirred daily. The contents of Lactobacillus, Bacillus subtilis, yeast and Aspergillus in fermented wet wheat bran were 7.78, 5.62, 5.24 and 4.93 log CFU/g, respectively, which were determined according to ISO (Citation2013). Fresh samples of wet wheat bran were dried in a fluidized bed.
Determination of nutrient composition
The moisture (AOAC, 934.01), dietary fibre (AOAC, 993.21), crude fibre (AOAC, 962.09), crude ash (AOAC, 942.05), crude fat (AOAC, 920.39), and crude protein (AOAC, 990.03) were determined according to the AOAC method (AOAC, Citation2007). The total phosphorus was determined by an ultraviolet spectrophotometer (MAPADA P9, Shanghai, China), and gross energy was measured by the oxygen bomb calorimeter (IKA C6000, Germany), according to the AOCS method (AOCS, Citation2009). The total polyphenols were measured by Folin-phenol method using gallic acid as a calibration curve (Emmons et al., Citation1999). The trichloroacetic acid-soluble protein was measured according to Zhang and Shen (Citation2013). Crude polysaccharides were measured by phenol-sulfuric acid method with glucose as the reference (Dubois et al., Citation1956). The mannan was determined by high performance liquid chromatography with a standard curve derived from gradient concentrations of mannose solution (Waters e2695, USA). The specific determination conditions were as follows: 250 nm UV wavelength, mobile phase consisted of acetonitrile and ammonium formate at a ratio of 2:8, the column size was C18 (250 mm × 4.6 mm × 5μm), and the sample volume was 10 μL. The three mycotoxins were determined by high performance liquid chromatography (Waters e2695, USA), using methanol, acetonitrile and water as mobile phases, the chromatographic column was C18, in which C18 (150 mm × 4.6 mm × 5 μm) was used for zearalenone (ZEN) and deoxynivalenol (DON), and C18 (250 mm × 4.6 mm × 5 μm) was used for aflatoxin B1 (AFB1) (Herzallah, Citation2009; Rahmani et al., Citation2010).
Birds and experimental management
Laying hens were raised and handled in accordance with the guidelines of the Animal Ethics Committee of the Academy of National Food and Strategic Reserves Administration (Beijing, China). The animal experiment was performed at the Wuqing base of the National Food and Strategic Reserves Administration (Tianjing, China).
A total of 225 18-week-old Hy-Line brown-egg laying hens were selected and randomly divided into 3 treatments with 5 replicates per treatment and 15 laying hens per treatment. Hens in three groups were fed a corn-soybean meal basal diet and two experimental diets, in which the basal diet was supplemented with 10% wheat bran or 10% fermented wheat bran to partly replace soybean meal with iso-nitrogen and iso-energy principle. The composition and nutrient levels of three diets () met or exceeded the nutrient requirements of Chinese feeding standard of chicken (NY/T, Citation2004). The experiment lasted for 27 weeks and was divided into four stages (18∼27 weeks, 28∼34 weeks, 35∼39 weeks, and 40∼44 weeks).
Table 1. Ingredients and nutrient level of experimental diets for laying hens.
Laying hens were kept in battery cages with 16 h of light, and the room temperature was controlled at 20°C∼25°C, humidity 30%∼60%. According to the management procedures, regular ventilation, disinfection, and vaccination were carried out.
Laying performance
Eggs were collected every afternoon. The egg number and weight, laying rate, broken egg rate, feed intake and feed to egg ratio were all recorded and calculated at 18∼27 weeks, 28∼34 weeks, 35∼39 weeks and 40∼44 weeks.
Egg quality
Three eggs were randomly selected from each replicate at the 27th, 34th and 39th weeks to determine egg quality. Each egg was weighed individually. The egg shell thickness Gauge and Egg Force Reader (ORKA Food Technology Ltd, Ramat Hasharon, Israel) were used to measure the eggshell strength and thickness, respectively. The egg shape index (ratio of longitudinal diameter to transverse diameter) was measured by Vernier Callipers (Syntek, China). The Egg Analyzer (ORKA Food Technology Ltd, Ramat Hasharon, Israel) was used to measure yolk colour, albumen height and Haugh unit.
Serum biochemical parameters
Blood was collected from the wing veins of randomly selected laying hens before slaughter. The blood was centrifuged to obtain the serum, which was stored in the refrigerator at −20°C. Analyses were performed using an automatic biochemical analyzer (Mairui BS-420 autoanalyzer, Shenzhen, China). Serum triglyceride (TG), high density lipoprotein (HDL), trimethyl aminoxide (TMAO), total cholesterol (TC), uric acid (UA), albumin (ALB), globulin (GLB), total protein (TP), calcium (Ca) and phosphorus (P) were determined by the colorimetric method. The kits were purchased from a commercial company (BioSino Bio-Technology and Science Inc, Beijing, China).
Serum reproductive hormone
The serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), progesterone (P4) and gonadotropin-releasing hormone (GnRH) in laying hens were determined by ELISA diagnostic kits from the Beijing Sino-UK Institute of Biological Technology (Beijing, China).
Serum immunology and inflammatory factors
The serum immunoglobulin and lipopolysaccharide (LPS) levels were determined by colorimetry using kits (BioSino Bio-Technology and Science Inc, Beijing, China), and the serum levels of interleukin-2 (IL-2), interleukin-10 (IL-10) and interferon-γ (IFN-γ) were analyzed by kits from Beijing Sino-UK Institute of Biological Technology (Beijing, China).
Immune indexes of jejunal mucosa
The jejunum was collected and the content was removed. Mucosa was scraped with glass slides, put in a 1.5 mL centrifuge tube, and stored in a refrigerator at −20°C. After thawing, normal saline was added at a proportion of 1:9, and then homogenized with a homogenizer (Wheaton, USA), and centrifuged for 10 min at 4000 rpm, 4°C. The supernatant was collected for assay (Chen et al., Citation2014). The secretory immunoglobulin A (sIgA, CSB-E10097Ch) and interleukin-2 (IL-2, CSB-E06755Ch) were determined by ELISA kits (CUSABIO, Wuhan, China).
Statistical analysis
The statistical analysis was performed with SPSS 20.0 software. The indexes of wheat bran before and after fermentation were analyzed by an independent T-test. Laying performance and egg quality were analyzed by two-way ANOVA, and multiple comparisons were performed by the Duncan method. There was statistical significance at P≤ 0.05.
Results
Nutrient composition of wheat bran and fermented wheat bran
The changes in the nutrient composition of wheat bran by fermentation are shown in . After fermentation, the contents of crude protein, trichloroacetic acid-soluble protein, crude ash, gross energy, soluble dietary fibre, insoluble dietary fibre, total dietary fibre, mannan and total polyphenols in wheat bran were significantly increased (P < 0.05), while the levels of crude fat, crude fibre, aflatoxin B1 and zearalenone levels were effectively reduced (P < 0.05).
Table 2. Composition changes of wheat bran before and after fermentation (%, air-dry basis).
Laying performance
The effects of different dietary treatments and different production periods on the laying performance of laying hens were displayed in . There were no significant effects on the laying performance parameters among all the groups (P > 0.05). With the laying time increasing, the egg production, feed conversion ratio and broken egg rate of laying hens decreased, whereas the average daily feed intake, average egg weight and laying rate were increased (P < 0.05).
Table 3. Effects of fermented wheat bran on laying performance ofhens/
Egg quality
The egg quality indexes of 3 experimental treatments and 3 experimental periods were summarized in . Different dietary treatments and experiment periods had no effect on eggshell strength or Haugh unit of laying hens (P > 0.05). The egg weight, eggshell thickness, yolk colour and albumen height at 34 and 39 weeks were significantly higher than those at 27 weeks (P < 0.05). The egg shape index at 34 weeks was significantly lower than that at 27 weeks, while the egg shape index at 39 weeks was significantly higher (P < 0.05). The albumen height of laying hens at 39 weeks was significantly higher than that at 27 and 34 weeks (P < 0.05). Compared to the control group, supplementation of wheat bran or fermented wheat bran significantly increased egg yolk colour (P < 0.05).
Table 4. Effect of fermented wheat bran on egg quality of laying hens.
Serum biochemical parameters
The effects of fermented wheat bran on serum biochemical parameters of laying hens were presented in . Supplementation of wheat bran or fermented wheat bran significantly affected the levels of TMAO, ALB, TP and P in the serum of laying hens (P < 0.05). Compared to the control group, ALB, TP and P levels in the serum of laying hens supplemented with fermented wheat bran were significantly increased (P < 0.05). Compared to the control group, serum TMAO concentrations in the wheat bran group were significantly increased, but no significant effect was found on TMAO in the fermented wheat bran group (P < 0.05).
Table 5. Effects of fermented wheat bran on serum metabolites of laying hens.
Serum reproductive hormones
The effect of fermented wheat bran on serum reproductive hormone levels was displayed in . Compared to the control group, supplementation of wheat bran significantly increased the levels of E2 and GnRH in the serum of laying hens (P < 0.05), while the supplementation of fermented wheat bran had no significant effects on the levels of reproductive hormones (P > 0.05).
Table 6. Effects of fermented wheat bran on serum hormone levels of laying hens.
Serum immunoglobulin and inflammatory factors
The levels of immunoglobulin and inflammatory factors were shown in . Supplementation of wheat bran or fermented wheat bran significantly affected serum levels of IgA, IgM, IL-2 and IFN-γ(P < 0.05). Compared to the control group, supplementation of wheat bran significantly increased serum IgM levels (P < 0.05), but significantly decreased serum IL-2 and IFN-γ levels of laying hens (P < 0.05). The supplementation of wheat bran increased the serum IgA content of laying hens, but the difference was not significant compared to the control group (P > 0.05).
Table 7. Effects of fermented wheat bran on serum immunology and inflammatory factors of laying hens.
Immune indexes of jejunal mucosa
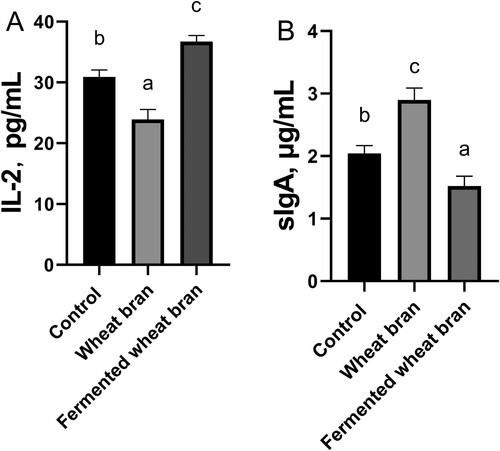
The effects of fermented wheat bran on IL-2, sIgA and IFN-γ in the jejunal mucosa of laying hens were presented in . Supplementation of wheat bran significantly increased the level of IL-2 in the jejunum mucosa (P < 0.05), but significantly decreased the level of sIgA in the jejunum mucosa (P < 0.05). The addition of fermented wheat bran significantly decreased the level of IL-2 in jejunum mucosa (P < 0.05), but significantly increased the level of sIgA in jejunum mucosa (P < 0.05).
Figure 2. Effects of wheat bran and fermented wheat bran on IL-2(A) and sIgA(B) contents of jejunal mucosa of laying hens. abc Means among without the same letter are significantly different(P < 0.05). Values are expressed as the mean ± SE (n = 5). IL-2,interleukin-2; sIgA,secretory immunoglobulin A.
Discussion
Wheat bran contains 15% crude protein, which can be used as an alternative protein or energy feedstuff in animal diets (Balandrán-Quintana et al., Citation2015). However, the high level of dietary fibre, arabinoxylan and phytic acid in wheat bran may reduce nutrient digestion and absorption (Noblet & Goff, Citation2001; Stevenson et al., Citation2012). Generally, the proportion of wheat bran added to the diet is not more than 8% in practice. After solid-state fermentation of wheat bran, the contents of aroma substances and phenols were increased, while arabinoxylan, phytic acid and acidity decreased, which may increase the nutritional value of wheat bran and application proportion in poultry diets (Spaggiari et al., Citation2020). There were two main reasons for the relative increase of crude protein level of wheat bran after solid-state fermentation: 1) Fermented material concentration effect at the expense of carbohydrates loss in the process of microbial fermentation. This is probably the main reason and can be assessed by dry matter recovery rate; 2) The bacterial protein synthesis by microorganisms using non-protein nitrogen, but this cause is hard to accurately analyze (Rozan et al., Citation2018). The reduction of crude fibre can be attributed to cellulase degradation by specific microorganisms during fermentation (Oladapo Olukomaiya et al., Citation2019). The combined action of fermentation can break down the lignocellulose in the cell wall and release polyphenols, thus the content of total polyphenols in fermented wheat bran were increased (Yin et al., Citation2018).
In the present study, the diet supplemented with 10% wheat bran or fermented wheat bran maintained the same nutritional level by adjusting diet composition, which had no effect on egg weight and laying rate. Wanzenbck et al. (Citation2020) and Huang et al. (Citation2021) also reported that dietary supplementation of 10% wheat bran or fermented wheat bran had no negative effect on the laying performance and egg quality of laying hens. Laying performance is affected by multiple factors, such as breed, laying cycle, light duration, temperature, nutrient level, environmental conditions and age. Consistent with the results of this study, the feed conversion rate of laying hens was decreased, while the laying rate was increased after 28 weeks of age (Bovera et al., Citation2014), which originated from the high yield period of laying hens at about 28 weeks of age.
Egg quality is a parameter to evaluate laying performance and economic benefits (Iskender et al., Citation2017). This study indicated yolk colour was more yellow in the wheat bran group or fermented wheat bran group than that in the control group, which might be owing to the presence of corn gluten meal in the feed formula, which is in favour of pigment deposition (Galobart et al., Citation2004). Compared with 28-week-old eggs, 40-week-old eggs had higher egg weight and yolk colour (Padhi et al., Citation2013), which was consistent with the egg quality characteristics of 34-week and 39-week-old laying hens compared with 27-week-old eggs in this experiment.
TMAO, as an intermediate product of lipid metabolism, is also one of the parameters to indicate lipid metabolism. It was reported that TMAO is related to cholesterol metabolism and vascular sclerosis, and its content is positively correlated with cardiovascular disease (Ufnal et al., Citation2015; Yang et al., Citation2019). Compared to wheat bran, long-term supplementation of fermented wheat bran could significantly reduce the level of TMAO, which may be related to the increase in active components of wheat bran and the changes of intestinal microorganisms during fermentation (Coutinho-Wolino et al., Citation2021). As an important index of protein metabolism, the level of total protein in serum is positively correlated with protein deposition, which can promote animal growth, and albumin also plays an important role in maintaining animal nutrition. In this study, it was found that supplementation of fermented wheat bran could effectively increase the levels of total protein and albumin in the serum of laying hens. This might be related to the increase of trichloroacetic acid-soluble protein levels and the decrease of phytic acid content in the fermentation process. However, Lin and Lee (Citation2020a) reported that diet supplemented with of 5% or 10% fermented wheat bran had no significant effect on TP and ALB in the serum of broilers, which might be attributed to the different fermentation process of wheat bran. Calcium and phosphorus, as the constituent elements of eggshells, are mainly derived from blood and bones (De Vries et al., Citation2010). Supplementation of fermented wheat bran increased the content of serum phosphorus in laying hens, which was beneficial to the formation of the eggshell.
There is a close correlation between reproductive hormone level and the laying performance of laying hens (Cui et al., Citation2021; Onagbesan et al., Citation2006). Genetic diversity and endocrine hormone levels mostly limit egg production behaviours (Du et al., Citation2020). GnRH mainly stimulates egg-laying behaviour, whereas FSH and LH are related to the growth and development of follicles, and E2 and P4 act on the hypothalamus and pituitary to promote ovulation by promoting the release of LH (Hernandez & Ba Hr, Citation2003; Wang et al., Citation2013). Supplementation of wheat bran increased the serum E2 and GnRH levels of laying hens, while supplementation of fermented wheat bran had no effect on these hormones. From the level of reproductive hormones, supplementation of wheat bran or fermented wheat bran had no adverse effect on laying performance, which was consistent with the results of this study on laying performance.
Immunoglobulin levels are closely related to animal immune function, especially humoral immunity (Dalakas, Citation1997). In this study, supplementation of wheat bran increased the levels of IgA and IgM in the serum of laying hens, while supplementation of fermented wheat bran had no significant effect on the level of immunoglobulins in laying hens. Lin and Lee (Citation2020b) found that supplementation of wheat bran or fermented wheat bran had no effect on serum IgG levels. As the main component of the cell wall of gram-negative bacteria, LPS induces the release of inflammatory mediators from a variety of immune cells (Glauser, Citation1996). Cytokines play a regulatory role in immunity and inflammation by transmitting information. According to their role in the process of inflammation, cytokines can be classified into pro-inflammatory factors that induce deterioration and anti-inflammatory factors that promote healing (Dinarello, Citation2000). Th1-cells release pro-inflammatory compounds such as IL-2 and IFN-γ, whereas Th2-cells secrete anti-inflammatory substances such as IL-10 (Tayal & Kalra, Citation2008). In this study, the level of IL-2 and IFN-γ was decreased. So the supplementation of wheat branenhanced the anti-inflammatory ability of laying hens, which is related to the effect of arabinoxylan contained in wheat bran (Fadel et al., Citation2018; Li et al., Citation2015). Supplementation of fermented wheat bran had no effect on pro-inflammatory factors in the serum of broilers (Chuang et al., Citation2021), which was consistent with the results of this study.
Secreted IgA is produced on the surface of the mucosa that can remove viruses and pathogenic microorganisms. It has a protective effect by aggregating pathogens by adsorption on the intestinal tract of animals (Corthésy, Citation2010; Williams & Gibbons, Citation1972). Shang et al. (Citation2020) found that dietary supplementing with 3% fermented wheat bran could increase the level of sIgA in the jejunal mucosa of broilers, while decreasing the level of several pro-inflammatory factors, which was consistent with the current results. In this study, it was found that the jejunal mucosa sIgA level increased and the IL-2 level decreased after the supplementation of fermented wheat bran. The enhancement of jejunal immunity may be related to the increase of small peptides and the changes of intestinal microorganisms during fermentation (Sugiharto & Ranjitkar, Citation2019).
Conclusions
In summary, long-term dietary supplementation of 10% fermented wheat bran could improve the immune performance and laying performance of laying hens by influencing serum biochemical, reproductive hormone and inflammatory response. Moreover, fermented wheat bran could effectively promote protein and lipid metabolism of laying hens. More studies are needed to clarify the underlying mechanisms of fermented wheat bran in modulating the immune performance of laying hens.
Abbreviations
DF, dietary fibre; NSP, non-starch polysaccharides; ZEN, zearalenone; DON, deoxynivalenol; AFB1, aflatoxin B1; TG, Triglyceride; HDL, high density lipoprotein; TMAO, trimethyl aminoxide; TC, total cholesterol; UA, uric acid; ALB, albumin; GLB, globulin; TP, total protein; Ca, calcium; P, phosphorus; FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; P4, progesterone; GnRH, gonadotropin-releasing hormone; LPS, lipopolysaccharide; IL-2, interleukin-2; IL-10, interleukin-10; IFN-γ, interferon-γ; sIgA, secretory immunoglobulin A; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M.
Acknowledgments
We thank Jishan An for the preparation of solid-state fermented wheat bran, and associate professor Shuangshuang Guo is also appreciated for critical reading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- AOAC. (2007). Official methods of analysis of AOAC Int. AOAC Int.
- AOCS. (2009). Official methods and recommended practices of the AOCS (6th ed). American Oil Chemists Society.
- Apprich, S., Tirpanalan, Ö, Hell, J., Reisinger, M., Böhmdorfer, S., Siebenhandl-Ehn, S., Novalin, S., & Kneifel, W. (2014). Wheat bran-based biorefinery 2: Valorization of products. LWT - Food Science Technology, 56(2), 222–231. https://doi.org/10.1016/j.lwt.2013.12.003
- Balandrán-Quintana, R. R., Mercado-Ruiz, J. N., & Mendoza-Wilson, A. M. (2015). Wheat bran proteins: A review of their uses and potential. Food Reviews International, 31(3), 279–293. https://doi.org/10.1080/87559129.2015.1015137
- Banaszkiewicz, T. (2011). Nutritional value of soybean meal. Soybean and nutrition, 1–20. https://doi.org/10.1093/ps/79.11.1623
- Bovera, F., Iannaccone, F., Piccolo, G., Meo, C. D., Russo, F., Piscitelli, D., Attia, Y. A., Hassan, S., & Nizza, A. (2014). Effect of group size on performance and egg quality of laying hens during 20 to 36 weeks of age. Italian Journal of Animal Science, 13(1), 174–175. https://doi.org/10.4081/ijas.2014.3148
- Chen, Z., Xie, J., Wang, B., & Tang, J. (2014). Effect of γ-aminobutyric acid on digestive enzymes, absorption function, and immune function of intestinal mucosa in heat-stressed chicken. Poultry Science, 93(10), 2490–2500. https://doi.org/10.3382/ps.2013-03398
- Chu, Y. T., Lo, C. T., Chang, S. C., & Lee, T. T. (2016). Effects of trichoderma fermented wheat bran on growth performance, intestinal morphology and histological findings in broiler chickens. Italian Journal of Animal Science, 1–11. https://doi.org/10.1080/1828051X.2016.1241133
- Chuang, W. Y., Hsieh, Y. C., Lin, L. J., Chang, S. C., & Lee, T. (2021). Effects of Saccharomyces cerevisiae and phytase co-fermentation of wheat bran on growth, antioxidation, immunity and intestinal morphology in broilers. Animal Bioscience, 34(7), 1157–1168. https://doi.org/10.5713/ajas.20.0399
- Corthésy, B. (2010). Role of secretory immunoglobulin A and secretory component in the protection of mucosal surfaces. Future Microbiology, 5(5), 817–829. https://doi.org/10.2217/fmb.10.39
- Coutinho-Wolino, K. S., de F Cardozo, L. F., de Oliveira Leal, V., Mafra, D., & Stockler-Pinto, M. B. (2021). Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far? European Journal of Nutrition, 60(7), 3567–3584. https://doi.org/10.1007/s00394-021-02491-6
- Cui, Y. M., Wang, J., Zhang, H. J., Qi, G. H., & Wu, S. G. (2021). Effect of photoperiod on performance, ovarian morphology, reproductive hormone level, and hormone receptor mRNA expression in laying ducks. Poultry Science, 100(4), 100979. https://doi.org/10.1016/j.psj.2021.01.002
- Dalakas, M. C. (1997). Intravenous immune globulin therapy for neurologic diseases. Annals of Internal medicine, 1(26), 721–730. https://doi.org/10.7326/0003-4819-126-9-199705010-00008
- De Vries, S., Kwakkel, R. P., & Dijkstra, J. (2010). Dynamics of calcium and phosphorus metabolism in laying hens. In Phosphorus and calcium utilization and requirements in farm animals (pp. 133–150). CAB International. https://doi.org/10.1079/9781845936266.0133
- Dinarello, C. A. (2000). Proinflammatory cytokines. Chest, 118(2), 503–508. https://doi.org/10.1378/chest.118.2.503
- Du, Y., Liu, L., He, Y., Dou, T., & Ge, C. (2020). Endocrine and genetic factors affecting egg laying performance in chickens: A review. British Poultry Science, 61(5), 538–549. https://doi.org/10.1080/00071668.2020.1758299
- Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356. https://doi.org/10.1021/ac60111a017
- Emmons, C. L., Peterson, D. M., & Paul, G. L. (1999). Antioxidant capacity of oat (avena sativa L.) extracts. 2. In vitro antioxidant activity and contents of phenolic and tocol antioxidants. Journal of Agricultural Food Chemistry, 47, 4894–4898. https://doi.org/10.1021/jf990530i
- Fadel, A., Plunkett, A., Li, W., Ranneh, Y., Tessu Gyamfi, V. E., Salmon, Y., Nyaranga, R. R., Ashworth, J., & Ganji, V. (2018). Arabinoxylans from rice bran and wheat immunomodulatory potentials: A review article. Nutrition Food Science, 00–00. https://doi.org/10.1108/NFS-06-2017-0111
- Galobart, J., Sala, R., Rincon-Carruyo, X., Manzanilla, E. G., Vila, B., & Gasa, J. (2004). Egg yolk color as affected by saponification of different natural pigmenting sources. Journal of Applied Poultry Research, 13(2), 328–334. https://doi.org/10.1093/japr/13.2.328
- Glauser, M. P. (1996). The inflammatory cytokines. Drugs, 52(Supplement 2), 9–17. https://doi.org/10.2165/00003495-199600522-00004
- Hell, J., Kneifel, W., Rosenau, T., & B?Hmdorfer, S. (2014). Analytical techniques for the elucidation of wheat bran constituents and their structural features with emphasis on dietary fiber – A review. Trends in Food Science Technology, 35(2), 102–113. https://doi.org/10.1016/j.tifs.2013.10.012
- Hernandez, A., & Ba Hr, J. (2003). Role of FSH and epidermal growth factor (EGF) in the initiation of steroidogenesis in granulosa cells associated with follicular selection in chicken ovaries. Reproduction, 125, 683. https://doi.org/10.1530/rep.0.1250683
- Herzallah, S. M. (2009). Determination of aflatoxins in eggs, milk, meat and meat products using HPLC fluorescent and UV detectors. Food Chemistry, 114(3), 1141–1146. https://doi.org/10.1016/j.foodchem.2008.10.077
- Huang, C. M., Chuang, W. Y., Lin, W. C., Lin, L. J., Chang, S. C., & Lee, T. T. (2021). Production performances and antioxidant activities of laying hens fed Aspergillus oryzae and phytase co-fermented wheat bran. Animal Bioscience, 34(3), 371–384. https://doi.org/10.5713/ajas.20.0116
- Iskender, H., Yenice, G., Dokumacioglu, E., Kaynar, O., HayiRli, A., & Kaya, A. (2017). Comparison of the effects of dietary supplementation of flavonoids on laying hen performance, egg quality and egg nutrient profile. British Poultry Science, 58(5), 550–556. https://doi.org/10.1080/00071668.2017.1349297
- ISO. (2013). Microbiology of food and animal feeding stuffs. Author.
- Kim, S. W., Less, J. F., Wang, L., Yan, T., Kiron, V., Kaushik, S. J., & Lei, X. G. (2019). Meeting global feed protein demand: Challenge, opportunity, and strategy. Annual Review of Animal Biosciences, 7(1), 1. https://doi.org/10.1146/annurev-animal-020518-114902
- Li, W., Zhang, S., & Smith, C. (2015). The molecular structure features-immune stimulatory activity of arabinoxylans derived from the pentosan faction of wheat flour. Journal of Cereal Science, 62, 81–86. https://doi.org/10.1016/j.jcs.2014.12.002
- Lin, W. C., & Lee, T. T. (2020a). Effects of laetiporus sulphureus-fermented wheat bran on growth performance, intestinal microbiota and digesta characteristics in broiler chickens. Animals, 10(9), 1457. https://doi.org/10.3390/ani10091457
- Lin, W. C., & Lee, T. T. (2020b). Laetiporus sulphureus fermented wheat bran enhanced the broiler growth performance by improving the intestinal microflora and inflammation status. Poultry Science, 99, 7. https://doi.org/10.1016/j.psj.2020.04.011
- Noblet, J., & Goff, G. L. (2001). Effect of dietary fibre on the energy value of feeds for pigs. Animal Feed Science and Technology, 90(1-2), 35–52. https://doi.org/10.1016/S0377-8401(01)00195-X
- NY/T. (2004). Nutrient requirements of Chinese feeding standard of chicken. The Ministry of Agriculture of the People’s Republic of China.
- Oladapo Olukomaiya, C. F., Mereddy, R., Li, X., & Sultanbawa, Y. (2019). Solid-state fermented plant protein sources in the diets of broiler chickens: A review. Animal Nutrition, 5(4), 319–330. https://doi.org/10.1016/j.aninu.2019.05.005
- Onagbesan, O. M., Metayer, S., Tona, K., Williams, J., & Bruggeman, V. (2006). Effects of genotype and feed allowance on plasma luteinizing hormones, follicle-stimulating hormones, progesterone, estradiol levels, follicle differentiation, and egg production rates of broiler breeder hens. Poult, 85(7), 1245–1258. https://doi.org/10.1093/ps/85.7.1245
- Onipe, O. O., Jideani, A. I., & Beswa, D. (2015). Composition and functionality of wheat bran and its application in some cereal food products. International Journal of Food Science Technology, 50(12), 2509–2518. https://doi.org/10.1111/ijfs.12935
- Padhi, M. K., Chatterjee, R. N., Haunshi, S., & Rajkumar, U. (2013). Effect of age on egg quality in chicken. Indian Journal of Poultry Science, 48(1), 122–125.
- Prückler, M., Siebenhandl-Ehn, S., Apprich, S., H? Ltinger, S., Haas, C., Schmid, E., & Kneifel, W. (2014). Wheat bran-based biorefinery 1: Composition of wheat bran and strategies of functionalization. LWT - Food Science Technology, 56(2), 211–221. https://doi.org/10.1016/j.lwt.2013.12.004
- Rahmani, A., Jinap, S., & Soleimany, F. (2010). Validation of the procedure for the simultaneous determination of aflatoxins ochratoxin A and zearalenone in cereals using HPLC-FLD. Food additives contaminants - part A chemistry, analysis, control. Exposure Risk Assessment, 27, 1683–1693. https://doi.org/10.1080/19440049.2010.514951
- Rozan, P., Villaum, C., Bau, H. M., Schwertz, A., Nicolas, J. P., & Mejean, L. (2018). Detoxication of rapeseed meal by rhizopus oligosporus sp-T3: A first step towards rapeseed protein concentrate. International Journal of Food Science & Technology, 31(1), 85–90. https://doi.org/10.1111/j.1365-2621.1996.17-315.x
- Shang, Q. H., Liu, S. J., He, T. F., Liu, H. S., & Piao, X. S. (2020). Effects of wheat bran in comparison to antibiotics on growth performance, intestinal immunity, barrier function and microbial composition in broiler chickens. Poultry Science, 99(10), 4929–4938. https://doi.org/10.1016/j.psj.2020.06.031
- Spaggiari, M., Ricci, A., Calani, L., Bresciani, L., Neviani, E., Dall’Asta, C., Lazzi, C., & Galaverna, G. (2020). Solid state lactic acid fermentation: A strategy to improve wheat bran functionality. LWT, 118, 108668. https://doi.org/10.1016/j.lwt.2019.108668
- Stevenson, L., Phillips, F., O'Sullivan, K., & Walton, J. (2012). Wheat bran: Its composition and benefits to health, a European perspective. International Journal of Food Sciences and Nutrition, 63(8), 1001–1013. https://doi.org/10.3109/09637486.2012.687366
- Sugiharto, S., & Ranjitkar, S. (2019). Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Animal Nutrition, 5(1), 1–10. https://doi.org/10.1016/j.aninu.2018.11.001
- Tayal, V., & Kalra, B. S. (2008). Cytokines and anti-cytokines as therapeutics — An update. European Journal of Pharmacology, 579(1-3), 1–12. https://doi.org/10.1016/j.ejphar.2007.10.049
- Teng, P. Y., Chang, C. L., Huang, C. M., Chang, S. C., & Lee, T. T. (2017). Effects of solid-state fermented wheat bran by bacillus amyloliquefaciens and saccharomyces cerevisiae on growth performance and intestinal microbiota in broiler chickens. Italian Journal of Animal Science, 1–11. https://doi.org/10.1080/1828051X.2017.1299597
- Tu, J., Zhao, J., Liu, G., Tang, C., & Xiao, H. (2020). Solid state fermentation by fomitopsis pinicola improves physicochemical and functional properties of wheat bran and the bran-containing products. Food Chemistry, 328, 127046. https://doi.org/10.1016/j.foodchem.2020.127046
- Ufnal, M., Zadlo, A., & Ostaszewski, R. (2015). Tmao: A small molecule of great expectations. Nutrition, 31(11-12), 1317–1323. https://doi.org/10.1016/j.nut.2015.05.006
- Wang, X. J., Yan, L., Song, Q. Q., Guo, Y. Y., & Hai, L. (2013). Corticosterone regulation of ovarian follicular development is dependent on the energy status of laying hens. Journal of Lipid Research, 54(7), 1860. https://doi.org/10.1194/jlr.M036301
- Wanzenbck, E., Zitz, U., Steinbauer, C., Kneifel, W., & Schedle, K. (2020). A diet containing native or fermented wheat bran does not interfere with natural microbiota of laying hens. Animal, 14, 1–9. https://doi.org/10.1017/S1751731119003343
- Williams, R. C., & Gibbons, R. J. (1972). Inhibition of bacterial adherence by secretory immunoglobulin A: A mechanism of antigen disposal. Science, 177(4050), 697–699. https://doi.org/10.1126/science.177.4050.697
- Yang, S., Li, X., Yang, F., Zhao, R., & Wu, M. (2019). Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: Inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Frontiers in Pharmacology, 10, 1360. https://doi.org/10.3389/fphar.2019.01360
- Yin, Z., Wu, W., Sun, C., Lei, Z., Chen, H., Liu, H., Chen, W., Ma, J., Min, T., Zhang, M., & Wu, H. (2018). Comparison of releasing bound phenolic acids from wheat bran by fermentation of three Aspergillus species. International Journal of Food Science & Technology, 53(5), 1120–1130. https://doi.org/10.1111/ijfs.13675
- Zhang, M., & Shen, S. (2013). Effective protein extraction protocol for proteomics studies of Jerusalem artichoke leaves. Journal of Separation Science, 36(13), 2203–2209. https://doi.org/10.1002/jssc.201300199
- Zhao, H. M., Guo, X. N., & Zhu, K. X. (2017). Impact of solid state fermentation on nutritional, physical and flavor properties of wheat bran. Food Chemistry, 217, 28–36. https://doi.org/10.1016/j.foodchem.2016.08.062