ABSTRACT
Arsenic (As), an environmental pollutant, is a highly poisonous metalloid. Accumulated evidence has shown the association between As exposure and elevated risk of the development of coronary heart disease (CHD). Calycosin, a beneficial flavonoid, has demonstrated cardioprotective activities, including those against CHD, in preclinical studies. The anti-As-related CHD activity and mechanism of calycosin have not yet been elucidated. Here, we aimed to determine the core biotargets and molecular mechanisms of calycosin against As-interrelated CHD via integrated bioinformatic analysis, including network pharmacology and molecular docking. The network pharmacology data demonstrated 41 intersection genes of calycosin against As-related CHD, prior to the identification of 15 core targets. Additional in silico investigation indicated that mitogen-activated protein kinase-3 (MAPK3), epidermal growth factor receptor (EGFR), and interleukin-6 (IL6) were the primary pharmacological targets of calycosin for the treatment of As-related CHD. In addition, the therapeutic effects can be realized via cardioprotection-associated signaling pathways for reducing As-induced myocardial toxicity and impairment and boosting CHD functional reparation. In summary, calycosin mediates potent pharmacological effects in As-related CHD therapy functioning via multiple targets and multiple pathways. The results may eventually aid in promoting future clinical application after experimental verification.
1. Introduction
Typically, occupational or environmental exposure to metalloids, such as, is a health hazard. This challenge is compounded owing to the omnipresence of such metalloids (El-Ghiaty and El-Kadi, Citation2021). In natural systems, As-contaminated food and water chains can be one of the primary sources of wildlife or botanical exposure to As. Accumulation of As in the human body may be mediated via different routes, especially by consumption of As-contaminated food and water (Santhana Kumar et al., Citation2021). The potent cytotoxic, neurotoxic, and genotoxic effects induced by As exposure are evident (Carmona et al., Citation2021; Qian et al., Citation2021; Yang et al., Citation2021b). Recently, accumulating evidence has suggested that As exposure is associated with the risk of the development of cardiovascular diseases (Karachaliou et al., Citation2021). Dietary exposure to inorganic As may induce the development of coronary heart disease (CHD) via a foodborne chain (Oberoi et al., Citation2019). A nested case–control study in China indicated that CHD incidence is closely related to As levels in plasma (Yuan et al., Citation2017). Hence, early or timely reduction of As cytotoxicity may aid in the suppression of cardiotoxic effects. Calycosin, a beneficial bioactive component separated from Astragalus membranaceus, has demonstrated evident pharmacological activities, comprising neuroprotection (Yu et al., Citation2021), anti-neoplastic action (Deng et al., Citation2021), and cardioprotection (Zhai et al., Citation2020) in preclinical studies. A preclinical study investigating the underlying pharmacological mechanisms demonstrated that anti-cardiotoxic effects of calycosin are associated with the regulation of autophagy (Lu et al., Citation2021) and inhibition of oxidative stress (Huang et al., Citation2020). However, no interrelated data corresponding to anti-As-related CHD actions and mechanisms of calycosin were explored. The network pharmacology approach may be employed to decipher bioinformatic findings of bioactive compounds used for the treatment of various disorders (Su et al., Citation2019; Li et al., Citation2021b). The findings of our previous bioinformatic studies performed using network pharmacology analysis have revealed anti- osteosarcoma (Pan et al., Citation2021), anti-meningitis (Nong et al., Citation2020), and anti-cerebral ischemia/reperfusion injury (Yu et al., Citation2021) activities of calycosin, a promising isoflavone. Here, we investigated pharmacological targets and signaling mechanisms of calycosin in the treatment of As-related CHD via a group of network pharmacology and molecular docking assays, aiming to aid further clinical validation.
2. Methods
2.1. Identification of target genes of calycosin and as-related CHD
The data were submitted to Comparative Toxicogenomics Database and SwissTargetPrediction databases to determine preliminary genes. After excluding some replicative parts, these candidate targets were re-imported to the UniProt database, in which the protein species of the common targets was set to “Homo sapiens”, and the target gene source was set to “reviewed” for normalizing gene symbols. The candidate genes of As-related CHD were determined by searching GeneCards (Stelzer et al., Citation2016) and Online Mendelian Inheritance in Man (OMIM) databases. All potential genes associated with calycosin and As-related CHD were examined with Venn mapping to analyze common genes associated with the effects of calycosin against As-related CHD using the “Venn diagram” packet in the R-language tool (Li et al., Citation2021a).
2.2. Analysis of protein–protein interaction network and core targets
All common genes were submitted again to the STRING online tool using gene symbol formats (Szklarczyk et al., Citation2015). The protein–protein interaction network map was generated through parametric screening. The R-language software was employed to determine the frequency of common genes and to further identify the core targets. Using the network analyzer plug-in in the Cytoscape tool, topological parameters, such as the median and maximum degrees of freedom in the network, were determined, and the core targets were identified according to the Degree value (Li et al., Citation2021c).
2.3. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment assays
The GO enrichment terms including biological processes (BP), cellular components (CC), and molecular functions (MF), were assayed via the Database for Annotation, Visualization and Integrated Discovery (DAVID) online database, in which the “goplot” R package was used to plot a bubble chart and circle graph of the core targets (Dennis et al., Citation2003). Using the KEGG database, all enriched core targets were transformed as visible signaling pathway diagrams (Kanehisa and Goto, Citation2000).
2.4. Network construction using bioinformatic data
The calycosin-target gene-As-related CHD network was summarized and constructed accordingly, in which this schematic diagram aimed to exhibit the preclinical perspective of the treatment of As-related CHD using calycosin (Yang et al., Citation2021a).
2.5. Molecular docking analysis of calycosin and As-related CHD
In order to computationally verify the core targets identified, molecular docking analysis was implemented. Briefly, the three-dimensional structure of the core protein was downloaded from the PDB database (Pietruś et al., Citation2022). It was then was uploaded to the AutoDock Tools programme for adding hydrogen atoms, computing the total charge, and determining the atomic type. AutoGrid4 was applied for spatial positioning and molecular docking, as detailed in previous reports (Li et al., Citation2021d).
3. Results
3.1. Identification of candidate genes of calycosin and As-related CHD
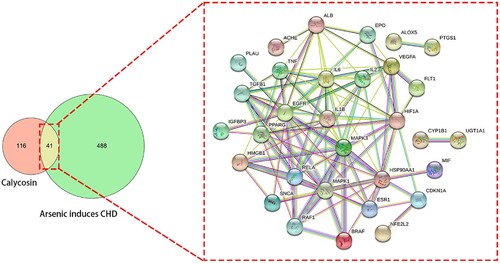
Statistically, a total of 157 and 529 candidate target genes of calycosin and As-related CHD were identified, respectively. As revealed in the Venn diagram, 41 intersection genes of calycosin against As-related CHD were identified. These shared genes were used for the visualization in the interaction network map ().
3.2. Identification of core targets of calycosin against As-related CHD
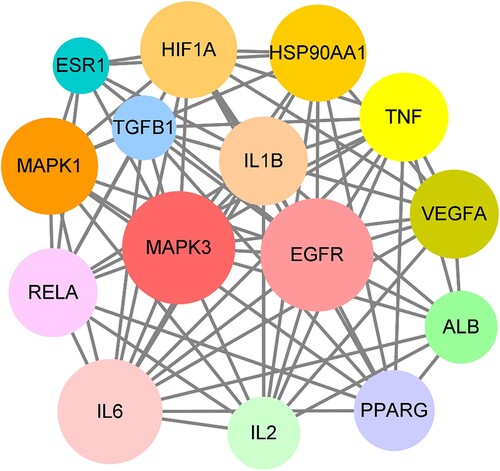
The Cytoscape tool was used to determine the parametric interconnection for screening and ascertaining the top potential genes of calycosin against As-related CHD. These core targets were identified completely, including MAPK3, EGFR, IL6, MAPK1, HSP90AA1, HIF1A, IL1B, VEGFA, TNF, RELA, PPARG, ALB, IL2, TGFB1, and ESR1, respectively (; Supplementary Table S1).
3.3. Go and KEGG pathway enrichment findings
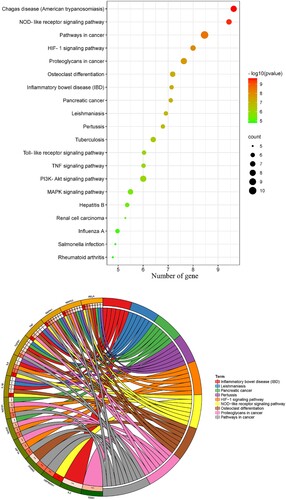
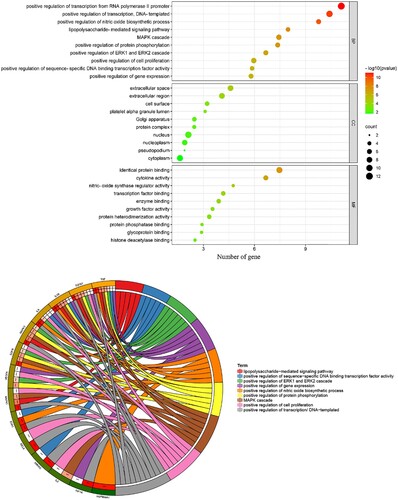
The enrichment analysis performed using core targets suggested that GO entries of all categories were harvested. Among these GO terms, the biological processes primarily comprised positive regulation of the transcription-mediated via RNA polymerase II promoter, positive regulation of DNA-templated transcription, positive regulation of nitric oxide biosynthetic process, lipopolysaccharide-mediated signaling pathway, MAPK cascade, positive regulation of protein phosphorylation, positive regulation of ERK1 and ERK2 cascade, positive regulation of cell proliferation, positive regulation of sequence-specific DNA binding transcription factor activity, and positive regulation of gene expression. The cellular components might involve extracellular space, extracellular region, cell surface, platelet alpha granule lumen, Golgi apparatus, protein complex, nucleus, nucleoplasm, pseudopodium, and cytoplasm. The following molecular functions were involved: identical protein binding, cytokine activity, nitric-oxide synthase regulator activity, transcription factor binding, enzyme binding, growth factor activity, protein heterodimerization activity, protein phosphatase binding, glycoprotein binding, and histone deacetylase binding ((a, b); Supplementary Table S2). The pathway enrichment analysis indicated that a number of 81 KEGG terms were screened and identified (P < 0.05), as shown in (a, b) (Supplementary Table S3). The following pharmacological mechanisms were observed: Chagas disease (American trypanosomiasis), NOD-like receptor signaling pathway, pathways in cancer, HIF-1 signaling pathway, proteoglycans in cancer, osteoclast differentiation, inflammatory bowel disease, pancreatic cancer, leishmaniasis, pertussis, tuberculosis, toll-like receptor signaling pathway, TNF signaling pathway, PI3K-Akt signaling pathway, MAPK signaling pathway, hepatitis B, renal cell carcinoma, influenza A, Salmonella infection, and rheumatoid arthritis. In addition, calycosin–core target–As-related CHD network maps were integrated and visualized ().
Figure 3. GO-determined characteristics of calycosin against As-related CHD, as shown in the bubble diagram (A) and the circle chart (B).
3.4. Molecular docking validation
PDB IDs including 6GES, 5UGC, and 1ALU were identified and used for the molecular docking analyses of MAPK3, EGFR, and IL6, respectively. In the 6GES structure (MAPK3), the parameters set by the active cavity box model are listed as follows: x = 40, y = 40, z = 40; spacing = 0.375; and center-x, -y, and -z were 60.717, 20.955, and 18.71, respectively. The root mean square deviation (RMSD) of the original ligand was 3.51 Å. The hydrogen bond between the original ligands in 6H3 and 6GES interacted with the amino acid residues of MET-125, ASP-128, ASN-171, SER-170, and ASP-184. The free docking energy was −9.83 Kcal/mol. Calycosin and the amino acid residues, namely, ASP-128, MET-125, and ASP-123 formed hydrogen bonds, and the free docking energy was −7.34 Kcal/mol ((a)).
Figure 6. Molecular docking mode chart of calycosin against As-related CHD, including (A) MAPK3 and 6GES; (B) EGFR and 5UGC; (C) IL6 and 1ALU.
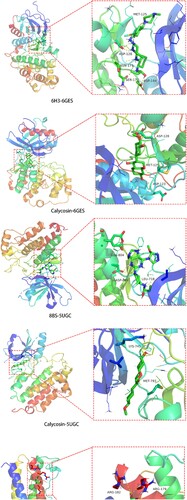
In the 5UGC of EGFR, the parameters set by the active cavity box model are listed as follows: x = 40, y = 40, z = 40; spacing = 0.375; and center-x, -y, and -z were −12.571, 14.653, and −25.527, respectively. The RMSD of the original ligand was 3.96 Å. The hydrogen bond between the original ligands in 8BS and 5UGC interacted with the amino acid residues of GLU-804, ASP-800, and LEU-718. The free docking energy was −6.06 Kcal/mol. Calycosin and the amino acid residues, namely, LYS-745, MET-793 formed hydrogen bonds, and the free docking energy was −8.42 Kcal/mol ((b)).
In the 1ALU of IL6, the parameters set by the active cavity box model were x = 40, y = 40, z = 40, spacing = 0.375, and center-x, -y, and -z were −7.547, −12.764, and −0.134, respectively. The RMSD of the original ligand was 1.62 Å. The hydrogen bond between the original ligands in TLA and 1ALU interacted with the amino acid residues of ARG-182, ARG-179, and GLN-175. The free docking energy was −0.74 Kcal/mol. The calycosin and the amino acid residues, namely, ASP-26, ARG-30, ARG-182, ARG-179, and GLN-175 formed hydrogen bonds, and the free docking energy was −5.58 Kcal/mol ((c)).
4. Discussion
CHD is a marked public health challenge around the world. If not controlled in a timely fashion, CHD may lead to myocardial asthenia, heart failure, and even death (Greenland and Lloyd-Jones, Citation2022), with a lower quality of life among patients. As, a well-known trioxide toxin, is involved in the development of cardiopathological disorders, such as arrhythmia, ischemia, and heart failure (Alamolhodaei et al., Citation2015). Thus, As exposure-induced cardiotoxicity can worsen cardiac conditions in patients with CHD. In routine clinical management, salicylic acid or statins can be prescribed to patients with CHD. However, certain undesirable side effects have been detected, such as indigestion, dizziness or statin-caused myositis, rhabdomyolysis, and hyperglycemia. Therefore, exploring a safe and effective compound, especially a natural ingredient, for the treatment of As-related CHD is essential. Calycosin is a well-studied naturally-occurring compound demonstrating potent cardiovascular protection activities. Nevertheless, the therapeutic effects and molecular mechanisms of calycosin for the treatment of As-related CHD have not been reported. Using network pharmacology analysis in this study, we identified that 41 common targets may be associated with the underlying pharmacological effects mediated by calycosin for the treatment of As-related CHD. Moreover, a total of 15 core targets of calycosin against As-related CHD were identified for further investigation. These core targets used for enrichment analysis revealed that anti-As-related CHD functions of calycosin were characterized by positive regulation of cell proliferation, metabolism, protein phosphorylation, and transcription factor activity. Additional KEGG analyses demonstrated that anti-As-related CHD mechanisms of calycosin were evidently implicated in NOD-like receptor signaling pathway, HIF-1 signaling pathway, toll-like receptor signaling pathway, TNF signaling pathway, PI3K-Akt signaling pathway, and MAPK signaling pathway. In-silico data obtained using molecular docking suggested that calycosin could bind to the following identified core targets (less than −5.0 kcal/mol): MAPK3, EGFR, and IL6. The docked molecules may be potential pharmacological targets for calycosin against As-related CHD. The MAPK molecular pathway is significantly associated with cardiovascular diseases, including CHD (Zhang et al., Citation2020). MAPK3, another term as Erk1, is a primary MAPK activated in response to extracellular stimuli, such as growth factors, cytokines, and mitogens (Roskoski, Citation2012). MAPK3 has been associated with the effects of certain compounds isolated in traditional Chinese medicine, such as rutin, against CHD (Lv et al., Citation2018). EGFR, a functional factor receptor, can activate certain kinase pathways located in the cell, inducing cell proliferation (Sharma et al., Citation2021). Accumulated evidence shows that EGFR activity may be involved in mediating cardiac fibrosis via signaling crosstalk (Zou et al., Citation2021). IL6, a circulating pleiotropic cytokine, is strongly associated with the development of inflammation (Jarlborg and Gabay, Citation2022). IL-6 may be involved in the detection of coronary vascular disease in women (Gurzău et al., Citation2021). Collectively, calycosin-mediated anti-As-related CHD targets were associated with main biological functions, including gene transcription, myocardial proliferation, cardiac metabolism, or reconstruction. The anti-As-related CHD effects of calycosin were primarily mediated via the regulation of NOD-like receptor signaling pathway, toll-like receptor signaling pathway, TNF signaling pathway, PI3K-Akt signaling pathway, and MAPK signaling pathway.
Our preclinical findings obtained using bioinformatic analysis elucidated pharmacological activities and therapeutic mechanisms of potential cardioprotection activity of calycosin against As-related CHD. The mechanism of the anti-As-related CHD effect showed that the therapeutic actions were mediated through some cardioprotection-associated signaling pathways associated with the alleviation of As-induced myocardial toxicity or damage and promoting CHD functional recuperation. Accordingly, calycosin may be clinically applied in combination therapy to enhance the therapeutic efficacy for CHD, including As-related CHD.
5. Conclusion
Herein, bioinformatic findings based on network pharmacology and molecular docking assays helped elucidate the biological network targets of calycosin against As-related CHD, comprising core targets. The pharmacological pathways/mechanisms involved were also revealed, which may pave the way for future clinical applications.
Declaration of interest
The authors declare that they have no conflict of interest.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alamolhodaei, N. S., Shirani, K., & Karimi, G. (2015). Arsenic cardiotoxicity: An overview. Environmental Toxicology and Pharmacology, 40(3), 1005–1014. https://doi.org/10.1016/j.etap.2015.08.030
- Carmona, A., Roudeau, S., & Ortega, R. (2021). Molecular mechanisms of environmental metal neurotoxicity: A focus on the interactions of metals with synapse structure and function. Toxics, 9(9), 198. https://doi.org/10.3390/toxics9090198
- Deng, M., Chen, H., Long, J., Song, J., Xie, L., & Li, X. (2021). Calycosin: A review of its pharmacological effects and application prospects. Expert Review of Anti-Infective Therapy, 19(7), 911–925. https://doi.org/10.1080/14787210.2021.1863145
- Dennis, G., Sherman, B. T., Hosack, D. A., Yang, J., Gao, W., Lane, H. C., & Lempicki, R. A. (2003). David: Database for annotation, visualization, and integrated discovery. Genome Biology, 4(5), 3. https://doi.org/10.1186/gb-2003-4-5-p3
- El-Ghiaty, M. A., & El-Kadi, A. O. S. (2021). Arsenic: Various species with different effects on cytochrome P450 regulation in humans. EXCLI Journal, 20, 1184–1242.
- Greenland, P., & Lloyd-Jones, D. M. (2022). Role of coronary artery calcium testing for risk assessment in primary prevention of atherosclerotic cardiovascular disease: A review. JAMA Cardiology, 7(2), 219. https://doi.org/10.1001/jamacardio.2021.3948
- Gurzău, D., Sitar-Tăut, A., Caloian, B., Guşetu, G., Comşa, H., Frîngu, F., Zdrenghea, D., & Pop, D. (2021). The role of IL-6 and ET-1 in the diagnosis of coronary microvascular disease in women. Journal of Personalized Medicine, 11(10), 965. doi:10.3390/jpm11100965
- Huang, J., Shen, H., Jiang, M., Huang, L., Yuan, Y., & Wang, Q. (2020). Calycosin reduces infarct size, oxidative stress and preserve heart function in isoproterenol-induced myocardial infarction model. Pakistan Journal of Pharmaceutical Sciences, 33, 1341–1347.
- Jarlborg, M., & Gabay, C. (2022). Systemic effects of IL-6 blockade in rheumatoid arthritis beyond the joints. Cytokine, 149, 155742. https://doi.org/10.1016/j.cyto.2021.155742
- Kanehisa, M., & Goto, S. (2000). Kegg: Kyoto encyclopedia of genes and genomes. Nucleic Acids Research, 28(1), 27–30. https://doi.org/10.1093/nar/28.1.27
- Karachaliou, C., Sgourou, A., Kakkos, S., & Kalavrouziotis, I. (2021). Arsenic exposure promotes the emergence of cardiovascular diseases. Reviews on Environmental Health, https://doi.org/10.1515/reveh-2021-0004
- Li, R., Guo, C., Li, Y., Qin, Z., & Huang, W. (2021a). Therapeutic targets and signaling mechanisms of vitamin C activity against sepsis: A bioinformatics study. Briefings in Bioinformatics, 22, bbaa079.
- Li, R., Li, Y., Liang, X., Yang, L., Su, M., & Lai, K. P. (2021b). Network pharmacology and bioinformatics analyses identify intersection genes of niacin and COVID-19 as potential therapeutic targets. Briefings in Bioinformatics, 22(2), 1279–1290. https://doi.org/10.1093/bib/bbaa300
- Li, R., Wu, K., Li, Y., Liang, X., Lai, K. P., & Chen, J. (2021c). Integrative pharmacological mechanism of vitamin C combined with glycyrrhizic acid against COVID-19: Findings of bioinformatics analyses. Briefings in Bioinformatics, 22(2), 1161–1174. https://doi.org/10.1093/bib/bbaa141
- Li, Y., Yu, S., Li, Y., Liang, X., Su, M., & Li, R. (2021d). Medical significance of uterine corpus endometrial carcinoma patients infected With SARS-CoV-2 and pharmacological characteristics of plumbagin. Frontiers in Endocrinology, 12, 714909. https://doi.org/10.3389/fendo.2021.714909
- Lu, X., Lu, L., Gao, L., Wang, Y., & Wang, W. (2021). Calycosin attenuates doxorubicin-induced cardiotoxicity via autophagy regulation in zebrafish models. Biomedicine & Pharmacotherapy, 137, 111375. https://doi.org/10.1016/j.biopha.2021.111375
- Lv, L., Yao, Y., Zhao, G., & Zhu, G. (2018). Rutin inhibits coronary heart disease through ERK1/2 and Akt signaling in a porcine model. Experimental and Therapeutic Medicine, 15, 506–512.
- Nong, Y., Liang, Y., Liang, X., Li, Y., & Yang, B. (2020). Pharmacological targets and mechanisms of calycosin against meningitis. Aging (Albany NY), 12(19), 19468–19492. https://doi.org/10.18632/aging.103886
- Oberoi, S., Devleesschauwer, B., Gibb, H. J., & Barchowsky, A. (2019). Global burden of cancer and coronary heart disease resulting from dietary exposure to arsenic, 2015. Environmental Research, 171, 185–192. https://doi.org/10.1016/j.envres.2019.01.025
- Pan, Q., Wu, K., Tan, J., Li, Y., Liang, X., & Su, M. (2021). Anti-neoplastic characteristics and potential targets of calycosin against bisphenol A-related osteosarcoma: Bioinformatics analysis. Bioengineered, 12(1), 4278–4288. https://doi.org/10.1080/21655979.2021.1956401
- Pietruś, W., Kafel, R., Bojarski, A. J., & Kurczab, R. (2022). Hydrogen bonds with fluorine in ligand-protein complexes-the PDB analysis and energy calculations. Molecules, 27(3), 1005. https://doi.org/10.3390/molecules27031005
- Qian, Q., Chen, Y., Wang, J. Q., Yang, D. Q., Jiang, C., Sun, J., Dong, J., & Li, G. C. (2021). Use of the alkaline comet assay for monitoring genotoxic effects of arsenic in human populations. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 867, 503368. https://doi.org/10.1016/j.mrgentox.2021.503368
- Roskoski, R. (2012). Erk1/2 MAP kinases: Structure, function, and regulation. Pharmacological Research, 66(2), 105–143. https://doi.org/10.1016/j.phrs.2012.04.005
- Santhana Kumar, V., Raman, R. K., Talukder, A., Mahanty, A., Sarkar, D. J., Das, B. K., Bhowmick, S., Samanta, S., Manna, S. K., & Mohanty, B. P. (2021). Arsenic bioaccumulation and identification of Low-arsenic-accumulating food fishes for aquaculture in arsenic-contaminated ponds and associated aquatic ecosystems. Biological Trace Element Research, https://doi.org/10.1007/s12011-021-02858-0
- Sharma, B., Singh, V. J., & Chawla, P. A. (2021). Epidermal growth factor receptor inhibitors as potential anticancer agents: An update of recent progress. Bioorganic Chemistry, 116, 105393. https://doi.org/10.1016/j.bioorg.2021.105393
- Stelzer, G., Rosen, N., Plaschkes, I., Zimmerman, S., Twik, M., Fishilevich, S., Stein, T. I., Nudel, R., Lieder, I., Mazor, Y., Kaplan, S., Dahary, D., Warshawsky, D., Guan-Golan, Y., Kohn, A., Rappaport, N., Safran, M., & Lancet, D. (2016). The GeneCards suite: From gene data mining to disease genome sequence analyses. Current Protocols in Bioinformatics, 54(1), 1.30.1–1.30.33. https://doi.org/10.1002/cpbi.5
- Su, M., Guo, C., Liu, M., Liang, X., & Yang, B. (2019). Therapeutic targets of vitamin C on liver injury and associated biological mechanisms: A study of network pharmacology. International Immunopharmacology, 66, 383–387. https://doi.org/10.1016/j.intimp.2018.11.048
- Szklarczyk, D., Franceschini, A., Wyder, S., Forslund, K., Heller, D., Huerta-Cepas, J., Simonovic, M., Roth, A., Santos, A., Tsafou, K. P., Kuhn, M., Bork, P., Jensen, L. J., & von Mering, C. (2015). String v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Research, 43(D1), D447–D452. https://doi.org/10.1093/nar/gku1003
- Yang, L., Yang, J., Liang, X., Huang, W., Zhang, X., & Li, R. (2021a). Uncovering antiobesity-related hypertension targets and mechanisms of metformin, an antidiabetic medication. Bioengineered, 12(1), 4757–4767. https://doi.org/10.1080/21655979.2021.1954581
- Yang, Y., Wei, S., Zhang, B., & Li, W. (2021b). Recent progress in environmental toxins-induced cardiotoxicity and protective potential of natural products. Frontiers in Pharmacology, 12, 699193. https://doi.org/10.3389/fphar.2021.699193
- Yu, S., Wu, K., Liang, Y., Zhang, H., Guo, C., & Yang, B. (2021). Therapeutic targets and molecular mechanism of calycosin for the treatment of cerebral ischemia/reperfusion injury. Aging (Albany NY), 13(12), 16804–16815. https://doi.org/10.18632/aging.203219
- Yuan, Y., Xiao, Y., Feng, W., Liu, Y., Yu, Y., Zhou, L., Qiu, G., Wang, H., Liu, B., Liu, K., Yang, H., Li, X., Min, X., Zhang, C., Xu, C., Zhang, X., He, M., Hu, F. B., Pan, A., & Wu, T. (2017). Plasma metal concentrations and incident coronary heart disease in Chinese adults: The dongfeng-tongji cohort. Environmental Health Perspectives, 125(10), 107007. https://doi.org/10.1289/EHP1521
- Zhai, J., Tao, L., Zhang, S., Gao, H., Zhang, Y., Sun, J., Song, Y., & Qu, X. (2020). Calycosin ameliorates doxorubicin-induced cardiotoxicity by suppressing oxidative stress and inflammation via the sirtuin 1–NOD-like receptor protein 3 pathway. Phytotherapy Research, 34(3), 649–659. https://doi.org/10.1002/ptr.6557
- Zhang, G., Wang, S., Gu, Y., Song, L., Yu, S., & Feng, X. (2020). Tai Chi improves coronary heart disease risk by inactivating MAPK/ERK pathway through serum miR-126. Evidence-Based Complementary and Alternative Medicine, 2020, 4565438.
- Zou, Y., Pan, L., Shen, Y., Wang, X., Huang, C., Wang, H., Jin, X., Yin, C., Wang, Y., Jia, J., Qian, J., Zou, Y., Gong, H., & Ge, J. (2021). Cardiac Wnt5a and Wnt11 promote fibrosis by the crosstalk of FZD5 and EGFR signaling under pressure overload. Cell Death & Disease, 12(10), 877. https://doi.org/10.1038/s41419-021-04152-2