ABSTRACT
Chronic exposure to airborne particulate matter (PM) causes respiratory damage in humans owing to oxidative stress and inflammation. Chrysanthemum zawadskii Herbich var. latilobum (Maxim.) Kitamura (CZL) has been used in traditional medicine to treat several inflammatory diseases; however, studies on inflammatory pulmonary diseases are scarce. This study investigated the protective effects of CZL extract against PM10-induced lung injury in BALB/c mice. Cell type specific signaling pathways were explored using A549 and RAW264.7 cell lines. CZL extract noticeably attenuated PM10-induced lung injury and inflammatory cell infiltration in a mouse model. Protein markers, such as p-AKT, p-ERK, and p-NF-κB for PM10 induced lung inflammation were effectively reduced in CZL extract-treated mice and cells. Furthermore, CZL extracts considerably reduced the generation of reactive oxygen species and nitric oxide in cells. Collectively, CZL extract effectively reduced PM10-induced lung injury by suppressing pulmonary inflammation, potentially due to its anti-inflammatory and antioxidant properties.
Introduction
Particulate matter 10 (PM10; inhalable particles with diameters of 10 μm and smaller) is an indicator of air quality and consists of various toxic substances, including heavy metals, acid oxides, organic pollutants, bacteria, and viruses (Harrison & Yin, Citation2000). Owing to its small particle size, it is easily inhaled and accumulates in airways, thereby causing damage to the human respiratory and circulatory systems (Cho et al., Citation2018). Several studies have shown that PM10 induces inflammation-mediated lung damage via oxidative stress from excessive reactive oxygen species (ROS) (Kim et al., Citation2017; Xu et al., Citation2011) and phagocytic oxidative stress (Han et al., Citation2021; Misiukiewicz-Stepien & Paplinska-Goryca, Citation2021). PM10-induced oxidative damage induces the release of various cytokines and chemokines (Lee et al., Citation2018; Weiden et al., Citation2012; Yan et al., Citation2018), which are risk factors that potentially lead to inflammatory tissue damage.
Plant-based traditional medicines have been used in Asian countries to treat or prevent PM10-induced lung disease (Cui et al., Citation2021; Ko et al., Citation2020). For instance, Chrysanthemum zawadskii Herbich var. latilobum (Maxim.) Kitamura (CZL) is effective in treating respiratory diseases, such as pneumonia, bronchitis, and pharyngitis (Hyun et al., Citation2011; Uehara et al., Citation2012). CZL, widely cultivated in East Asia and Eastern Europe, is commonly used as a traditional medicine in Korea owing to its diverse bioactive properties (Kwon et al., Citation2006; Shim et al., Citation2012; Kim et al., Citation2001). Moreover, other biological properties (e.g. anti-inflammation and anti-oxidative stress) have been reported (Kim, Citation2017; Kim et al., Citation2012; Singh et al., Citation2005; Suh et al., Citation2013; Wu et al., Citation2011). Kim et al. (Citation2015) and You and Moon (Citation2016) reported that CZL extract showed anti-inflammatory effects by suppressing inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in RAW264.7 macrophage induced by lipopolysaccharide (LPS). Wu et al. (Citation2011) reported that CZL extracts exerted anti-inflammatory, anti-oxidative, and detoxification properties through potent inhibitory effects on NF-κB-mediated inflammation and activation of the Nrf2-ARE-anti-oxidative stress signaling pathway.
Despite promising pharmacological benefits, studies on the protective potential of CZL against PM-induced lung injury are limited. To this end, this study investigated whether (1) CZL water extract alleviated inflammation-mediated respiratory injury in response to acute exposure to PM10 in mice, (2) identified the key signaling pathways responsible for such protection, and (3) validated the key signaling pathway using human lung epithelial cell line and macrophage cell line in vitro models.
Materials and methods
Materials
Dried CZL was purchased from a local market (Jeongeup, Republic of Korea) and extracted in distilled water at 80°C for 8 hr using a reflux condenser with a solid-to-liquid ratio of 1:10 (w/v). The extracted material was then filtered, lyophilized, and stored at −80°C until further analysis.
Animal study
7-week-old BALB/c male mice were purchased from Raon Bio, Inc. (Yong-in, Republic of Korea). All animals were housed in polypropylene cages (five mice per cage) in a temperature-controlled room (24 ± 2°C) with a relative humidity of 50–60% and a 12 hr light/dark cycle. During the experiment, mice had free access to food and water. After a week of acclimation, a total of 20 mice were randomly assigned to four groups: negative control (CON [n = 5); saline), PM10 control (PM10 [n = 5); PM10 10 mg/kg), positive control (P-CON [n = 5); naringin 100 mg/kg + PM10), and CZL water extract (CZL [n=5)]; CZL 200 mg/kg + PM10). The 2018 Teklad Global 18% Protein Rodent Diet (Raon Bio Inc.) was used as the basal diet for all groups. Saline, naringin, and CZL were orally administered once daily for one week. On the last day of CZL intervention (i.e. day 7), 10 mg/kg PM10 was intranasally administered to PM10, P-CON, and CZL mice. The CON mice were nasally administered with saline. After 6 hr of exposure to PM10, the mice were euthanized by intraperitoneal injection of avertin (tribromoethanol; 200 mg/kg); bronchoalveolar lavage fluid (BALF) and lung tissues were collected. The harvested lung tissues were stored at either −80°C or 4% paraformaldehyde. All animal care and experimental procedures were approved by the ethics committee of Korea University (approval number: KUIACUC-2020-0044).
Histological analysis
The left lobe of lung tissue was fixed in 4% paraformaldehyde, dehydrated in ethanol, and embedded in paraffin. Subsequently, 3 μm tissue sections were prepared using a microtome for hematoxylin and eosin (H&E) staining. The stained images were visualized under an inverted microscope (Olympus BH 2, Tokyo, Japan; magnification, 200×).
Quantification of TNF-α and IL-1β in BALF
The levels of TNF-α and IL-1β in BALF were quantified using specific ELISA kits (R&D Systems, Minneapolis, MN, USA) per the manufacturer's instructions. After the substrate reaction, colour development was stopped, and the intensity of colour was assayed at 450 nm using a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA).
Cell culture
The A549 human alveolar epithelial cell line and the RAW264.7-macrophage cell line were obtained from the Korean Cell Line Bank (Seoul, Republic of Korea) and maintained at 37°C in a humidified CO2 incubator. A549 cells and RAW264.7 macrophage were maintained in Roswell Park Memorial Institute medium (Gibco, Waltham, MA, USA) and high-glucose Dulbecco's Modified Eagle Medium (Gibco), respectively. All growth media were supplemented with 10% (v/v) fetal bovine serum (Gibco) and 1% (v/v) penicillin/streptomycin solution (Gibco).
Nitric oxide (NO) analysis
RAW264.7 macrophage (2×105 cells/well) were plated on a 24-well plate for 24 hr, and then, 100 ng/mL of lipopolysaccharide (LPS) and the sample solution was co-treated for 24 hr. The concentration of NO was measured using Griess reagent. Equal volumes of supernatant and Griess reagent were mixed (i.e. 50 μL each) and incubated for 15 min at room temperature. The absorbance was measured at 540 nm using a microplate reader (Bio-Rad Laboratories). A standard curve of sodium nitrate was used to calculate the NO concentrations of the samples. The sample concentrations were determined based on a cytotoxicity test (Supplemental File 1).
Intracellular levels of ROS
A549 cells (1×104 cells/well) were plated in a 96-well plate for 24 hr, and then, 200 μg/mL of PM10 and sample diluent were co-treated for 60 min. A fluorescent probe (Sigma-Aldrich, St. Louis, MO, USA) was then loaded onto the cells and incubated for 15 min. After incubation, the cells were immediately placed in a spectrofluorometer (Perkin Elmer, Waltham, MA, USA) to determine ROS generation at excitation and emission wavelengths of 535 and 485 nm, respectively.
Western blot analysis
Total protein was extracted from the lung tissue and cells ((PM10 (200 mg/mL), LPS (100 ng/mL), and CZL (0.5, 1, 2 mg/mL)) were co-treated for 6 hr), and protein concentrations were measured using a bicinchoninic acid protein assay kit (Sigma-Aldrich). The adjusted protein samples (20 μg) were separated by 10% and 12% sodium dodecyl salt-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Boston, MA, USA). Membranes were blocked with 5% skim milk at room temperature for 1 hr, followed by incubation with primary antibodies (anti-β-actin and CypB, dilution 1:5000; anti-pAKT, AKT, pERK, ERK, pNF-κB, NF-κB, iNOS, COX-2, TNF-α, IL-1β, and IL-6, dilution 1: 2000) (Cell Signaling, Danvers, MA, USA; Santa Cruz Biotechnology, Dallas, TX, USA; Abcam, Cambridge, UK) at 4°C for 16 hr. After five consecutive washes with Tris-buffered saline-Tween buffer, the membranes were probed with horseradish peroxidase-conjugated secondary antibodies for 1 hr at room temperature. The bands were developed using enhanced chemiluminescence reagents (Bio-Rad Laboratories) and visualized using an Image Quant LAS-4000 chemiluminometer (GE Healthcare, Chicago, IL, USA). The captured bands were quantified using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed using the IBM SPSS software (version 25.0; IBM Corp, Armonk, NY, USA). Data are expressed as mean ± standard deviation (SD) from three independent experiments unless otherwise specified. Differences between groups were analyzed with one-way analysis of variance (ANOVA) followed by Dunnett’s multi-range test, where p < 0.05 was considered to indicate a significant difference.
Results
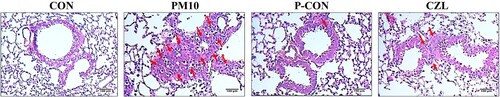
Effects of CZL extract on PM10-induced lung tissue damage
Although there were no changes in body weight (Supplemental file 2), PM10-induced morphological damage in mice lung tissues was reversed by the CZL extract treatment. Specifically, PM10 significantly impaired lung tissue morphology, with increased alveolar wall thickness, edema, and inflammatory cell infiltration, all of which were markedly suppressed in the lungs of CZL extract-treated mice ().
Effects of CZL extract on PM10-induced pulmonary inflammation
PM10 increased TNF-α in BALF by approximately 8-fold, and CZL extracts significantly decreased TNF-α levels to levels similar to those in CON or P-CON. In addition, the PM10 administrated group increased the level of IL-1β by approximately 2-fold compared to the CON group, while the CZL extract-treated group restored the level of IL-1β to the normal range as much as the CON group ((a)). Next, we examined key proteins involved in inflammatory signaling pathways, such as AKT, ERK, NF-κB, iNOS, COX-2, TNF-α, IL-1β, and IL-6. Phosphorylation of AKT, ERK, and NF-κB increased in PM10-treated mice, whereas CZL extract treatment dramatically lowered protein expression compared to CON mice ((b)). Additionally, iNOS, COX-2, TNF-α, IL-1β, and IL-6 levels were increased in PM10 mice compared to CON mice and were restored by CZL extract treatment ((b)).
Figure 2. Effects of CZL extract treatment on inflammatory protein expression in PM10-treated mice (A, B) and A549 cells (C). Data are expressed as the mean ± SD. Statistical significance was calculated using one-way ANOVA followed by Dunnett’s post-hoc test. *p< 0.05; **p< 0.01 vs. PM10 group.
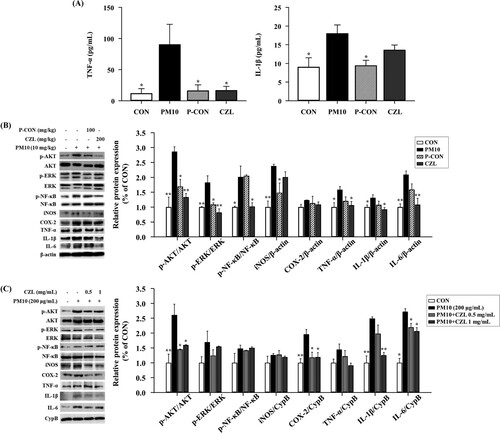
To validate the observations of our in vivo study, the same inflammatory protein markers were assessed in the A549 cells. Overall, our in vitro results were consistent with our in vivo findings; although phosphorylation of AKT, ERK, and NF-κB was increased, albeit NF-κB was marginally changed ((c)). Phosphorylated AKT was significantly decreased by CZL extract treatment regardless of exposure level. However, only low-dose CZL (0.5 mg/mL) was effective against phosphorylated ERK and NF-κB. In addition, iNOS, COX-2, TNF-α, IL-1β, and IL-6 levels were restored by PM10-induced stimulation in a dose-dependent manner in CZL extract-treated cells, which is in line with the results in lung tissues.
Effects of CZL extract on NO production and macrophage inflammation in RAW264.7 cells
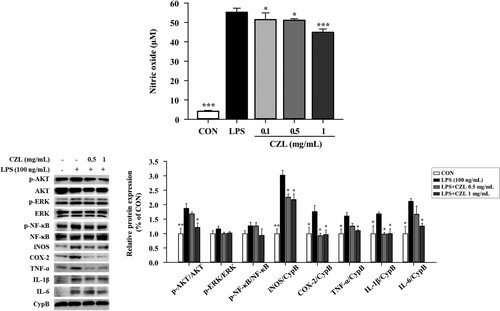
The inhibitory effects of the CZL extract on LPS-induced NO production were evaluated. CZL extract treatment reduced NO production in vitro in a dose-dependent manner ((a)). Furthermore, the effects of CZL extract on the expression of inflammatory proteins in RAW264.7 macrophage were confirmed. Similar to the aforementioned mice and A549 cell studies, CZL extract treatment decreased phosphorylation of AKT, ERK, and NF-κB on LPS treatment in RAW264.7 macrophage ((b)). In addition, the levels of remaining proteins (e.g. iNOS, COX-2, TNF-α, IL-1β, and IL-6) were effectively reduced in CZL-treated cells.
Figure 3. Effects of CZL extract treatment on NO release (A) and inflammatory protein expression (B) in LPS-treated RAW264.7 macrophage. Data are expressed as the mean ± SD. Statistical significance was calculated using one-way ANOVA followed by Dunnett’s post-hoc test. *p< 0.05; **p< 0.01; ***p< 0.001 vs. LPS group.
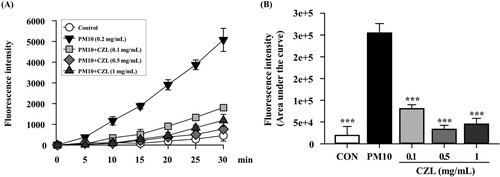
Effects of CZL extract on PM10-induced ROS generation in A549 cells
The regulatory effects of CZL extract on PM10-induced ROS production were assessed. PM10 group had dramatically increased intracellular levels of ROS compared to the CON group, and CZL extract treatment significantly reduced ROS production in a dose-dependent manner, especially at 0.5 and 1 mg/mL ().
Discussion
CZL has traditionally been used in Europe and East Asia to prevent or treat pneumonia, bronchitis, and pharyngitis. However, the exact cellular and molecular mechanisms involved remain unclear. Therefore, we investigated the effect of CZL extract on the expression of anti-inflammatory and anti-oxidative stress proteins in PM-induced lung injuries using in vitro and in vivo approaches.
PM10 causes lung dysfunction and respiratory disease symptoms, partially explaining inhalation toxicant-induced mortality and hospitalization rates (Ko et al., Citation2021; Rice et al., Citation2013; Tsai et al., Citation2017; Zhang et al., Citation2014; Zhang et al., Citation2015). Likewise, in our experimental conditions, PM10 resulted in histological changes in mouse lung tissues and increased the inflammatory response in BALF, a known marker for lung inflammation ( and (a)). On the other hand, CZL extract treatment significantly prevented PM10-induced histological damage and inflammatory responses (i.e. TNF-α and IL-1β) in BALF, which was enriched with non-cellular and cellular contents (e.g. cytokines and epithelial cells) from the lung alveoli (Domagala-Kulawik, Citation2020). Thus, elevated levels of inflammatory cytokines in BALF are surrogate markers of lung inflammation. Next, the activation of NF-κB mediated by AKT or ERK was assessed in PM10-treated mouse lung and A549 lung cells, proving that CZL extract reduces inflammatory responses via AKT and ERK ((b,c)). Specifically, phosphorylated AKT or ERK are known to trigger the activation of NF-κB, thereby stimulating the expression of inflammatory genes (e.g. iNOS and TNF-α) (Chen et al., Citation2016). Our corroborating data also showed that downstream inflammatory markers corresponding to AKT and ERK-meditated NF-κB activation; iNOS, COX-2, TNF-α, IL-1β, and IL-6 were effectively downregulated by CZL extract treatment in both mouse lung tissue and the A549 human lung cell line.
As PM10 not only contains endotoxins but also induces endotoxins, LPS was used to induce inflammation (Jones et al., Citation2021). In addition, the activation of macrophages by endotoxins (i.e. LPS) is a major inflammatory induction mechanism (Pyle et al., Citation2017). Notably, NO plays a key role in inflammatory responses (Soufli et al., Citation2016), and cytokine-activated macrophages trigger NO release (Baig et al., Citation2015). In this study, CZL extract treatment decreased NO production in a dose-dependent manner in RAW264.7 mouse macrophage cell line against LPS induction ((a)). Moreover, CZL extract treatment suppressed the expression of proinflammatory cytokines in LPS-treated RAW264.7 macrophages ((b)), which might be due to excessively produced ROS by LPS. LPS-induced ROS activates ERK-and AKT-mediated NF-κB activation (Ngabire et al., Citation2018), and we demonstrated that CZL extract treatment highly suppressed phosphorylation of AKT and ERK in LPS-treated RAW264.7 macrophages, although there was a marginal change in NF-κB expression ((b)).
Several studies have reported that PM10 induces inflammatory lung injury by increasing ROS levels in lung tissues and lung cells (García-Cuellar et al., Citation2020; Hwang et al., Citation2021), and ROS lowering agent may be one of the mitigating strategies to alleviate PM10-induced lung injury. In the present study, our results of ABTS and DPPH assays showed that CZL extract had a strong radical scavenging capacity equivalent to 40.27 ± 0.41 mg and 38.15 ± 1.06 mg of vitamin C, respectively (Supplementary file 3). Additionally, the CZL extract contained 27.10 mg of gallic acid, equivalent to the total polyphenol content (Supplementary file 3). ROS-scavenging assays in cell line models, such as cellular antioxidant assays, were performed because test tube-level assays are difficult to apply to elucidate biological effects. We successfully induced ROS overproduction in A549 cells using PM10, and CZL extract treatment significantly suppressed ROS levels for PM10 (). Although we did not characterize the bioactivity profile of CZL, the polyphenolic compounds in CZL are likely to be one of the prophylactic effect. In fact, studies have been conducted to identify the major bioactive substances of CZL, and acacetin-7-O-β-D-rutinoside (≈55 mg/g) and 4,5-Di-O-caffeoylquinic acid (≈16 mg/g) are the most abundant bioactive compounds and are well known to have anti-oxidative properties (Lee et al., Citation2021). In addition, small amounts of the following compounds have been reported to be present in CZL: 3,5-Di-O- caffeoylquinic acid (≈6 mg/g) and apigenin-7-O-β-D-glucuronide (2 mg/g) (Lee et al., Citation2021).
Taken together, we demonstrated the prophylactic effects of CZL extract on PM10-induced lung injury and proposed key inflammatory signaling pathways regulated by CZL extract treatment using various models (e.g. in vivo and in vitro). Specifically, treatment with the CZL extract effectively prevented macrophage-induced inflammatory responses by reducing the phosphorylation of AKT, ERK, and NF-κB. However, there are limitations: (1) a high dose of PM10 (10 mg/kg) was used; (2) there is a lack of mechanistic studies. Considering this was an acute study, 10 mg/kg was chosen to induce the phenotype. Further chronic studies with achievable PM doses are required. As for mechanistic studies, it is difficult to identify the specific mechanisms of crude extracts of plant-based materials due to their complicated composition. Therefore, we performed a literature search to estimate the major bioactive compounds in CZL, warranting mechanistic studies to understand the roles of individual bioactive compounds in CZL against PM10-induced lung injury.
Acknowledgements
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01603104)” from the Rural Development Administration, and a grant (Graduate School Education Program of Regulatory Sciences for Functional Food, 21153MFDS604) from ministry of food and drug safety of the Republic of Korea.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Baig, M. S., Zaichick, S. V., Mao, M., de Abreu, A. L., Bakhshi, F. R., Hart, P. C., Saqib, U., Deng, J., Chatterjee, S., & Block, M. L. (2015). NOS1-derived nitric oxide promotes NF-κB transcriptional activity through inhibition of suppressor of cytokine signaling-1. Journal of Experimental Medicine, 212(10), 1725–1738. https://doi.org/10.1084/jem.20140654
- Chen, B., Liu, J., Ho, T., Ding, X., & Mo, Y. (2016). ERK-mediated NF-κB activation through ASIC1 in response to acidosis. Oncogenesis, 5(12), e279–e279. https://doi.org/10.1038/oncsis.2016.81
- Cho, C., Hsieh, W., Tsai, C., Chen, C., Chang, H., & Lin, C. (2018). In vitro and In vivo experimental studies of PM2.5 on disease progression. International Journal of Environmental Research and Public Health, 15(7), 1380. https://doi.org/10.3390/ijerph15071380
- Cui, Y., Lin, Y., Meng, X., Ma, J., Deng, H., Liu, X., He, X., & Zhao, J. (2021). Cyanidin-3-galactoside from Aronia melanocarpa ameliorates PM10 induced pulmonary injury by modulating M1/M2 macrophage polarization and NRF2/Sirt1 MAPK signaling. Journal of Functional Foods, 78(1), 104363. https://doi.org/10.1016/j.jff.2021.104363
- Domagala-Kulawik, J. (2020). The relevance of bronchoalveolar lavage fluid analysis for lung cancer patients. Expert Review of Respiratory Medicine, 14(3), 329–337. https://doi.org/10.1080/17476348.2020.1708720
- García-Cuellar, C. M., Chirino, Y. I., Morales-Bárcenas, R., Soto-Reyes, E., Quintana-Belmares, R., Santibáñez-Andrade, M., & Sánchez-Pérez, Y. (2020). Airborne particulate matter (PM10) inhibits apoptosis through PI3K/AKT/FoxO3a pathway in lung epithelial cells: The role of a second oxidant stimulus. International Journal of Molecular Sciences, 21(2), 473. https://doi.org/10.3390/ijms21020473
- Han, H., Oh, E. Y., Lee, J. H., Park, J. W., & Park, H. J. (2021). Effects of particulate matter 10 inhalation on lung tissue RNA expression in a murine model. Tuberculosis and Respiratory Diseases, 84(1), 55–66. https://doi.org/10.4046/trd.2020.0107
- Harrison, R. M., & Yin, J. (2000). Particulate matter in the atmosphere: Which particle properties are important for its effects on health? Science of The Total Environment, 249(1-3), 85–101. https://doi.org/10.1016/S0048-9697(99)00513-6
- Hwang, L., Ko, I., Jin, J., Kim, S., Kim, C., Hwang, J., Choi, C. W., & Chang, B. S. (2021). Attenuation effect of polydeoxyribonucleotide on inflammatory cytokines and apoptotic factors induced by particulate matter (PM10) damage in human bronchial cells. Journal of Biochemical and Molecular Toxicology, 35(2), e22635. https://doi.org/10.1002/jbt.22635
- Hyun, M., Lee, Y., & Park, Y. (2011). Antioxidative activity and flavonoid content of Chrysanthemum zawadskii flowers. Korean Journal of Horticultural Science and Technology, 29(1), 68–73.
- Jones, N., Blagih, J., Zani, F., Rees, A., Hill, D. G., Jenkins, B. J., Bull, C. J., Moreira, D., Bantan, A. I., & Cronin, J. G. (2021). Anomalous collapses of nares strait ice arches leads to enhanced export of Arctic sea ice. Nature Communications, 12(1), 1–13. https://doi.org/10.1038/s41467-020-20314-w
- Kim, H. (2017). Extracts of Chrysanthemum zawadskii attenuate oxidative damage to vascular endothelial cells caused by a highly reducing sugar. Cytotechnology, 69(6), 915–924. https://doi.org/10.1007/s10616-017-0110-7
- Kim H, Choi M, Park M, Seo Y. 2017. Predictive and prognostic biomarkers of respiratory diseases due to particulate matter exposure. Journal of Cancer Prevention. 22(1): 6–15. https://doi.org/10.15430/JCP.2017.22.1.6
- Kim, S. J., Lee, K., Choi, H., Ha, T. J., Nam, J. H., Hong, S. Y., Chang, D. C., & Kim, K. S. (2015). Anti-inflammatory effects of flavonoids in Korean chrysanthemum species via suppression of inducible nitric oxide synthase and cyclooxygenase-2 in LPS-induced RAW 264.7 macrophages. Food Science and Biotechnology, 24(3), 975–985. https://doi.org/10.1007/s10068-015-0125-9
- Kim Y, Han J, Sung J, Sung M, Choi Y, Jeong H, Lee J. 2012. Anti-inflammatory activity of chrysanthemum zawadskii var. Latilobum leaf extract through haem oxygenase-1 induction. Journal of Functional Foods. 4(2):474-479. https://doi.org/10.1016/j.jff.2012.02.007
- Kim, Y. Y., Lee, S. Y., & Yim, D. S. (2001). Biological activities of linarin from Chrysanthemum zawadskii var. latilobum. Yakhak Hoeji, 45, 604–610.
- Ko, H. M., Choi, S., Kim, Y., An, E., Lee, S., Kim, K., Jung, H., & Jang, H. (2020). Effect of Rosa laevigata on PM10-induced inflammatory response of human lung epithelial cells. Evidence-Based Complementary and Alternative Medicine, 2020, 2893609.
- Ko, H. M., Lee, S., Jee, W., Jung, J. H., Kim, K., Jung, H., & Jang, H. (2021). Gancaonin N from Glycyrrhiza uralensis attenuates the inflammatory response by downregulating the NF-κB/MAPK pathway on an acute pneumonia in vitro model. Pharmaceutics, 13(7), 1028. https://doi.org/10.3390/pharmaceutics13071028
- Kwon, H., Ha, T., Hwang, S., Jin, Y., Nam, S., Park, K., & Yang, M. (2006). Cytotoxic flavonoids from the whole plants of Chrysanthemum zawadskii Herbich var. latilobum Kitamura. Journal of Life Science, 16(5), 746–749. https://doi.org/10.5352/JLS.2006.16.5.746
- Lee, H., Kim, Y. I., Nirmala, F. S., Jeong, H. Y., Seo, H., Ha, T. Y., Jung, C. H., & Ahn, J. (2021). Chrysanthemum zawadskil Herbich attenuates dexamethasone-induced muscle atrophy through the regulation of proteostasis and mitochondrial function. Biomedicine & Pharmacotherapy, 136, 111226. https://doi.org/10.1016/j.biopha.2021.111226
- Lee, J., Seok, J. K., & Boo, Y. C. (2018). Ecklonia cava extract and dieckol attenuate cellular lipid peroxidation in keratinocytes exposed to PM10. Evidence-Based Complementary and Alternative Medicine, 2018, 8248323.
- Misiukiewicz-Stepien, P., & Paplinska-Goryca, M. (2021). Biological effect of PM10 on airway epithelium-focus on obstructive lung diseases. Clinical Immunology, 227, 108754. https://doi.org/10.1016/j.clim.2021.108754
- Ngabire, D., Seong, Y., Patil, M. P., Niyonizigiye, I., Seo, Y. B., & Kim, G. (2018). Anti-inflammatory effects of Aster incisus through the inhibition of NF-κB, MAPK, and AKT pathways in LPS-stimulated RAW 264.7 macrophages. Mediators of Inflammation, 2018, 4675204. https://doi.org/10.1155/2018/4675204
- Pyle, C. J., Akhter, S., Bao, S., Dodd, C. E., Schlesinger, L. S., & Knoell, D. L. (2017). Zinc modulates endotoxin-induced human macrophage inflammation through ZIP8 induction and C/EBPβ inhibition. PloS one, 12(1), e0169531.
- Rice, M. B., Ljungman, P. L., Wilker, E. H., Gold, D. R., Schwartz, J. D., Koutrakis, P., Washko, G. R., O’Connor, G. T., & Mittleman, M. A. (2013). Short-term exposure to air pollution and lung function in the Framingham heart study. American Journal of Respiratory and Critical Care Medicine, 188(11), 1351–1357. https://doi.org/10.1164/rccm.201308-1414OC
- Shim, S., Kang, H., Sun, H., Lee, Y., Park, J., Chun, S., Song, Y., & Byun, D. (2012). Isolation and identification of flavonoids from Gujeolcho (Chrysanthemum zawadskii var. Latilobum) as inhibitor of histamine release. Food Science and Biotechnology, 21(2), 613–617. https://doi.org/10.1007/s10068-012-0079-0
- Singh, R. P., Agrawal, P., Yim, D., Agarwal, C., & Agarwal, R. (2005). Acacetin inhibits cell growth and cell cycle progression, and induces apoptosis in human prostate cancer cells: Structure–activity relationship with linarin and linarin acetate. Carcinogenesis, 26(4), 845–854. https://doi.org/10.1093/carcin/bgi014
- Soufli, I., Toumi, R., Rafa, H., & Touil-Boukoffa, C. (2016). Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World Journal of Gastrointestinal Pharmacology and Therapeutics, 7(3), 353–360. https://doi.org/10.4292/wjgpt.v7.i3.353
- Suh, K. S., Rhee, S. Y., Jung, W. W., Kim, N. J., Jang, Y. P., Kim, H. J., Kim, M. K., Choi, Y. K., & Kim, Y. S. (2013). Chrysanthemum zawadskii extract protects osteoblastic cells from highly reducing sugar-induced oxidative damage. International Journal of Molecular Medicine, 32(1), 241–250. https://doi.org/10.3892/ijmm.2013.1371
- Tsai, M., Hsu, L., Lee, C., Chiang, Y., Lee, M., How, J., Wu, C., Huang, C., & Lee, I. (2017). Resveratrol inhibits urban particulate matter-induced COX-2/PGE2 release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-κB. The International Journal of Biochemistry & Cell Biology, 88, 113–123. https://doi.org/10.1016/j.biocel.2017.05.015
- Uehara, A., Nakata, M., Kitajima, J., & Iwashina, T. (2012). Internal and external flavonoids from the leaves of Japanese Chrysanthemum species (Asteraceae). Biochemical Systematics and Ecology, 41(2012), 142–149. https://doi.org/10.1016/j.bse.2011.12.020
- Weiden, M. D., Naveed, B., Kwon, S., Segal, L. N., Cho, S. J., Tsukiji, J., Kulkarni, R., Comfort, A. L., Kasturiarachchi, K. J., & Prophete, C. (2012). Comparison of WTC dust size on macrophage inflammatory cytokine release in vivo and in vitro. PloS one, 7(7), e40016. https://doi.org/10.1371/journal.pone.0040016
- Wu, T., Khor, T. O., Saw, C. L. L., Loh, S. C., Chen, A. I., Lim, S. S., Park, J. H. Y., Cai, L., & Kong, A. T. (2011). Anti-inflammatory/anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. The AAPS Journal, 13(1), 1–13. https://doi.org/10.1208/s12248-010-9239-4
- Xu, Z., Xu, X., Zhong, M., Hotchkiss, I. P., Lewandowski, R. P., Wagner, J. G., Bramble, L. A., Yang, Y., Wang, A., & Harkema, J. R. (2011). Amorphous nanosilica induce endocytosis-dependent ROS generation and DNA damage in human keratinocytes. Particle and Fibre Toxicology, 8(1), 1–14. https://doi.org/10.1186/1743-8977-8-1
- Yan, F., Wu, Y., Liu, H., Wu, Y., Shen, H., & Li, W. (2018). Atf3 is positively involved in particulate matter-induced airway inflammation in vitro and in vivo. Toxicology Letters, 287, 113–121. https://doi.org/10.1016/j.toxlet.2018.01.022
- You, S., & Moon, J. (2016). A study on anti-oxidative, anti-inflammatory, and melanin inhibitory effects of Chrysanthemum sibiricum extract. Journal of the Korean Oil Chemists' Society, 33(4), 762–770. https://doi.org/10.12925/jkocs.2016.33.4.762
- Zhang, R., Dai, Y., Zhang, X., Niu, Y., Meng, T., Li, Y., Duan, H., Bin, P., Ye, M., & Jia, X. (2014). Reduced pulmonary function and increased pro-inflammatory cytokines in nanoscale carbon black-exposed workers. Particle and Fibre Toxicology, 11(1), 1–14. https://doi.org/10.1186/s12989-014-0073-1
- Zhang, Y., He, M., Wu, S., Zhu, Y., Wang, S., Shima, M., Tamura, K., & Ma, L. (2015). Short-term effects of fine particulate matter and temperature on lung function among healthy college students in Wuhan, China. International Journal of Environmental Research and Public Health, 12(7), 7777–7793. doi:10.3390/ijerph120707777