?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
With advances in the study of the human microbiome, the fact that lactate is the smallest chiral molecule in nature has drawn attention. However, there have been few comparative studies on the classification of L-lactate and D-lactate. In this study, we investigated the safety and efficacy of D-lactate as an immunomodulator of food origin. In terms of safety evaluation, both lactates were found to promote cell proliferation and repair. D-lactate was well tolerated for 30 d at doses up to 2000 mg/kg in mice. In terms of immune function evaluation, unexpectedly, D-lactate was found to enhance immunity by increasing the immune organ index, ear swelling index, lymphocyte conversion rate, haemolysis rate and macrophage phagocytosis rate and had a dose-dependent effect. D-lactate showed stronger immune regulation effects than L-lactate. This study proves the safety and the immunoregulation capacity of D-lactate and provides a mechanistic basis for the bioactivity of fermented foods.
1. Introduction
Lactic acid bacteria has received considerable attention of medical and biological research in recent years for its improvement on health through its metabolites consumption (Bezkorovainy, Citation2001; Garrote et al., Citation2000; Golowczyc et al., Citation2007; Holzapfel et al., Citation1995; Kakisu et al., Citation2012; Sanders et al., Citation2013). However, the current mechanisms are mostly limited to short-chain fatty acids and extracellular polysaccharides and the role of lactate as the main metabolite of lactate bacteria has been neglected (Iraporda et al., Citation2015; Jayasimhan & Mariño, Citation2019; Schilderink et al., Citation2013; Wenzel et al., Citation2020). Lactate has long been regarded as a metabolic product produced by adenosine triphosphate (ATP) during anaerobic glycolysis. A rise in lactate levels in the blood is considered an early sign of microcirculatory failure (Ge et al., Citation2008; Samuelsson, Citation1983). Surprisingly, lactate has only recently been identified as an important energy source and metabolic substrate (Hui et al., Citation2017). Further, lactate can also act as signalling molecules to influence metabolic activity (Ahmed et al., Citation2010; Certo et al., Citation2020; Colegio et al., Citation2014; Ge et al., Citation2008; Harada et al., Citation2018; Lauritzen et al., Citation2014; Morland et al., Citation2015). In fact, lactate Ringer's fluid is commonly used in clinical practice for rapid rehydration and improves the prognosis of acute pancreatitis and organ failure (Wu et al., Citation2011).
With advances in the study of the human microbiome, the fact that lactate is the smallest chiral molecule in nature has drawn attention again. Lactate, produced by lactate bacteria fermentation in the intestinal tract, has two configurations: left-handed, L (+) and dextral, D (−). Nevertheless, the classification of L-lactate and D-lactate have not been adequately studied. Since the kidneys are the main excretory organ of D-lactate, and the liver and kidney functions of children are not fully developed, L-lactate and D-lactate are restricted in infant food (Flint et al., Citation2015). In fact, D-lactate can be producted by many bacterial fermentation, especially by lactobacillus and bifidobacteria, both of which are key contributors to the health-promoting activity of the gut microbiome (Cristescu et al., Citation2008; Wu et al., Citation2018; Imaoka et al., Citation2008; O’Mahony et al., Citation2005; Peran et al., Citation2007; Sheu et al., Citation2002).
Compared with studies on mixed-racemic lactate or L-lactate alone, there has been little research on the biological activity of D-lactate. Using sterile mice, McDonald recently found that supplementation of D-lactate or the intestinal microbes that produced D-lactate can significantly promote the ability of Kupffer cells to kill pathogens and reshape the intravascular immune firewall to prevent dissemination of blood-borne bacteria (McDonald et al., Citation2020). Consequently, studies of different functional activity of lactate may need to be considered attributing to the different configurations of lactate. So far, there are rare evaluations of the differences in biological activity between L-lactate and D-lactate. Since D-lactate is widely consumed in fermented foods, the health-related biological activities of D-lactate should be further evaluated. Here, we hypothesise that D-lactate, a dietary constituent and a bacterial metabolite, acts as a new signalling molecule to improve immune regulation and comprehensively evaluate the safety and efficacy of D-lactate.
2. Materials and methods
2.1. Materials and cells
Creatinine (CRE) determination kit, urea nitrogen (BUN) determination kit, alkaline phosphatase (ALP) determination kit, aspartate aminotransferase (AST) kit and alanine aminotransferase (ALT) kit were purchased from Nanjing Jiancheng Institute of Biological Engineering. The other chemical reagents, including L-lactate, D-lactate and corresponding sodium lactate were from Sigma-Aldrich unless indicated otherwise. Sheep red blood cell (SRBC) and Indian ink were purchased from Nanjing Senbeijia Biological Co., LTD. YAC-1 cells were from Cell Bank of Chinese Academy of Science (Shanghai, China).
2.2. Experimental animals
SPF male C67BL/6 mice with body weight of (18 ± 2) g were purchased from Shanghai Slack Experimental Animal Co., LTD., license number: SCXK (Shanghai) 2017-0002. The animals were maintained in a 12/12 h light/dark cycle room with a temperature of 24 ± 1°C and a humidity of 50 ± 10%, adaptive feeding for seven days. All procedures related to the animals and their care were in line with the internationally accepted principles as found in the Guidelines for Keeping Experimental Animals issued by the government of China. The experiment of toxicological evaluation and immune regulation evaluation was approved by the Experimental Animal Welfare and Ethics Committee of Jiangnan University, license number: Jn.NO0330C0800615; Jn.NO1015S0281102.
2.3. Toxicological evaluation method
2.3.1. In vitro toxicological evaluation
The MTS method was used to determine cell proliferation. HCT116 cells at the logarithmic growth stage were laid on 96-well plates with 3000–5000 cells per well density. Five multiple holes were set and the edges of the 96-well plates were supplemented with sterile PBS. After 18 h, gradient concentrations of L-lactate and D-lactate were added into the DMEM (final concentrations were 0, 0.1, 1, 10, 20, 40, 60, 80, 100 mM, respectively). After 48 h, 20 μL MTS was added to each well, and cultured at 37°C for 4 h and the absorbance value at 570 nm was detected.
For the scratch mobility experiment, HCT116 cells were taken as 2 × 105 at a 2 mL concentration in 6-well plates overnight. When the cells covered about 90% of the 6-well plate, scratch with 200 μL pipette head perpendicular to the plate, rinse the cells with PBS to remove the scratched cells. Blank control group and administration group (20 mM L-lactate, 20 mM D-lactate), adding serum-free DMEM medium corresponding to the group. The hole plate was photographed 0, 12 and 24 h. The widths of the scratch were measured as D0 h, D12 h and D24 h and calculated according to Equation (1).
(1)
(1)
2.3.2. In vivo toxicological evaluation
Animal grouping: After 1 week of adaptive feeding, C57/BL6 mice were randomly divided into control group (Con, n = 10), positive control group (PC, 25 mg/kg Levamisole hydrochloride, n = 10), low-dose L-lactate intervention group (LL, 1000 mg/kg sodium L-lactate, n = 10), high-dose L-lactate intervention group (HL, 2000 mg/kg sodium L-lactate, n = 10), low-dose D-lactate intervention group (LD, 1000 mg/kg sodium D-lactate, n = 10) and high-dose D-lactate intervention group (HD, 2000 mg/kg sodium D-lactate, n = 10). Animals were given 200 μL regular intragastric administration for 30 days. During the whole experiment period, the basic condition and body weight of mice were recorded every day. The liver, spleen and thymus of mice were removed and weighed. These tissue samples were stored in the refrigerator at −80°C for future use. Creatinine (CRE), urea nitrogen (BUN), alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) kits were tested according to the instructions. Thymus index, Liver index and spleen index were calculated according to Equations (2)–(4).
(2)
(2)
(3)
(3)
(4)
(4)
2.4. Evaluation of immune regulation ability
2.4.1. Animal feeding and grouping
The grouping of C57BL/6 mice was the same as 2.3.2 (n = 48). Mice were randomly divided into 6 groups. From day 1 to day 30, mice received intragastrical administration once daily. All of groups were subjected to immunosuppression by intraperitoneal injection of cyclophosphamide CTX (60 mg/kg/d) at day 1, 2, 3 and 10. 8 mice in each group were used for: (a) transformation test of spleen lymphocytes; (b) ear swelling test; and (c) haemolytic plaque test and phagocytosis test of peritoneal macrophages. Twenty-four hours after the last administration, mice were sacrificed by cervical dislocation and subjected to the following analyses.
2.4.2. Primary cells
Spleen lymphocyte isolation: mice spleen was removed aseptically, crushed gently with a syringe plug on a 200-mesh stainless steel net. Cells were cultured for 4 h, and the unadherent cell suspensions were spleen lymphocytes. After trypan blue staining, the percentage of viable cells was over 95%.
Peritoneal macrophages isolation: mice were immersed in 75% ethanol solution for 3 min and then intraperitoneally injected 5 mL phosphate buffered saline (PBS) (0.05 mol/L, pH 7.0). The abdominal fluid was sucked into the centrifugal tube with a pipette and collected repeatedly for three times. The peritoneal fluid obtained was centrifuged at 1000 × g for 8 min. After 4 h, unadherent cells were removed and purified peritoneal macrophages were collected.
2.4.3. Evaluation of cellular immune function
Transformation test of spleen lymphocytes: Spleen cells were prepared aseptic to prepare spleen cell suspension, and the cell concentration was adjusted to 1 × 107/mL. Add 50 μL spleen cell suspension to 96-well cell culture plate, 50 μL 10 μg/mL ConA was added to each experimental well. Cells were cultured for 48 h. 10 μL 5 mg/mL MTT was added to each well to culture for another 3 h. Then 100 μL sodium dodecyl sulfate–hydrochloric acid (SDS–HCL) was added to each well for 2 h. The optical density at 570 nm was determined, and the transformation rate of splenic lymphocytes was calculated according to Equation (5).
(5)
(5)
Ear swelling test: delayed hypersensitivity reaction induced by 2, 4-dinitrochlorobenzene (DNCB) as described in previous studies (Hirasawa et al., Citation2009; Sugiura et al., Citation2017). DNCB solution was prepared fresh, 50 mg DNFB with 5 mL of pre-prepared acetone sesame oil solution (acetone: sesame oil = 1:1) . For sensitisation stage: the abdominal skin of mice was depilated with barium sulphide, and sensitised with DNFB solution 50 μL evenly. For allergy stage: Five days later, 10 μL of DNFB solution was evenly applied to both sides of ear. The mice were sacrificed for cervical dislocation 36 h after allergic attack, and the left and right ear shells were cut off. The ear piece with diameter of 8 mm was removed with a hole punch and weighed to compare the weight of left and right auricle of mice in different groups.
2.4.4. Evaluation of humoral immunity function
Haemolytic plaque test: mice was intraperitoneally injected with 0.2 mL 2% SRBC saline suspension. After five days of SRBC immunisation, the spleen cell suspension of 1 × 107 cells /mL was prepared on complete medium RPMI 1640. Agarose was added into 100 mL sterilised normal saline as the bottom culture medium. In addition, dissolved agarose Hanks, and added 0.5 mL/test tube with a constant temperature. 50 μL 20%SRBC normal saline suspension and 200 μL spleen cell suspension were added into the above test tubes successively, mixed and poured into the six-well plate.
The culture plate prepared and placed at 37°C, 5% CO2 incubator for 1 h, then add complete medium to each well, continue to incubate for 2 h, automatic image analyzer read the haemolysis plaque image analysis software counted the number of haemolysis plaque. The average number of parallel pores was taken as the value of haemolysis plaque of the sample.
2.4.5. Evaluation methods of non-specific immune function
Phagocytosis test of peritoneal macrophages by using fluorescent microspheres: mice were injected intraperitoneously with 0.2 mL 2% sheep red blood cells four days before the experiment to activate macrophages. The primary peritoneal macrophages were sterile extracted and adjusted to 6 × 105/mL. Fluorescent microspheres were incubated with 10 mL 1% BSA at 37°C for 30 min away from light, and were pre-treated with ultrasound for 5 min. The pre-treated fluorescent microspheres were added in peritoneal macrophages (1:10). After incubation, discarded the supernatant and gently washed the supernatant twice with PBS. After removing the supernatant, added 0.3 mL PBS buffer at 4°C to scrape the lower adherent cells and went through 75 mm filter before machine analysis.
Gate setting: the FSC–SSC two-dimensional scatter plot was set up. By adjusting the voltage values of FSC and SSC, gates were set for macrophages to define the group of macrophages analyzed. The fluorescence intensity of macrophages was detected in the fluorescence pathway of the light emitted by the fluorescence microspheres. 5000 macrophages were obtained from each sample, and the data were displayed in the 2d scatter plot. In scatter plot, the macrophages that did not phagocytose fluorescent microspheres and those that phagocytose fluorescent microspheres could be delineated by setting a gate as the figure below.
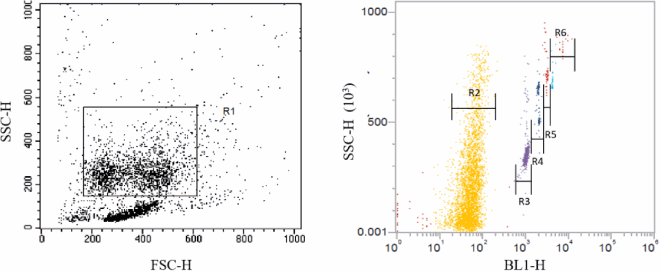
Gate setting: R1: total macrophage; R2: macrophages without any fluorescent microspheres; R3: macrophages that engulf one fluorescent microsphere; R4: macrophages that engulf two light microspheres; R5: macrophages that engulf three fluorescent microspheres; and R6: macrophages that engulf four or more fluorescent microspheres.
2.5. Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0 Software. Multiple group comparisons were done by a one-way analysis of variance (ANOVA), followed by the Bonferroni procedure for the comparison of means. Student’s t-test was used to compare two independent groups. Data are the means ± S.E.M. Differences were regarded as statistically significant if p ≤ 0.05 versus Control.
3. Results
3.1. Toxicological evaluation of chiral lactate
3.1.1. Toxicological evaluation of chiral lactate in vitro
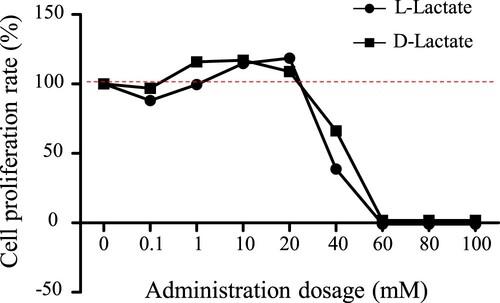
The most suitable concentration of lactate for cell assay was confirmed first. As shown in , we compared the cytotoxicity of L-lactate and D-lactate at concentrations of 0, 0.1, 1, 10, 20, 40, 60, 80 and 100 mM. Below concentrations of 20 mM, both were found to enhance cell proliferation. More than 40 mM lactate produced cellular apoptosis, which may resulted from low pH levels. These results suggested that instead of lactate itself lactate sodium should be used in vivo experiments to prevent acidic corrosion of animal mucosal tissues.
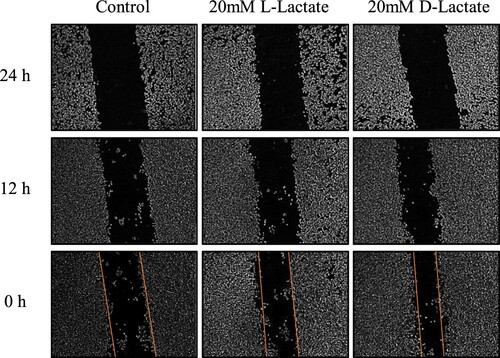
Based on the results of cytotoxicity test, 20 mmol/L of L-lactate and D-lactate were used for subsequent experiments in vitro. The effects of the two kinds of chiral lactate on cellular repair were evaluated by cell scratch test. The mobility of the control group, the L-lactate group and the D-lactate group were 12.6%, 25.3% and 29.2%, respectively (). Compared with the control group, both L-lactate and D-lactate significantly increased the repair ability of cells (p < 0.05). Toxicological evaluation In vitro demonstrated that, at 20 mmol/L, neither L-lactate nor D-lactate had any detrimental effects on cell proliferation or repairation.
3.1.2. Toxicological evaluation of chiral lactate in vivo
For toxicological evaluation in vivo, we performed with a 30-day feeding experiment using two different concentrations of lactate (1000 mg/kg or 2000 mg/kg) in mice. Body weight and immune organ indexs were used as indicators of the basic physiological status of experimental mice. As shown in , the body weights of mice treated with both lactates remained at normal levels (27.7 ± 1.3 g). In addition, the spleen and liver index clearly showed that D-lactate could increase the immune organ index better than L-lactate. The effects of HD group in spleen and liver index were similar to PC group treated with levamisole hydrochloride, which is known to be beneficial for immune regulation (Jie et al., Citation2015; Lei et al., Citation2007). What's more, we also evaluated renal function, represented by BUN and CRE, and liver function, represented by ALP, ALT and AST shown in . Neither L-lactate nor D-lactate damaged liver or kidney functions at either of the two concentrations.
Table 1. Effect of lactate enantiomers intake on toxicological indexes in mice.
In summary, toxicological evaluation results indicated the safety of L-lactate and D-lactate at 40 mM in vitro and 2000 mg/kg in vivo.
3.2. Immunomodulatory function evaluation of chiral lactate
Since different chiral lactates have different effects on the immune organ indexes mentioned above, we further evaluated the immunoregulatory ability of L-lactate and D-lactate in terms of cellular immunity, humoral immunity and non-specific immunity on cyclophosphamide-treated immunosuppression mice.
3.2.1. Effects of chiral lactates on the cellular immune function of mice
The effects of L-lactate and D-lactate on the splenic lymphocyte transformation and delayed allergic reaction induced by dinitrofluorobenzene (DNCB) were evaluated. Compared with control group, the proliferation rate of spleen lymphocytes in mice of PC group was significantly increased after ConA induction (). Although there was no significant difference between LD group and control group, the 2000 mg/kg D-lactate, consistent with the PC group, significantly promoted the proliferation of lymphocytes (p < 0.05). However, neither the 1000 mg/kg nor 2000 mg/kg L-lactate showed a significant increase in lymphocyte proliferation.
Table 2. Effect of lactate enantiomers intake on immune function indexes in mice.
DNCB binds to proteins in the abdominal wall to form antigens and stimulates T lymphocytes' proliferation and conversion into sensitised lymphocytes. The migration of these isensitised lymphocytes can lead to ear swelling in mice. Five days after first stimulating, DNCB was smeared again on mice ears to prompt antigen attack. The degree of ear swelling peaked after 36 h, reflected the extent of the delayed allergic reaction. As shown in , D-lactate significantly increased the ear swelling index in a dose-dependent manner (p < 0.01). Compared with L-lactate, the intake of D-lactate could enhance the cellular immunity of mice.
3.2.2. Effect of chiral lactates on the humoral immune function of mice
Hemolytic plaque test was used for evaluation of mice humoral immunity. Spleen cell suspensions were mixed with SRBC around antibody secreting cells, resulting in visible hemolytic plaques with complement participation. Similar with outcomes of cellular immunity, groups treated with D-lactate could increase the number of hemolytic plaque (). Although high doses of L-lactate also enhanced humoral immunity, D-lactate showed a higher dose-dependent effect. Thus, the intake of D-lactate could enhance the humoral immunity of mice.
3.2.3. Effect of chiral lactates on the non-specific immune function of mice
Macrophages are important to innate immunity and phagocytosis is macrophages most critical and fundamental function (Geissmann et al., Citation2010; Gordon et al., Citation2014; Varin & Gordon, Citation2009; Mantovani et al., Citation2004). In order to confirm the immunomodulatory effects of L-lactate and D-lactate on non-specific immunologic functions of mice, we next evaluated the phagocytosis function of peritoneal macrophages by using flow cytometry and fluorescent microspheres. As shown in , the primary peritoneal macrophages of mice from each group were isolated.
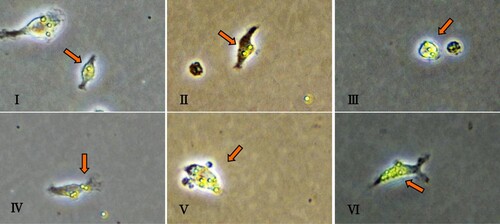
After being shielded from light, primary peritoneal macrophages were co-cultured with fluorescent microspheres for one hour and observed under a fluorescence microscope ().
Figure 5. a. Primary peritoneal macrophages phagocytic fluorescent microspheres; b. Phagocytosis of peritoneal macrophages based on fluorescent microspheres imaging. The arrow represents a primary peritoneal macrophage. i. Phagocytosis of a microsphere into a cell; ii. Phagocytosis of two microspheres into a cell; iii. Phagocytosis of three microspheres into a cell; iv. Phagocytosis of four microspheres into a cell; v. Phagocytosis of five microspheres into a cell; and vi. More than six microspheres engulfing a cell.
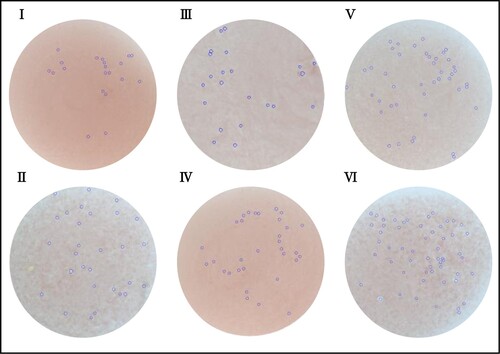
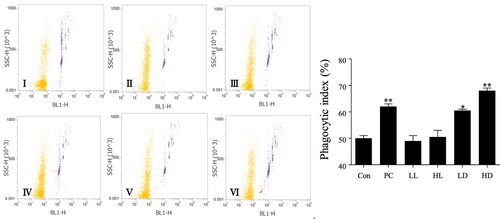
Compared with the control group, the PC group showed a significantly higher rate of peritoneal macrophages phagocytosis. The effect of D-lactate intake (LD group and HD group) on the phagocytic activity of peritoneal macrophages was better than that of L-lactate intake (LL group and HL group) and showed a dose-dependent pattern (p < 0.001) (). The intake of D-lactate could enhance the non-specific immunity of mice. Overall, our results show that D-lactate can improve the cellular, humoral and non-specific immune responses of immunosuppression mice.
Figure 6. Effects of lactate enantiomers on phagocytosis test of peritoneal macrophages. I. Control group; II. Positive control group; III. L-lactate 1000 mg/kg group; IV. L-lactate 2000 mg/kg group; V. D-lactate 1000 mg/kg group; and VI. D-lactate 2000 mg/kg group. The values for each group are expressed as mean ± standard deviation (n = 6). *Indicates that the group was significantly different from the control group (Con) (*p < .05; **p < .01; ***p < .001).
4. Discussion
In this study, we provide evidence that dietary D-lactate may be a previously unrecognised nutrient. The findings of this study shed light on how fermented foods profoundly affect the health of the host, and D-lactate production may, therefore, be an important target for lactate fermentation in the future.
The studies outlined in this report also offer some resolution to the controversy regarding the safety of D-lactate. Although the official recommended limits on dietary intake of D-lactate were lifted in 1974, there is still widespread mistrust of dietary D-lactate (De Ross & Katan, Citation2000; de Vrese et al., Citation1990, Citation1991). The MTT assay and scratch assay results showed that L-lactate and D-lactate concentrations below 40 mM have no significant inhibitory effect on cell proliferation and repair. The 30-day feeding test showed D-lactate (2000 mg/kg) to be well tolerated in mice, with no significant nephrotoxicity or hepatotoxicity observed. D-lactate is widely found in fermented foods that have been eaten since ancient times. Therefore, there is no cause for safety concerns about the consumption of foods containing D-lactate, especially among healthy adults. D-lactate was found to enhance immunity by improving spleen size, ear swelling index, lymphocyte conversion rate, haemolysis rate and macrophage phagocytosis rate, and showed more effectively than L-lactate.
Pharmacologically speaking, the use of any drug has benefits and risks. Food safety is also relative but can be defined as an acceptable risk that generally does not cause harm to health (McMeekin et al., Citation1997; Henson & Caswell, Citation1999; Uhlig and Gowik, Citation2018; Wileyblackwell, Citation2013). From the perspective of nutrition, D-lactate is a physiological isomer, and a large intake of D-lactate may increase the metabolic burden on the body. The main factor that may limit daily D-lactate intake is the risk of lactate poisoning. However, it is important to note that direct evidence of a correlation between lactate poisoning and D-lactate intake has never been found. In view of the above analysis, we suggest that the potential clinical significance of serum lactate and D-lactate requires careful re-evaluation.
This study leads to further speculation that D-lactate might be recognised as a food nutrient. Whats more, key protein levels should be further checked to reveal the mechanism of such difference. G-protein-coupled receptors (GPCRs) are sensors for small molecules such as fatty acids, sugars and endogenous intermediate metabolites from microbes or food sources, with profound effects on a variety of biological processes (Blad et al., Citation2012). Among these receptors, GPR81 (or HCA1), a lactate-specific receptor, is reported to be expressed in intestinal tissues and mediates macrophage-dependent anti-inflammatory effects in hepatitis and pancreatitis (Hoque et al., Citation2014; Iraporda et al., Citation2015; Offermanns, Citation2014, Citation2011). Whether GPR81 contributes to the bioactivity of D-lactate remains to be confirmed, and the related mechanisms of GPR81 require a further exploration. On the other hand, although our experiments demonstrated the unexpected immunomodulatory activity of D-lactate, the role of L-lactate can not be ignored, especially L-lactate has been proved to play important roles in anti-inflammatory activity and important energy metabolism. D- and L-lactate also exist synergically in fermented foods represented by yogurt. To explore the optimal ratio of D-lactate and L-lactate will be an important direction for further research.
This is the first study to investigate the cellular, humoral and non-specific immunomodulatory activities of L-lactate and D-lactate. Altogether, the potential of D-lactate as a food immunomodulator is clear. This study proves the safety and the immunoregulation capacity of D-lactate and provides a mechanistic basis for the bioactivity of fermented foods.
Authors’ Contributions
H.L., H.Z. and W.C. designed research; Y.Y., R.X. and Z.Y. performed research; Y.Y., X.L. and H.L. analyzed data; Y.Y, R.X. Z.Y., H.Z., H.L. and W.C. interpreted data; and Y.Y. and H.L. wrote the paper.
Acknowledgements
This work was supported by the National Youth 1000 Talents Plan, the Jiangsu Specially-appointed Professor Programme, Jiangsu Province Recruitment Plan for High-level, Innovative and Entrepreneurial Talents (Innovative Research Team) and Collaborative innovation centre of food safety and quality control in Jiangsu Province.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
Additional information
Funding
References
- Ahmed, K., Tunaru, S., Tang, C., Müller, M., Gille, A., Sassmann, A., Hanson, J., & Offermanns, S. (2010). An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metabolism, 11(4), 311–319. https://doi.org/10.1016/j.cmet.2010.02.012
- Bezkorovainy, A. (2001). Probiotics: Determinants of survival and growth in the gut. The American Journal of Clinical Nutrition, 73(2 Suppl), 399S–405S. https://doi.org/10.1093/ajcn/73.2.399s
- Blad, C. C., Tang, C., & Offermanns, S. (2012). G protein-coupled receptors for energy metabolites as new therapeutic targets. Nature Reviews Drug Discovery, 11(8), 603–619. https://doi.org/10.1038/nrd3777
- Certo, M., Tsai, C. H., Pucino, V., Ho, P. C., & Mauro, C. (2020). Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nature Reviews Immunology, 2021(21), 151–161. https://doi.org/10.1038/s41577-020-0406-2
- Colegio, O. R., Chu, N. Q., Szabo, A. L., Chu, T., Rhebergen, A. M., Jairam, V., Cyrus, N., Brokowski, C. E., Eisenbarth, S. C., Phillips, G. M., Cline, G. W., Phillips, A. J., & Medzhitov, R. (2014). Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature, 513(7519), 559–563. https://doi.org/10.1038/nature13490
- Cristescu, M. E., Innes, D. J., Stillman, J. H., & Crease, T. J. (2008). D- and L-lactate dehydrogenases during invertebrate evolution. BMC Evolutionary Biology, 8(1), 268. https://doi.org/10.1186/1471-2148-8-268
- De Ross, N. M., & Katan, M. B. (2000). Effects of probiotic bacteria on diarrhea, lipid metabolism, and carcinogenesis: A review of papers published between 1988 and 1998. The American Journal of Clinical Nutrition, 71(2), 405–411. https://doi.org/10.1093/ajcn/71.2.405
- de Vrese, M., & Barth, C. A. (1991). Postprandial plasma D-lactate concentrations after yogurt ingestion. Zeitschrift für Ernährungswissenschaft, 30(2), 131–137. https://doi.org/10.1007/BF01610068
- de Vrese, M., Koppenhoefer, B., & Barth, C. A. (1990). D-lactic acid metabolism after an oral load of DL-lactate. Clinical Nutrition, 9(1), 23–28. https://doi.org/10.1016/0261-5614(90)90069-5
- Flint, H. J., Duncan, S. H., Scott, K. P., & Louis, P. (2015). Links between diet, gut microbiota composition and gut metabolism. Proceedings of the Nutrition Society, 74(01), 13–22. https://doi.org/10.1017/S0029665114001463
- Garrote, G. L., Abraham, A. G., & De Antoni, G. L. (2000). Inhibitory power of kefir: The role of organic acids. Journal of Food Protection, 63(3), 364–369. https://doi.org/10.4315/0362-028X-63.3.364
- Ge, H., Weiszmann, J., Reagan, J. D., Gupte, J., Baribault, H., Gyuris, T., Chen, J. L., Tian, H., & Li, Y. (2008). Elucidation of signaling and functional activities of an orphan GPCR, GPR81. Journal of Lipid Research, 49(4), 797–803. https://doi.org/10.1194/jlr.M700513-JLR200
- Geissmann, F., Manz, M. G., Jung, S., Sieweke, M. H., Merad, M., & Ley, K. (2010). Development of monocytes, macrophages, and dendritic cells. Science, 327(5966), 656–661. https://doi.org/10.1126/science.1178331
- Golowczyc, M. A., Mobili, P., Garrote, G. L., Abraham, A. G., & De Antoni, G. L. (2007). Protective action of Lactobacillus kefir carrying S-layer protein against Salmonella enterica serovar Enteritidis. International Journal of Food Microbiology, 118(3), 264–273. https://doi.org/10.1016/j.ijfoodmicro.2007.07.042
- Gordon, S., Helming, L., & Estrada, F. (2014). Alternative activation of macrophages: Concepts and prospects. Springer.
- Harada, N., Hirano, I., Inui, H., & Yamaji, R. (2018). Stereoselective effects of lactate enantiomers on the enhancement of 3T3-L1 adipocyte differentiation. Biochemical and Biophysical Research Communications, 498(1), 105–110. https://doi.org/10.1016/j.bbrc.2018.02.198
- Henson, S. J., & Caswell, J. A. (1999). Food safety regulation: An overview of contemporary issues. Food Policy, 24(6), 589–603. https://doi.org/10.1016/S0306-9192(99)00072-X
- Hirasawa, N., Ohsawa, Y., Katoh, G., Shibata, K., Ishihara, K., Seyama, T., Tamura, S., Hong, J., & Ohuchi, K. (2009). Modification of the picryl chloride-induced allergic dermatitis model in mouse ear lobes by 12-O-tetradecanoylphorbol 13-acetate, and analysis of the role of histamine in the modified model. International Archives of Allergy and Immunology, 148(4), 279–288. https://doi.org/10.1159/000170381
- Holzapfel, W. H., Geisen, R., & Schillinger, U. (1995). Biological preservation of foods with reference to protective cultures, bacteriocins and food-grade enzymes. International Journal of Food Microbiology, 24(3), 343–362. https://doi.org/10.1016/0168-1605(94)00036-6
- Hoque, R., Farooq, A., Ghani, A., Gorelick, F., & Mehal, W. Z. (2014). Lactate reduces liver and pancreatic injury in toll-like receptor– and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology, 146(7), 1763–1774. https://doi.org/10.1053/j.gastro.2014.03.014
- Hui, S., Ghergurovich, J. M., Morscher, R. J., Jang, C., Teng, X., Lu, W., Esparza, L. A., Reya, T., Zhan, L., Yanxiang Guo, J., White, E., & Rabinowitz, J. D. (2017). Glucose feeds the TCA cycle via circulating lactate. Nature, 551(7678), 115–118. https://doi.org/10.1038/nature24057
- Imaoka, A., Shima, T., Kato, K., Mizuno, S., Uehara, T., Matsumoto, S., Setoyama, H., Hara, T., & Umesaki, Y. (2008). Anti-inflammatory activity of probiotic Bifidobacterium: Enhancement of IL-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of IL-8 secretion in HT-29 cells. World Journal of Gastroenterology, 14(16), 2511–2516. https://doi.org/10.3748/wjg.14.2511
- Iraporda, C., Errea, A., Romanin, D. E., Cayet, D., Pereyra, E., Pignataro, O., Sirard, J. C., Garrote, G. L., Abraham, A. G., & Rumbo, M. (2015). Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology, 220(10), 1161–1169. https://doi.org/10.1016/j.imbio.2015.06.004
- Jayasimhan, A., & Mariño, E. (2019). Dietary SCFAs, IL-22, and GFAP: The three musketeers in the gut-neuro-immune network in type 1 diabetes. Frontiers in Immunology, 19(10), 2429–2439. http://doi.org/10.3389/fimmu.2019.02429
- Jie, Z., Chun-Yan, L., Jing-Ping, L., Ren, G., Hui, W., Juan, P., & Sheng-Lan, L. (2015). Immunoregulation on mice of Low immunity and effects on five kinds of human cancer cells of Panax japonicus polysaccharide. Evidence-Based Complementray and Alternative Medicine, 8(4), 839697. http://doi.org/10.1155/2015/839697
- Kakisu, E., Abraham, A. G., Farinati, C. T., Ibarra, C., & De Antoni, G. L. (2012). Lactobacillus plantarum isolated from kefir protects vero cells from cytotoxicity by type-II shiga toxin from Escherichia coli O157:H7. Journal of Dairy Research, 80(1), 1–8. http://doi.org/10.1017/S0022029912000659
- Lauritzen, K. H., Morland, C., Puchades, M., Holm-Hansen, S., Hagelin, E. M., Lauritzen, F., Attramadal, H., Storm-Mathisen, J., Gjedde, A., & Bergersen, L. H. (2014). Lactate receptor sites Link neurotransmission, neurovascular coupling, and brain energy metabolism. Cerebral Cortex, 2014(10), 2784–2795. http://doi.org/10.1093/cercor/bht136
- Lei, X. L., Zhao, S. J., Fan, X. P., Wu, H. M., & Yang, Z. J. (2007). Immunoregulation effects of polysaccharides isolated from octopus dollfusi on immunosuppression mice. Chinese Journal of Marine Drugs, 26(5), 29–33. http://doi.org/10.1504/PCFD.2007.013013
- Mantovani, A., Sozzani, S., Locati, M., Schioppa, T., Saccani, A., Allavena, P., & Sica, A. (2004). Infiltration of tumours by macrophages and dendritic cells: tumour-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Novartis Found Symp. 256, 137–145. discussion 146-8, 259-69.
- McDonald, B., Zucoloto, A. Z., Yu, I., Burkhard, R., Brown, K., Geuking, M. B., & McCoy, K. D. (2020). Programing of an intravascular immune firewall by the gut microbiota protects against pathogen dissemination during infection. Cell Host & Microbe, 28(5), 660–668. http://doi.org/10.1016/j.chom.2020.07.014
- McMeekin, T. A., Brown, J., Krist, K., Miles, D., Neumeyer, K., Nichols, D. S., Olley, J., Presser, K., Ratkowsky, D. A., Ross, T., Salter, M., & Soontranon, S. (1997). Quantitative microbiology: A basis for food safety. Emerging Infectious Diseases, 3(4), 541–549. https://doi.org/10.3201/eid0304.970419
- Morland, C., Lauritzen, K. H., Puchades, M., Holm-Hansen, S., Andersson, K., Gjedde, A., Attramadal, H., Storm-Mathisen, J., & Bergersen, L. H. (2015). The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: Expression and action in brain. Journal of Neuroscience Research, 93(7), 1045–1055. http://doi.org/10.1002/jnr.23593
- Offermanns, S. (2014). Free Fatty Acid (FFA) and Hydroxy Carboxylic Acid (HCA) receptors. Annual Review of Pharmacology and Toxicology, 54(1), 407–434. https://doi.org/10.1146/annurev-pharmtox-011613-135945
- Offermanns, S., Colletti, S. L., Lovenberg, T. W., Semple, G., Wise, A., & IJzerman, A. P. (2011). International union of basic and clinical pharmacology. LXXXII: Nomenclature and classification of hydroxy-carboxylic acid receptors (GPR81, GPR109A, and GPR109B). Pharmacological Reviews, 63(2), 269–290. https://doi.org/10.1124/pr.110.003301
- O’Mahony, L., McCarthy, J., Kelly, P., Hurley, G., Luo, F., Chen, K., O’Sullivan, G. C., Kiely, B., Collins, J. K., Shanahan, F., & Quigley, E. M. (2005). Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology, 128(3), 541–551. https://doi.org/10.1053/j.gastro.2004.11.050
- Peran L., Camuesco D., Comalada M., Bailon E., Henriksson A., Xaus J., Zarzuelo A., & Galvez J. (2007). A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. Journal of Applied Microbiology, 103(4), 836–844. https://doi.org/10.1111/j.1365-2672.2007.03302.x
- Samuelsson, B. (1983). Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Advances in Prostaglandin, Thromboxane, and Leukotriene Research, 11(1), 1–10. https://doi.org/10.1016/0262-1746(83)90104-X
- Sanders, M. E., Guarner, F., Guerrant, R., Holt, P. R., Quigley, E. M., Sartor, R. B., Sherman, P. M., & Mayer, E. A. (2013). An update on the use and investigation of probiotics in health and disease. Gut, 62(5), 787–796. https://doi.org/10.1136/gutjnl-2012-302504
- Schilderink, R., Verseijden, C., & de Jonge, W. J. (2013). Dietary inhibitors of histone deacetylases in intestinal immunity and homeostasis. Frontiers in Immunology, 226(4), 1–11. http://doi.org/10.3389/fimmu.2013.00414
- Sheu, B. S., Wu, J. J., Lo, C. Y., Wu, H. W., Chen, J. H., Lin, Y. S., & Lin, M. D. (2002). Impact of supplement with Lactobacillus- and Bifidobacterium-containing yogurt on triple therapy for Helicobacter pylori eradication. Alimentary Pharmacology & Therapeutics, 16(9), 1669–1675. https://doi.org/10.1046/j.1365-2036.2002.01335.x
- Sugiura, Y., Usui, M., Katsuzaki, H., Imai, K., & Miyata, M. (2017). Anti-inflammatory effects of 6,6'-bieckol and 6,8'-bieckol from Eisenia arborea on mouse ear swelling. Food Science and Technology Research, 23(3), 475–480. https://doi.org/10.3136/fstr.23.475
- Uhlig, S., & Gowik, P. (2018). Efficient estimation of the limit of detection and the relative limit of detection along with their reproducibility in the validation of qualitative microbiological methods by means of generalized linear mixed models. Journal of Consumer Protection and Food Safety, 13(1), 79–87. https://doi.org/10.1007/s00003-017-1130-0
- Varin, A., & Gordon, S. (2009). Alternative activation of macrophages: Immune function and cellular biology. Immunobiology, 214(7), 630–641. https://doi.org/10.1016/j.imbio.2008.11.009
- Wenzel, T. J., Gates, E. J., Ranger, A. L., & Klegeris, A. (2020). Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Molecular and Cellular Neuroscience, 105(6), 103493. https://doi.org/10.1016/j.mcn.2020.103493
- Wileyblackwell. (2013). Comprehensive reviews in food science and food safety. Wiley-Blackwell.
- Wu, B., Yu, Q., Zheng, S., Pedroso, M. M., Guddat, L. W., He, B., & Schenk, G. (2018). Relative catalytic efficiencies and transcript levels of three d- and two l-lactate dehydrogenases for optically pure d-lactate production in Sporolactobacillus inulinus. Microbiologyopen, 8(5), e00704. http://doi.org/10.1002/mbo3.704
- Wu, B. U., Hwang, J. Q., Gardner, T. H., Repas, K., Delee, R., Yu, S., Smith, B., Banks, P. A., & Conwell, D. L. (2011). Lactated Ringer's solution Reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clinical Gastroenterology and Hepatology, 9(8), 710–717.e1. https://doi.org/10.1016/j.cgh.2011.04.026