ABSTRACT
This research was designed to assess the influence of a synbiotic – yucca extract compound preparation (MCC) on production performance, faecal microbiota and immune in laying hens. A total of 180, 27-week-old healthy Hy-Line Brown laying hens were divided into 2 treatments with 6 replicates (15 birds/per replicates), including control group (CN), and treatment group with 3.34 g MCC/kg diet (MCC). The results revealed that supplementation of MCC in diet resulted in a greater laying rate in hens. The concentrations of serum IL-2, IL-6 and TNF-α were lower than CN group, whereas the concentrations of IgG and IL-10 in serum were higher in the MCC group. Furthermore, at the genus levels, the abundance of Romboutsia, and Helicobacter was lower, however, the abundance of Lactobacillus was higher when the hens were fed MCC. Therefore, the addition of MCC positively impacted the production performance, immune and faecal microbial diversity of laying hens.
Introduction
Microbial antibiotic resistance resulting from overuse of antibiotics, is a major threat to global health, food security and development (Van Boeckel et al., Citation2019). The widespread use of antibiotics in poultry farming not only increases antibiotic residues in livestock products but also leads to the development of drug-resistant bacteria(Al-Khalaifa et al., Citation2019). The use of antibiotics in laying hens increases egg safety risks and reduces egg quality, so there is a crying need to find effective alternatives to these antibiotics (Xiang et al., Citation2019). Probiotics, prebiotics, herbs/botanicals and plant extracts have been pointed to partially replace antibiotics (Adhikari et al., Citation2019; Alagawany et al., Citation2016; Chen et al., Citation2020; Ding et al., Citation2019).
Nevertheless, single antibiotic-substitutes have limited effect in alleviating inflammation and injury of tissues and organs. The combination of probiotics, prebiotics, organic acids and phytobiotics is being used in the antibiotics-free feed of broiler chickens (Chen et al., Citation2020; Ren et al., Citation2019). A combination of Bacillus subtilis, Lactobacillus casei and Candida utilise, has been shown to improve production performance, remain the stability of gut microbiota, degrade mycotoxins and lessen histological lesions in broilers (Chang et al., Citation2019). Mi et al. (Citation2019) found that Pichia guilliermondii, B. subtilis and L. Plantarum in the respective ratio of 1:2:1 resulted in a 46% reduction of NH3 in faeces. However, the impact on the combination of probiotics, prebiotics and plant extracts has not been reported on laying hens.
Nowadays, some researches have pointed out that Lactobacillus Plantarum improved the laying rate and intestinal microbe of laying hens (Qiao et al., Citation2019), enhanced intestinal barrier function, immunity and inhibited apoptosis (Wu et al., Citation2019). The main function of Yucca extract is to reduce odorous substances. B. subtilis secretes multiple enzymes to improve nutrient utilization (Neijat et al., Citation2019). At the same time, it preferentially utilizes oxygen as it enters the intestine to provide an anaerobic environment for L. Plantarum. Fructo-oligosaccharides provide energy for L. Plantarum to promote their colonization.
Therefore, the aim of this study was to evaluate the influence of synbiotic – yucca extract compound preparation (MCC), composed of microencapsulated Lactobacillus Plantarum (MLP), Bacillus subtilis (BS), fructooligosaccharides (FOS) and Yucca Schidigera extract (DEO), on production performance, blood immune and antioxidant status, odorous substance and faecal microorganisms in laying hens. The purpose of this research was to develop a multifunctional compound probiotic improving production, immune performance and deodorant.
Materials and methods
All animal procedures were permitted by the Animal Ethics Committee Guidelines (registration number: 2020L02) of the Academy of National Food and Strategic Reserves Administration (ANFSRA, Beijing, China).
Preparation of the microbial-containing compound
The major components of MCC are MLP, BS, FOS and DEO. The MLP (1.0 × 1010 cfu/g) was prepared by our patented emulsion technology (ZL 201310218187.8), according to Dong et al. (Citation2016). BS (1.0 × 1011 cfu/g) was obtained from Beijing Smistyle Sci. &Tech. Development Co., Ltd. (Beijing, China). FOS was obtained from Baolingbao Biology Co., Ltd. (Shandong, China). DEO was generously provided by Alltech Biological Products (China) Co., Ltd, (Beijing, China).
Experimental design and diets
The poultry trial was carried out in the animal breeding base of ANFSRA (Wuqing District, Tianjin, China).
A total of 180, 27-week-old healthy laying hens (Hy-Line Brown) were divided into 2 treatments, each of which consisted of 6 replicates (15 birds/per replicates) at random for 12 weeks, including control group with basal diet (CN) and treatment group with basal diet + 3.34 g MCC/kg diet (MCC). The basal diet and corn and soybean meal diet were formulated according to NRC (Citation1994) and Chicken Feeding Standard (NY/T33-Citation2004). The composition and nutrient levels of the basal diet are shown in .
Table 1. Ingredients and nutrient composition of the basal diet. (as-fed basis).
The hens were placed in a three-story stepped cage, 3 hens per cage and given artificial light for 16 h/d and routine immunization. The room temperature was maintained at 21–24°C. All laying hens were free to feed and drink.
Laying performance and egg quality
Daily egg production, egg weight and the number of broken eggs were recorded. At the end of experiment, laying rate, average daily feed intake (ADFI), gain:feed and broken egg rate (the total number of broken eggs/the total number of eggs × 100) were calculated and egg quality with three eggs from each replicate was evaluated. The strength and thickness of eggshell were gauged with the Egg Force Reader and the thickness Gauge (ORKA Food Technology Ltd, Ramat Hasharon, Israel), respectively. Haugh unit, yolk colour and albumen height were determined with the Egg Analyzer (ORKA Food Technology Ltd, Ramat Hasharon, Israel).
Serum biochemical parameters and egg yolk antioxidant activity
After 12 weeks, one layer was randomly chosen from each replicate for wing vein blood collection. The serum samples were obtained through centrifuging the blood samples at 3000 × g for 10 min at 4°C, and stored at –20°C. The egg yolk samples were manually separated from two eggs per replicate with tweezers for determination of antioxidant properties, and frozen at −80°C. The concentration of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), total antioxidant capacity (T-AOC) and malondialdehyde (MDA) in serum and egg yolk were measured spectrophotometrically using the kits of Beijing Huaying Biotechnology Co. The concentrations of immunoglobulin and cytokines in serum were measured using chicken-specific ELISA kits with tumour necrosis factor-alpha (TNF-α), interleukin (IL-2, IL-6, IL-10) and immunoglobulin (IgA, IgM, IgG).
Faeces odorous substance and microflora diversity
After 12 weeks, faecal samples were randomly selected by repetition, rapidly frozen with liquid nitrogen, and stored at −80°C (Eren et al., Citation2015). Indole and skatole of faeces were measured using high-performance liquid chromatography as described by Knarreborg et al. (Citation2002). Eight samples of faecal content (4 from each treatment group) were randomly selected at the end of the experiment. The faecal microbiota diversity was analysed using high-throughput sequencing procedure by Novogene Co., LTD (Beijing, China). Briefly, the procedure included DNA extraction, 16S rRNA PCR amplification, amplicon sequencing and sequence data processing were used to analyse the faeces microbiota diversity (Zhang et al., Citation2019). Alpha diversity indices were calculated in QIIME from rarefied samples. The Shannon index, Simpson index and PD-whole-tree were used to evaluate diversity, while Chao1 index and ACE index were used to evaluate richness. The results of the β-diversity were visualized by a Principal Coordinates Analysis (PCoA) (Qiao et al., Citation2019). The relative abundance of top 13 and top 16 bacteria was compared at the family and genus levels, respectively.
Statistical analysis
The statistical analysis was performed with SPSS 20.0 software (SPSS Inc., Chicago, IL). The data were shown as means and SEM. Differences between mean values were compared using independent samples T-test and considered statistically significant at P < 0.05. PCoA and LEfSe were used to analyse discrepancy of structure of faecal microbiota. Correlation analysis between microorganisms and environmental factors (including production performance, immunity, antioxidant, deodorant performance) was accomplished by Pearson correlation analysis of SPSS 20.0, and the heatmap was plotted using Graphpad Prism8.
Results
Laying performance and egg quality
The effects of dietary supplementation of MCC on laying performance and egg quality were shown in . The laying rate of hens in MCC group was significantly higher (P < 0.05) than that in the CN group (). However, MCC group had no significant effects either on the ADFI or gain:feed in hens or the weight and mass of egg (P > 0.05). Although no significant difference in the broken egg rate was seen between hens in the CN and MCC groups, the egg breaking rate in MCC group was 62.97% lower than the CN group (P > 0.05).
Table 2. Effects of MCC on laying performance and egg quality in laying hens.
No significant differences were observed between the CN and MCC groups on egg quality, including eggshell thickness, albumen height, yolk colour, egg shape index, eggshell strength and Haugh unit at the end of the experimental period (, P > 0.05).
Immune status
As shown in , hens in the MCC group showed a diminution in the serum levels of IL-2, IL-6, TNF-α (P < 0.05), and an elevation in the levels of IgG and IL-10 (P < 0.05), compared with the CN group. However, the diet supplemented with MCC had no significant difference in serum IgM and IgA levels in hens (P > 0.05).
Table 3. Effects of MCC on serum immunoglobulin levels and immune cytokines in laying hens.
Serum and egg yolk antioxidant activity
Serum antioxidant indices were presented in . The levels of serum GSH-Px, SOD, T-AOC in hens fed the diet supplemented with MCC were higher than those in CN group (P < 0.05). In addition, the level of serum MDA in hens of MCC group was lower than that in hens of CN group (P < 0.05). There were no significant differences between the activities of GSH-Px and SOD in egg yolk (P > 0.05). However, the activity of T-AOC was significantly higher, and the activity of MDA was significantly lower in egg yolk of hens in MCC group, compared with those in the control group (P < 0.05).
Table 4. Effects of MCC on SOD, GSH-PX, MDA and T-AOC in laying hens.
Odorous substance and faecal microbiota diversity
The supplementation of MCC to the diet did not significantly affect the odour substance (indole and skatole) in faeces of hens (P > 0.05, ).
Table 5. Effects of MCC on skatole and indole in laying hens.
A total of 625,807 V3-V4 16S rRNA amplicon sequence reads were measured. The maximum and the minimum number of sequences per sample were 80,175 and 64,954, respectively. No significant differences were observed in Chao1 index, ACE index and Shannon index between the control group and the MCC group (P > 0.05), whereas the Simpson index was significantly higher in MCC group (P < 0.05) ().
Table 6. Effects of MCC on faecal microflora diversity in laying hens.
We detected 12 phyla, 17 classes, 28 orders, 53 families and 85 genera in the microbiota community from all faecal samples collected in this study. Results showed that Firmicutes was the dominant phylum in the faeces, comprising 86.4%–96.33% of all phyla (). Additionally, compared with those in CN group, the relative abundance of Lactobacillus in the faeces of hens in MCC group was significantly higher (P < 0.05), while the relative abundance of Romboutsia and Helicobacter was significantly lower at the genus level (P < 0.05, ).
Table 7. Effects of MCC on the distribution of faecal bacterial phyla in laying hens.
Table 8. Effects of MCC on the distribution of faecal bacterial genus in laying hens.
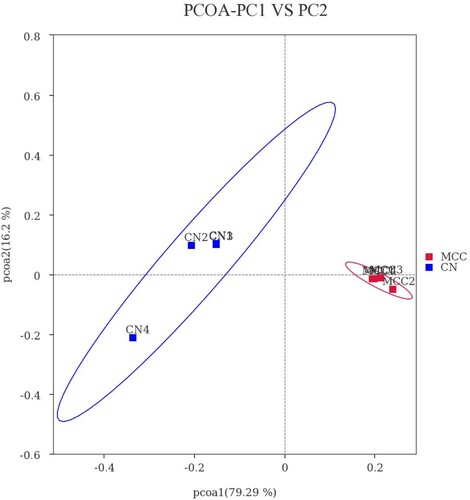
To analyse the discrepancy in structure of faecal microbiota between groups, we demonstrated the principal coordinates analysis (PCoA) plots in . The PCoA plot of the faecal microbiota was analysed based on the weighted UniFrac metric, measured as Adonis (P = 0.001) and Anosim (P = 0.025), which demonstrated that MCC supplementation changed (P < 0.05) the structure of faecal microbiota.
Figure 1. PCoA of the faecal bacterial population structures. The red and blue dots represent CN and MCC samples, respectively. It was generated with weighted Unifrac distance. CN: the control group; MCC: basal diet supplemented with MLP, BS, FOS, and DEO. MLP, microencapsulated Lactobacillus Plantarum; BS, Bacillus subtilis; FOS, fructooligosaccharides; DEO, Yucca Schidigera extract.
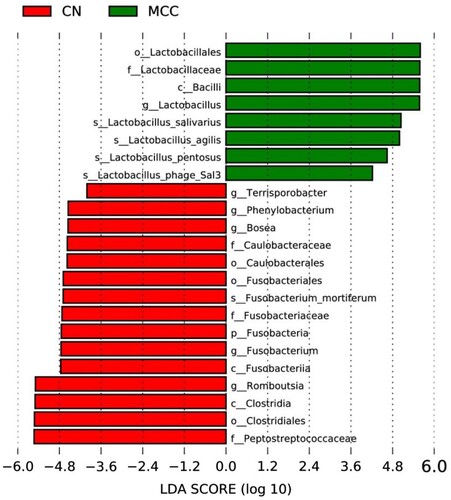
The LEfSe analysis provided us with the taxa representing significant differences in abundance between the faecal microbiota in hens of CN and MCC groups (). At the genus level, Lactobacillus belonging to the order Lactobacillales were observed to be enriched in MCC group while Romboutsia and Terrisporobacter belonging to Firmicutes, Phenylobacterium and Bosea belonging to Proteobacteria, and Fusobacterium were found to be enriched in CN group.
Figure 2. Effects of MCC on the microbial biomarkers of laying hens (n = 4). Linear discriminant analysis effect size (LEfSe) analysis shows differentially abundant genera as biomarkers determined using Kruskal–Wallis test (P < 0.05) with the logarithmic linear discriminant analysis (LDA) score > 4.0. CN: the control group; MCC: basal diet supplemented with MLP, BS, FOS and DEO. MLP, microencapsulated Lactobacillus Plantarum; BS, Bacillus subtilis; FOS, fructooligosaccharides; DEO, Yucca Schidigera extract.
Correlation analysis between microorganisms and production performance, immunity, antioxidant, deodorant properties
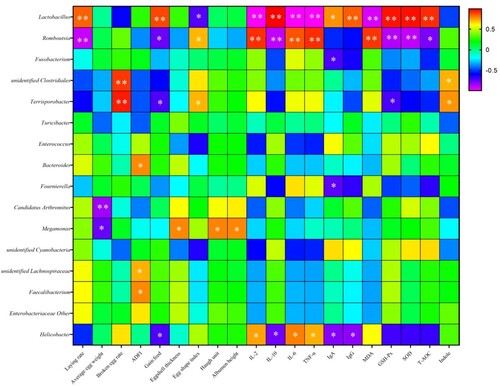
showed associations of faecal microorganisms with production performance, immunity, antioxidant and deodorant properties. Laying rate, gain:feed and antioxidant properties (SOD, T-AOC, GSH-Px) were significantly positively correlated with Lactobacillus, but negatively correlated with Romboutsia. The significantly positive correlations between the egg breaking rate, indoles and Terrisporobacter, Unidentified Clostridiales were also determined. Megamonas was negatively correlated with egg weight and positively correlated with eggshell thickness, Haugh unit and albumen height. The abundance of Bacteroides, Unidentified Lachnospiraceae and Faecalibacterium had a significantly positive correlation with feed intake. Egg shape index was negatively correlated with Terrisporobacter and Romboutsia, and positively correlated with Lactobacillus. Anti-inflammatory factors IL-10, IgA and IgG were negatively correlated with Helicobacter and positively correlated with Lactobacillus. The pro-inflammatory factors IL-2, IL-6 and TNF-α were positively correlated with Helicobacter and Romboutsia, and negatively correlated with Lactobacillus.
Figure 3. Associations of faecal microorganisms with productive, immune, antioxidant and deodorant properties. The depth of colours ranging from purple to red represents the magnitude of correlation. The OTU were organized according to their Pearson correlation coefficient. Significant correlations are noted by: *, 0.01 < P < 0.05; **, P < 0.01. ADFI = average daily feed intake.
Discussion
Many reports have shown that novel additives, such as probiotics or prebiotics, were regarded as alternatives for antibiotic growth promoters in feed (Alagawany et al., Citation2016; Yang et al., Citation2020). Nevertheless, the efficiency of the alternative novel additives hinges on a number of elements, such as absorption concentrations, daily diet, supplementation methods or the rearing environment (Patterson & Burkholder, Citation2003). In order to maximize the efficiency of these alternatives, the combination of a synergistic concept is a positive solution that may transcend their single application (Ren et al., Citation2019). The present research assessed the effects of MCC on production performance, egg quality, serum immunoglobulin and immune-related cytokine levels, serum oxidative marker concentrations, odorous substances contents and faecal microbiota in laying hens.
Several studies reported that diets supplemented with probiotics (L. Plantarum or B. subtilis) improved laying rate (Chen et al., Citation2020; Qiao et al., Citation2019; Yang et al., Citation2020). However, Xiang et al. (Citation2019) reported that the combination of probiotics (Saccharomyces boulardii and Pediococcus acidilactici) did not affect laying performance. Sheoran et al. (Citation2018) reported that supplementing hen diets with synbiotic (Lactobacillus fermentum, Bacillus spp., Saccharomyces cerevisiae manno-oligosaccharide of Saccharomyces cell wall and formic acid) improved egg production. In our study, dietary supplementation with MCC resulted in significantly higher laying rate, which may be owing to the enhancement of nutrient digestibility and absorption in the gastrointestinal tract of hens by probiotics and prebiotics (Tang et al., Citation2017; Xiang et al., Citation2019). Another possible explanation for this is that the supplement of MCC improved the immunity and antioxidant capacity of laying hens. In recent years, the demand for eggs has focused not on the quantity but on the quality of eggs. Neijat et al. (Citation2019) showed that the supplementation of B. subtilis improved the interior egg quality (albumen height and Haugh unit). The supplementation of synbiotic (isomaltooligosaccharide and PrimaLac) in the diets of laying hens had dramatically improved the ADFI, FCR, laying rate, weight, mass and size of egg (Tang et al., Citation2017). But in our research, the diet supplemented with MCC had no significant effects on average egg weight, egg mass, ADFI, gain:feed, Haugh unit, albumen height, yolk colour, egg shape index, eggshell strength and thickness during the experiment. This intriguing result could be attributed to the difference in the composition of compound probiotics and supplementation concentrations (Li et al., Citation2018). Moreover, the results of this study showed that laying rate was positively correlated with Lactobacillus, but negatively correlated with Romboutsia. Dietary MCC supplementation significantly increased the abundance of Lactobacillus and decreased the abundance of Romboutsia. This would explain why laying hens supplemented MCC had higher laying rate than those supplemented with the basic diet.
Immune and antioxidant performance of laying hens are important indexes to evaluate body health. The crucial response criteria for humoral immune response in humans and animals are evaluated by concentrations of immunoglobulins (Wang et al., Citation2018). Elevated levels of IgG and IgA expression showed a boosted immunity after probiotic feeding (Gao et al., Citation2017). Alagawany et al. (Citation2016) indicated that dietary supplementation of yucca exhibited remarkable improvement in IgG. However, Liu et al. (Citation2019) found that supplementation with B. subtilis improved IgM concentration, rather than IgA and IgG concentrations. Our present findings were in agreement with the observation of Ding et al. (Citation2019), who investigated that dietary supplementation with L. Plantarum 15–1 and FOS increased the serum level of IgG. In this study, the beneficial effects on humoral immune response may be attributed to the stimulation of B-cell-mediated immune response by the synbiotic, thereby enhancing the production of antibodies (Lammers et al., Citation2004).
Cytokines are small, molecular proteins with diverse biological activities, which are of importance in regulating immune and inflammatory responses (Dong et al., Citation2016). CD4+ T helper (Th) cells can differentiate into Th1 and Th2 cells when they encounter different foreign antigens, which in turn produce different cytokines. TNF-α and IL-2 are produced by Th1 cells, while IL-6 and IL-10 are produced by Th2 cells (Scheer et al., Citation2020). Wang et al. (Citation2020) found that the Yucca schidigera extract supplemented diets down-regulated mRNA levels of TNF-α, IL-1β, and IL-6 in the intestine of mirror carp. In our research, the MCC group showed a significant diminution in the serum levels of IL-2, IL-6 and TNF-α, and significant elevation in the level of IL-10 compared to the control group. The current results were in agreement with Adhikari et al. (Citation2019), who reported that the L. Plantarum treatments reduced IFN-γ and increased a key anti-inflammatory cytokine, IL-10. The observed suppression in pro-inflammatory cytokines could be attributed to the inhibition of the population of pathogenic bacteria and the improvement of the intestinal environment (Chen et al., Citation2020). In the current study, IL-10, IgA and IgG were negatively correlated with Helicobacter and positively correlated with Lactobacillus. The pro-inflammatory factors IL-2, IL-6 and TNF-α were positively correlated with Helicobacter and Romboutsia, but negatively correlated with Lactobacillus. Thus, Lactobacillus and Helicobacter might play essential roles in the regulation of immunity in laying hens by MCC.
Oxidative stress could generate multitudinous reactive oxygen species (ROS), give rise to injure of proteins and DNA, and apoptosis that occurs in cells/tissues, which ultimately affected the performance of laying hens (Liu et al., Citation2019). SOD is a metalloprotein enzyme that can eliminate excessive ROS in the body and monitor lipid oxidation status in the body by MDA (Alagawany et al., Citation2016; Chen et al., Citation2020). Supplementation of yucca extract in the diet significantly increased the levels of SOD and T-AOC and decreased the level of MDA (Alagawany et al., Citation2016). Dietary supplementation with B. subtilis increased SOD in the intestinal mucosa (Chen et al., Citation2020). However, some authors have pointed out that the supplementation with B. subtilis increased the activity of GSH-Px but did not affect SOD, T-AOC and MDA (Liu et al., Citation2019). The current results showed that the level of T-AOC was greater, and the level of MDA was lower in egg yolk and serum when hens were fed MCC, which were similar to the result of Wang et al. (Citation2018). The high antioxidant capacity in egg yolk may extend shelf life and also have a positive impact on human health. Lower MDA levels in serum were observed in MCC groups, showing that MCC likely relieved oxidative stress by inhibiting lipid peroxidation, which was consistent with previous studies (Wu et al., Citation2019). In this study, the levels of serum GSH-Px and SOD were higher in hens fed the diet supplemented with MCC. The synbiotic in MCC likely promotes scavenging ROS, promotes antioxidant capability of activating and translocates nuclear factors to activate the expression of various enzymes in the antioxidant defence system (Mohammed et al., Citation2019). Immune cells are sensitive to oxidative stress due to their membranes containing high concentrations of unsaturated fatty acids, which are highly sensitive to peroxidation. Therefore, MCC improved the body’s antioxidant capacity and reduced ROS, which may be one of the reasons for enhanced immune function. The improvement of immune and antioxidant properties may be responsible for ameliorating production performance and nutrient utilization. Furthermore, the antioxidant capacity (SOD, T-AOC, GSH-Px) was positively correlated with Lactobacillus, but negatively correlated with Romboutsia, indicating that changes in these microorganisms affected the antioxidant capacity of the laying hens. Therefore, the improvement of antioxidant properties may depend on the increase of Lactobacillus and the decrease of Romboutsia.
Ammonia, phenol, volatile fatty acids, sulphides, skatole and indole, are the most common odorants associated with animal manures (Mackie et al., Citation1998). It has been reported that the addition of probiotics to the diet can reduce ammonia emissions (Mi et al., Citation2019). The key role of Yucca schidigera extract in animal nutrition is to reduce blood ammonia-N, urea-N, and the ammonia content in the atmosphere and faecal odour in livestock excreta (Alagawany et al., Citation2016; Wang et al., Citation2020). Besides ammonia, skatole and indole are also the major odorants in animal manure (Li et al., Citation2019). Skatole is a tryptophan metabolite with a faecal odour (Yang et al., Citation2019). Liu et al. (Citation2018) showed the addition of inulin or soya bean oligosaccharide reduced the concentrations of indole and skatole and the rate of L- tryptophan degradation. However, the degradation effect of synbiotics on skatole and indole has not been studied. The current results showed that the addition of MCC to the diet did not significantly affect the odorous substances (indole and skatole), but compared with the control group, the MCC group had lower proportions of skatole and indole by 19.3% and 12.8%, respectively. It seems possible that these results are due to suppressed conversion of tryptophan into skatole by enhancing microbial activity and improving the digestibility of amino acids (Li et al., Citation2019). In this study, Pearson correlation analysis further found that Terrisporobacter exhibited markedly positive correlation with indoles, which suggested that dietary MCC supplementation reduced indoles by targeting intestinal microbiota.
Microbial diversity and composition are the critical elements to affect poultry health (Guo et al., Citation2018). The current study found that gut microbial diversity was greater in MCC group. Kitano and Oda (Citation2006) demonstrated that the host coped with environmental challenges by sheltering a wide range of bacteria. Hence, rich microbial diversity may enhance defences against pathogens. In our study, supplementation with MCC modulated the composition of faecal microbiota at different taxonomic levels. Firmicutes were the dominant phylum in the faeces, comprising 86.4–96.33% of all phyla. Guo et al. (Citation2018) indicated that bacteria within Firmicutes were involved in the digestion and absorption of diversified nutritional substances and built up host health, and the high relative abundance of Fusobacteria exerted detrimental effect by host. The relative abundance of Fusobacteria was frequently associated with tumour progression (Abed et al., Citation2017), and part of the flora of Fusobacteria is positively correlated with low-density lipoprotein cholesterol and total cholesterol (Koren et al., Citation2011). This research found that the addition of MCC to the diet resulted in lower relative content of Fusobacteria compared with the control group. Similar results were obtained by Guo et al. (Citation2018), where the authors showed that dietary supplementation of B. subtilis reduced the relative abundance of Fusobacteria. The reduction of Fusobacteria in this study predicts benefits to the host. Romboutsia and Lactobacillus appear to be correlated to beneficial effects on the host (Mangifesta et al., Citation2018). Ren et al. (Citation2019) demonstrated that significantly increased lactobacillus abundance by the supplementation of combinations of probiotics and phytobiotic. Our research showed that the relative abundance of Romboutsia were lower and the relative abundance of Lactobacillus were greater at the genus level in the birds of group MCC. The addition of MCC resulted in changes to the composition of beneficial bacteria, which may protect the host from pathogens through different competitive mechanisms (Yadav & Jha, Citation2019). Greater abundance of Helicobacter, is typically associated with carcinogenesis, such as gastric precancerous lesions (Mangifesta et al., Citation2018). Our research found that the relative abundance of Helicobacter were lower in the birds of MCC group. Helicobacter pylori infection-induced inflammation and damage were attributed to ROS production (Prazeres et al., Citation2019). The observed reduction in the relative abundance of Helicobacter could be attributed to significantly increased antioxidant capacity by reducing the production of ROS in the dietary supplementation of MCC. Therefore, the alteration of the microbial community, in particular the changes in abundance of potentially pathogenic bacteria may be conducive to maintaining the health and normal function of the intestinal tract and beneficially aid production performance (Guo et al., Citation2018). Overall, the increased abundance of Lactobacillus and the decreased abundance of Romboutsia could partially explain the observed effects of MCC supplementation on production performance, immunity and antioxidant properties of laying hens in the current study. Meanwhile, reduced abundance of Fusobacterium and Helicobacter can improve immunity.
Conclusions
In summary, this study suggested that dietary supplementation with MCC (MLP, BS, FOS, DEO) positively affected the egg production, immune status, oxidative status, and faecal microbial profile and diversity of laying hens. Furthermore, the addition of MCC to laying hen diets may favourably impact egg oxidative status.
Acknowledgements
This research was funded by Research Fund for National Non-profit Research Institution (grant number JY2016 and ZX1924), China Agriculture Research System of MOF and MARA (CARS-40-S20) and Beijing Agricultural Innovation Consortium 04-2021 (grant number BAIC04-2021).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abed, J., Maalouf, N., Parhi, L., Chaushu, S., Mandelboim, O., & Bachrach, G. (2017). Tumor targeting by Fusobacterium nucleatum: A pilot study and future perspectives. Frontiers in Cellular and Infection Microbiology, 7(7), https://doi.org/10.3389/fcimb.2017.00295
- Adhikari, P., Lee, C. H., Cosby, D. E., Cox, N. A., & Kim, W. K. (2019). Effect of probiotics on fecal excretion, colonization in internal organs and immune gene expression in the ileum of laying hens challenged with Salmonella Enteritidis. Poultry Science, 98(3), 1235–1242. https://doi.org/10.3382/ps/pey443
- Alagawany, M., Abd El-Hack, M. E., & El-Kholy, M. S. (2016). Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Yucca schidigera extract. Environmental Science and Pollution Research, 23(7), 6774–6782. https://doi.org/10.1007/s11356-015-5919-z
- Al-Khalaifa, H., Al-Nasser, A., Al-Surayee, T., Al-Kandari, S., Al-Enzi, N., Al-Sharrah, T., Ragheb, G., Al-Qalaf, S., & Mohammed, A. (2019). Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poultry Science, 98(10), 4465–4479. https://doi.org/10.3382/ps/pez282
- Chang, C. H., Teng, P. Y., Lee, T. T., & Yu, B. (2019). Effects of multi-strain probiotic supplementation on intestinal microbiota, tight junctions, and inflammation in young broiler chickens challenged with Salmonella enterica subsp. Enterica. Asian-Australasian Journal of Animal Sciences, 33(11), 1797–1808. https://doi.org/10.5713/ajas.19.0427
- Chen, J. F., Xu, M. M., Kang, K. L., Tang, S. G., He, C. Q., Qu, X. Y., & Guo, S. C. (2020). The effects and combinational effects of Bacillus subtilis and montmorillonite on the intestinal health status in laying hens. Poultry Science, 99(3), 1311–1319. https://doi.org/10.1016/j.psj.2019.11.016
- Ding, S., Wang, Y., Yan, W., Li, A., Jiang, H., & Fang, J. (2019). Effects of Lactobacillus plantarum 15-1 and fructooligosaccharides on the response of broilers to pathogenic Escherichia coli O78 challenge. PLoS One, 14(6), e0212079. https://doi.org/10.1371/journal.pone.0212079
- Dong, Z. L., Wang, Y. W., Song, D., Hou, Y. J., Wang, W. W., Qi, W. T., Yun, T. T., & Li, A. K. (2016). The effects of dietary supplementation of pre-microencapsulated Enterococcus fecalis and the extract of Camellia oleifera seed on growth performance, intestinal morphology, and intestinal mucosal immune functions in broiler chickens. Animal Feed Science and Technology, 212, 42–51. https://doi.org/10.1016/j.anifeedsci.2015.11.014
- Eren, A. M., Sogin, M. L., Morrison, H. G., Vineis, J. H., Fisher, J. C., Newton, R. J., & McLellan, S. L. (2015). A single genus in the gut microbiome reflects host preference and specificity. The ISME Journal, 9(1), 90–100. https://doi.org/10.1038/ismej.2014.97
- Gao, P., Ma, C., Sun, Z., Wang, L., Huang, S., Su, X., Xu, J., & Zhang, H. (2017). Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome, 5(1), 91. https://doi.org/10.1186/s40168-017-0315-1
- Guo, J. R., Dong, X. F., Liu, S., & Tong, J. M. (2018). High-throughput sequencing reveals the effect of Bacillus subtilis CGMCC 1.921 on the cecal microbiota and gene expression in ileum mucosa of laying hens. Poultry Science, 97(7), 2543–2556. https://doi.org/10.3382/ps/pey112
- Kitano, H., & Oda, K. (2006). Robustness trade-offs and host-microbial symbiosis in the immune system. Molecular Systems Biology, 2(2006), 0022. https://doi.org/10.1038/msb4100039
- Knarreborg, A., Beck, J., Jensen, M. T., Laue, A., Agergaard, N., & Jensen, B. B. (2002). Effect of non-starch polysaccharides on production and absorption of indolic compounds in entire male pigs. Animal Science, 74(3), 445–453. https://doi.org/10.1017/S1357729800052590
- Koren, O., Spor, A., Felin, J., Fak, F., Stombaugh, J., Tremaroli, V., Behre, C. J., Knight, R., Fagerberg, B., Ley, R. E., & Backhed, F. (2011). Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proceedings of the National Academy of Sciences, 108(Suppl 1), 4592–4598. https://doi.org/10.1073/pnas.1011383107
- Lammers, A., Klomp, M. E. V., Nieuwland, M. G. B., Savelkoul, H. F. J., & Parmentier, H. K. (2004). Adoptive transfer of natural antibodies to non-immunized chickens affects subsequent antigen-specific humoral and cellular immune responses. Developmental & Comparative Immunology, 28(1), 51–60. https://doi.org/10.1016/S0145-305X(03)00102-2
- Li, C. L., Wang, J., Zhang, H. J., Wu, S. G., Hui, Q. R., Yang, C. B., Fang, R. J., & Qi, G. H. (2018). Intestinal morphologic and microbiota responses to dietary bacillus spp. in a broiler chicken model. Frontiers in Physiology, 9(2018), 1968. https://doi.org/10.3389/fphys.2018.01968
- Li, X. Q., Jensen, B. B., & Canibe, N. (2019). The mode of action of chicory roots on Skatole production in entire male pigs is neither via reducing the population of Skatole-producing bacteria nor via increased butyrate production in the hindgut. Applied and Environmental Microbiology, 85(6), https://doi.org/10.1128/AEM.02327-18
- Liu, H. Y., Hou, R., Yang, G. Q., Zhao, F., & Dong, W. G. (2018). In vitro effects of inulin and soya bean oligosaccharide on skatole production and the intestinal microbiota in broilers. Journal of Animal Physiology and Animal Nutrition, 102(3), 706–716. https://doi.org/10.1111/jpn.12830
- Liu, X., Peng, C., Qu, X., Guo, S., Chen, J. F., He, C., Zhou, X., & Zhu, S. (2019). Effects of Bacillus subtilis C-3102 on production, hatching performance, egg quality, serum antioxidant capacity and immune response of laying breeders. Journal of Animal Physiology and Animal Nutrition, 103(1), 182–190. https://doi.org/10.1111/jpn.13022
- Mackie, R. I., and V, S. P. G., & H, V. (1998). Biochemical identification and biological origin of key odor components in livestock waste. Journal of Animal Science, 76(5), 1331–1342. https://doi.org/10.2527/1998.7651331x
- Mangifesta, M., Mancabelli, L., Milani, C., Gaiani, F., de’Angelis, N., de’Angelis, G. L., van Sinderen, D., Ventura, M., & Turroni, F. (2018). Mucosal microbiota of intestinal polyps reveals putative biomarkers of colorectal cancer. Scientific Reports, 8(1), 13974. https://doi.org/10.1038/s41598-018-32413-2
- Mi, J., Chen, X., & Liao, X. (2019). Screening of single or combined administration of 9 probiotics to reduce ammonia emissions from laying hens. Poultry Science, 98(9), 3977–3988. https://doi.org/10.3382/ps/pez138
- Mohammed, A. A., Jiang, S., Jacobs, J. A., & Cheng, H. W. (2019). Effect of a synbiotic supplement on cecal microbial ecology, antioxidant status, and immune response of broiler chickens reared under heat stress. Poultry Science, 98(10), 4408–4415. https://doi.org/10.3382/ps/pez246
- Neijat, M., Shirley, R. B., Barton, J., Thiery, P., Welsher, A., & Kiarie, E. (2019). Effect of dietary supplementation of Bacillus subtilis DSM29784 on hen performance, egg quality indices, and apparent retention of dietary components in laying hens from 19 to 48 weeks of age. Poultry Science, 98(11), 5622–5635. https://doi.org/10.3382/ps/pez324
- NRC. (1994). Nutrient requirements of poultry in Book Nutrient requirements of poultry. National Academy Press.
- NY/T33-2004. Nutrient requirements of Chinese feeding standard of chicken. The Ministry of Agriculture of the People’s Republic of China.
- Patterson, J. A., & Burkholder, K. M. (2003). Application of prebiotics and probiotics in poultry production. Poultry Science, 82(4), 627–631. https://doi.org/10.1093/ps/82.4.627
- Prazeres, L. D. K. T., Aragao, T. P., Brito, S. A., de Almeida, C. L. F., Silva, A. D., de Paula, M. M. F., Farias, J. S., Vieira, L. D., Damasceno, B. P. G. L., Rolim, L., Veras, B. O., Rocha, I. G., Neto, J. C. S., Bittencourt, M. L. F., Goncalves, R. D. R., Kitagawa, R. R., & Wanderley, A. G. (2019). Antioxidant and antiulcerogenic activity of the dry extract of pods of Libidibia ferrea Mart. ex Tul. (Fabaceae). Oxidative Medicine and Cellular Longevity, 2019(10094), 1–23. https://doi.org/10.1155/2019/1983137
- Qiao, H., Shi, H., Zhang, L., Song, Y., Zhang, X., & Bian, C. (2019). Effect of Lactobacillus plantarum supplementation on production performance and fecal microbial composition in laying hens. Open Life Sciences, 14(1), 69–79. https://doi.org/10.1515/biol-2019-0009
- Ren, H., Vahjen, W., Dadi, T., Saliu, E. M., Boroojeni, F. G., & Zentek, J. (2019). Synergistic effects of probiotics and phytobiotics on the intestinal microbiota in young broiler chicken. Microorganisms, 7(12), 684. https://doi.org/10.3390/microorganisms7120684
- Scheer, S., Runting, J., Bramhall, M., Russ, B., Zaini, A., Ellemor, J., Rodrigues, G., Ng, J., & Zaph, C. (2020). The Methyltransferase DOT1L controls activation and lineage integrity in CD4+ T cells during Infection and inflammation. Cell Reports, 33(11), 108505. https://doi.org/10.1016/j.celrep.2020.108505
- Sheoran, N., Maan, S., Kumar, A., Batra, K., Chaudhary, D., Sihag, S., Kumar, V., & Maan, N. S. (2018). Probiotic and prebiotic supplementation improving the production performance and immune characteristics of laying hens. Indian Journal of Animal Research, 52(10), 1433–1439. https://doi.org/10.18805/ijar.B-3394
- Tang, S. G. H., Sieo, C. C., Ramasamy, K., Saad, W. Z., Wong, H. K., & Ho, Y. W. (2017). Performance, biochemical and haematological responses, and relative organ weights of laying hens fed diets supplemented with prebiotic, probiotic and synbiotic. BMC Veterinary Research, 13(1), 248. https://doi.org/10.1186/s12917-017-1160-y
- Van Boeckel, T. P., Pires, J., Silvester, R., Zhao, C., Song, J., Criscuolo, N. G., Gilbert, M., Bonhoeffer, S., & Laxminarayan, R. (2019). Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science, 365(6459), eaaw1944. https://doi.org/10.1126/science.aaw1944
- Wang, L., Wu, D., Fan, Z., Li, H., Li, J., Zhang, Y., Xu, Q., Wang, G., & Zhu, Z. (2020). Effect of Yucca schidigera extract on the growth performance, intestinal antioxidant status, immune response, and tight junctions of mirror carp (Cyprinus carpio). Fish & Shellfish Immunology, 103, 211–219. https://doi.org/10.1016/j.fsi.2020.05.039
- Wang, W. W., Chen, J., Zhou, H., Wang, L., Ding, S. J., Wang, Y. W., Song, D., & Li, A. K. (2018). Effects of microencapsulated Lactobacillus plantarum and fructooligosaccharide on growth performance, blood immune parameters, and intestinal morphology in weaned piglets. Food and Agricultural Immunology, 29(1), 84–94. https://doi.org/10.1080/09540105.2017.1360254
- Wu, Y., Wang, B., Zeng, Z., Liu, R., Tang, L., Gong, L., & Li, W. (2019). Effects of probiotics Lactobacillus plantarum 16 and Paenibacillus polymyxa 10 on intestinal barrier function, antioxidative capacity, apoptosis, immune response, and biochemical parameters in broilers. Poultry Science, 98(10), 5028–5039. https://doi.org/10.3382/ps/pez226
- Xiang, Q., Wang, C., Zhang, H., Lai, W., Wei, H., & Peng, J. (2019). Effects of different probiotics on laying performance, egg quality, oxidative status, and gut health in laying hens. Animals, 9(12), 1110. https://doi.org/10.3390/ani9121110
- Yadav, S., & Jha, R. (2019). Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. Journal of Animal Science and Biotechnology, 10(1), 2. https://doi.org/10.1186/s40104-018-0310-9
- Yang, G., Zhang, P., Liu, H., Zhu, X., & Dong, W. (2019). Spatial variations in intestinal skatole production and microbial composition in broilers. Animal Science Journal, 90(3), 412–422. https://doi.org/10.1111/asj.13164
- Yang, J., Zhan, K., & Zhang, M. (2020). Effects of the use of a combination of two bacillus species on performance, egg quality, small intestinal mucosal morphology, and cecal microbiota profile in aging laying hens. Probiotics and Antimicrobial Proteins, 12(1), 204–213. https://doi.org/10.1007/s12602-019-09532-x
- Zhang, G. J., Li, B., Guo, F., Lin, J., Luan, M. Q., Liu, Y., & Guan, Y. T. (2019). Taxonomic relatedness and environmental pressure synergistically drive the primary succession of biofilm microbial communities in reclaimed wastewater distribution systems. Environment International, 124, 25–37. https://doi.org/10.1016/j.envint.2018.12.040
