ABSTRACT
Meat quality is damaged by traditional technologies. Researchers devised a revolutionary technique called plasma technology to meet the requirement for an effective cold processing method. Cold plasma (CP) used for meats and its products decontamination and storability extension has shown to be highly successful. The influence of CP on meat quality is important to its acceptability as an alternative meat processing technology. CP treatments have had effects on the color, pH, lipid oxidation, and microbial quality of different meat products. CP treatment offers crucial benefits over traditional processing methods because of its design adaptability, non-thermal behavior, relatively inexpensive, and environmental friendliness. CP processing is currently in its infancy and requires further investigation in meat and meat products to achieve its best extent. This paper discusses the fundamentals of CP technology, its effects on the quality of meats such as color, pH value, lipid oxidation, and microbial quality, and future perspectives.
1. Introduction
Meat is the main source of protein and has a high nutritional value for humans. Every year, the global consumption of meat (beef, chicken, pork, and lamb) is increasing (De Smet & Vossen, Citation2016; Zhang et al., Citation2018; Shi et al., Citation2021). In recent years, consumer food preferences have dramatically shifted, favoring ready-to-eat, fresh, minimally processed, and greater nutrient meat products with original color, texture, and flavor. The human diet depends on meat and meat products as a significant source of protein. Meat provides a great growth environment for foodborne microorganisms due to its intrinsic parameters like pH, water activity, and specific nutrients. For instance, Clostridium perfringens, induce vomiting, diarrhea, and stomach pain when present in meat products. Clostridium botulinum produces a neurotoxin that causes foodborne illnesses like botulism, which causes asphyxiation and muscle paralysis (Lee et al., Citation2018). The presence of microorganisms such as Enterobacteriaceae, Pseudomonas, and lactic acid bacteria in meat and meat products, and their proliferation, are the primary reason for meat spoilage whereas lipid oxidation is the second mechanism that induces meat deterioration (Dave & Ghaly, Citation2011). As a prominent food category, meat quality is evaluated by three important factors such as nutritional content, safety, and consumer acceptance (Clydesdale & Ahmed, Citation1978). The color of the meat and its flavor are also two crucial characteristics that have a direct effect on consumer acceptance. The appearance of meat and consumer purchase decisions are impacted by color because consumers often perceive the freshness of meat by its surface color (Mancini & Hunt, Citation2005). However, Lipid oxidation could have a direct impact on meat flavor and color, mainly in red meat, because the products of lipid oxidation result in the production of off-aromas and the oxidization of myofibrillar proteins. Beef minced meat is a well-known meat product on the market. It's more susceptible to oxidization and deterioration than intact meat because more meat surfaces are exposed to air and are therefore contaminated by microorganisms (Alp & Aksu, Citation2010). Huge quantities of raw meat and its products have been disposed of due to insufficient preservation technology (Zhao et al., Citation2018). Decontamination's main objective is to stop microbial proliferation which leads to food deterioration (Dave & Ghaly, Citation2011). Many adverse effects on food quality are exacerbated by traditional heat methods, such as the loss of essential food components or color degradation (Van Boekel et al., Citation2010). The meat industry recently investigated many innovative non-thermal antimicrobial techniques such as irradiation, pulsed electric fields, high hydrostatic pressure, pulsed ultraviolet, ultrasonication, and ozone, in order to ensure microbiological stability and safety of meats product. One of these is cold plasma (CP). An ionized gas produced under atmospheric conditions or low pressure is known as cold plasma. The potential of CP to produce a variety of bioactive agents, such as ROS (reactive oxygen species) and RNS (reactive nitrogen species) (such as ozone, hydroxyl radicals, superoxide, and nitric oxide), ultraviolet (UV), and charged particles, is a key property. These bio-active agents are highly powerful against microorganisms, suggesting that CP could be used to treat fresh meats (Han et al., Citation2016; Jayasena et al., Citation2015). CP technology has increased in popularity in the food manufacturing plants, notably in the meat and poultry industries, due to its successful microbicidal effectiveness and low operating temperature (60 °C) (Kim et al., Citation2013a). In recent years, many investigations have revealed that CP technology can be a valuable technique for the safety of different types of meat and meat products such as fresh meats (Bae et al., Citation2015), pork (Huang et al., Citation2019; Frohling et al., Citation2012), chicken (Dirks et al., Citation2012), raw poultry meat (Chaplot et al., Citation2019), beef (Kim et al., Citation2014) beef jerky (Yoo et al. (Citation2021), and processed meat products (Lee et al., Citation2018). For instance, some investigators reported that CP treatment significantly inactivated a wide number of microorganisms in different meats and its products, notably bacteria, fungus, and viruses (Choi et al., Citation2020; Gök et al., Citation2019; Kim et al., Citation2013b; Ulbin-Figlewicz et al., Citation2015). According to Lee et al. (Citation2016), CP treatment reduced the number of L. monocytogenes, Salmonella typhimurium, E. coli, and total aerobic bacteria, in chicken meat by 2.14, 2.71, 2.73, 3.36 Log10 CFU/g, respectively. Nevertheless, Huang et al. (Citation2019) found that CP treatment increased negative effects on meat colour in high oxygen MAP, resulting in a reduction in the a* value or redness of pork meat. Similarly, Jayasena et al. (Citation2015) found that extending the CP exposure period reduced the a* value in beef and pork when flexible thin layer CP treatment was applied. For meats processing and preservation with great quality, the cold plasma is being used (Misra & Jo, Citation2017; Lee et al., Citation2016; Jayasena et al., Citation2015; Huang et al., Citation2019) to bypass long-time processing, costly, and conventional techniques such as irradiation, high pressure, freezing, etc. These techniques have significant limitations, high costs of processing, higher setup cost, and rigorous operational considerations. CP is a progressive nonthermal technique used in the meat process industry for many advantages such as fast treatment, simple device operation, requires minimum power, operating at room temperature, maintaining the texture, flavor, and functional aspects of meat and meat products without water used (Misra & Jo, Citation2017; Lee et al., Citation2016). CP technology is expected to be widely used in the meat industry in the coming years for the cold treatment of meat and meat products (Misra & Jo, Citation2017). The many aspects of CP technology are discussed in this paper. This article reviews several CP technology aspects, including plasma formation, types of plasma devices, microbial inactivation mechanisms, effect on meat quality, and future prospects, in order to provide a better knowledge of novel CP technology.
2. Fundamentals of cold plasma technology
2.1 Plasma and cold plasma
Plasma is the fourth stage of matter, in which increases in the material's energy levels transform the material's phase from solid to liquid to gas, and finally to an ionized condition of the gas, ‘plasma,’ which has its own attributes. Cold plasma is comprised of a variety of excited atomic, molecular, radical species, and ionic, as well as a number of reactive species namely electrons, negative and positive ions, gas atoms, free-radicals, and molecules in an excited state, and electromagnetic radiation quanta as visible light and UV photons. However, when observing oscillations in ionized gas, Langmuir (Citation1928) invented the term “plasma,” describing it as the “region holding balanced charges of ions and electrons.” Plasma can be classed as low atmospheric and high-pressure plasmas. Additionally, plasma can also be classified as thermal and non-thermal, depending on the generation parameters. Besides this, non-thermal plasma could be generated at atmospheric pressure, referred to as atmospheric cold Plasma (ACP), or at low pressure, referred to as low-pressure cold plasma (LCP), and both plasmas produce similar reactive species and electron densities, resulting in similar microorganisms’ inhibition mechanisms (Bourke et al., Citation2017; Zhang et al., Citation2013). Thermal plasma is produced when heating gas at extremely high temperatures, that could reach thousands and thousands of Kelvins, at which chemical species, electrons, and ions are all in thermodynamic equilibrium. (Moreau et al., Citation2008; Banu et al., Citation2012; Scholtz et al., Citation2015). However, Non-equilibrium is a feature of Cold plasma, in which cooling of uncharged molecules and ions is significantly highly successful than the transfer of energy from electrons, leading to the gas remaining at a low temperature (Jayasena et al., Citation2015; Scholtz et al., Citation2015). Overall, “partially or entirely ionized gas holding myriads of reactive species like electrons, negatively charged ions, positively charged ions, free - radical, energized or non-excited atoms, and photons around at ambient temperature” can be described and at about rather than heating the whole gas mixture, almost all of the applied energy is cantered on electrons during the cold plasma formation. so, gas molecules generally maintain a temperature of approximately ambient. Thus, the Cold plasma technology is known as non-thermal technology.” Cold plasma’s effectiveness for use in meat processing areas where high temperatures are undesirable has been confirmed (Kim et al., Citation2013a; Wang et al., Citation2016; Roh et al., Citation2019). Furthermore, Cold plasma treatments are environmentally beneficial because use less water, leave no chemical residues, and use ambient air as a working gas (Misra & Jo, Citation2017).
2.2 Cold plasma formation
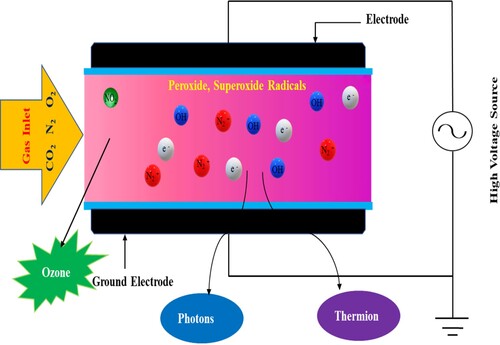
Electrical energy is delivered to carrier gases such as oxygen, air, nitrogen, or helium with the use of electrodes in the CP technique. A carrier gas, a sufficient power capacity source, and specific electrodes are all required for plasma formation. When an electric current is applied between electrodes (cathode and anode) spaced about 1 cm in the air at the voltage equal to roughly 30 kV/cm air breakdown threshold (Misra et al., Citation2016), atmospheric air is electrically broken and ignited, resulting in the formation of plasma (Misra et al., Citation2016). At higher powers, it usually begins as a narrow discharge seen as a spark and then transforms into a high-current hot flame. For air, atmospheric pressure, or vacuum, is maintained. At low pressure, this process takes less power, whereas, at atmospheric pressure, it requires more. Due to its minimal price and easy operation, AP plasma is the emphasis of most system development efforts in recent times. Thermodynamically, when molecules gain energy, the state of material transforms from solid to liquid to gas. Ionization of gas molecules occurs when energy is increased over a threshold value in a gas phase, resulting in a unique plasma state (Misra et al., Citation2016). Thermal, electric current, electromagnetic fields, nuclear, X-rays, radiofrequency, and other forms of energy are all possible (Wang et al., Citation2018; Stoica et al., Citation2014). These energies cause the gas molecules to split into an electron, spark ions, charged/neutral gas molecules, free radicals, and other species that are biologically active and also have the power to kill microbes. Chemical reactions are extremely complicated in plasma, including numerous species with lifetimes ranging from nanoseconds to hours (Gaens & Bogaerts, Citation2013) for a model of kinetic with 1880 processes and 84 species). The input gas to plasma has a variety of mono- to multi-atomic molecules, which adds to the complexity. Water droplets in the gas, or those supplied from food products put in the discharge, provide yet another layer of intricacy. With these facts in mind, it's clear that the chemistry of wet air plasma is by far the most complicated. Reactive oxygen (such as ozone O3, atomic oxygen O, excited oxygen O2, singlet oxygen O2, and superoxide anion O2 -) and nitrogen (such as atomic nitrogen N, excited nitrogen N2, and nitric oxide NO●) species are the most common reactive species generated. Nitric oxide (NO●), nitrogen dioxide radical (●NO2), peroxynitrite (ONOO-), peroxynitrous acid (OONOH), and alkylperoxynitrite are all examples of RNS found in humid air plasma (ROONO) (Arjunan et al., Citation2015). shows Cold Plasma formation in DBD. Different design and operating parameters generate distinct forms of reactive species and are thus associated with different levels of decontamination (Georgescu, Citation2015; Aboubakr et al., Citation2015). Many parameters influence the reactive species and its abundance in plasma, including the gas in which plasma is generated, the plasma source's setup, power input to the gas, duration of treatment, and humidity range (Misra & Jo, Citation2017).
2.3 Types of plasma devices
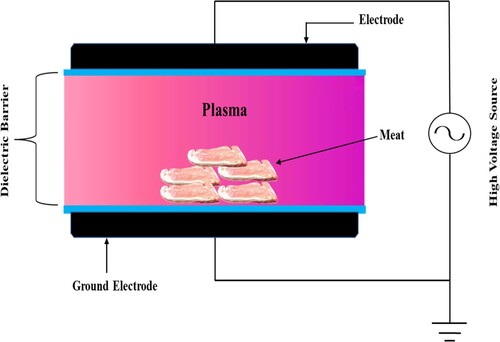
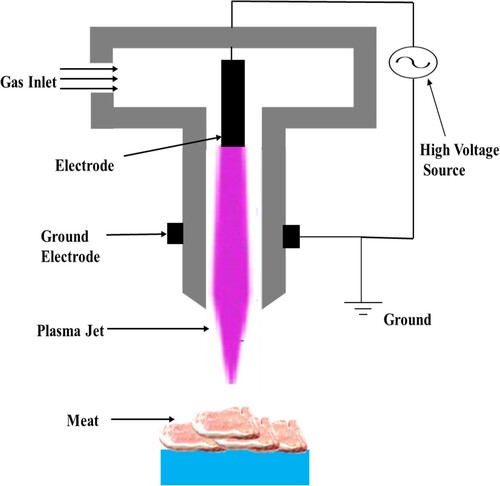
The much more popular method of generating cold plasma is to apply a powerful electromagnetic field to neutral gas and induce ionization. A number of electrical discharges can be used to generate cold plasma. Corona discharge, dielectric barrier discharge (DBD), gliding arc discharge, resistive barrier discharge system, plasma needle, and AP plasma jet are examples of these types of discharges. The chemical species generated, composition, and frequency of the chemical species generated are all affected by the type of plasma source used.
The two most common types of CP devices used in meat processing industries are the DBD and AP plasma jet. This is due to its uncomplicated design and flexibility to be altered to meet a variety of treatment needs. Plasma sources with a barrier discharge arrangement are commonly operated with an alternating current supply from the line (50-60 Hz) to radio frequencies. Barrier discharges are made up of two electrodes separated by a dielectric medium and operating at different voltages. The dielectric barrier between the electrodes restricts the flow of current, preventing an electric discharge and allowing the gas to be ionized inside the inter-electrode space. A dielectric barrier discharge can be generated depending on the electrode and barrier arrangements (). It's interesting to note that the topologies of a DBD make it a volumetric discharge, it has its own distinctive features. However, AP plasma beam electrodes are capacitance with a number of coupled devices, comprising two coaxial electrodes with a high-speed gas flow flowing through the center. shows a schematic illustration of the AP plasma jet device. The central electrode is activated by a 13.56 MHz RF power source, whereas the electrode at the outer side is grounded. Plasma is formed when free electrons accumulate across RF fields and collide with gas molecules in the surroundings. Such inelastic collisions create a lot of reactive species (excited atoms and molecules, as well as free radicals) at a faster flow rate through the device's nozzle, resulting in much more reactive species that could interact with the material surface within a few centimeters (Rossow et al., Citation2018; Lee et al., Citation2011).
2.4 Mechanism of microbial inactivation
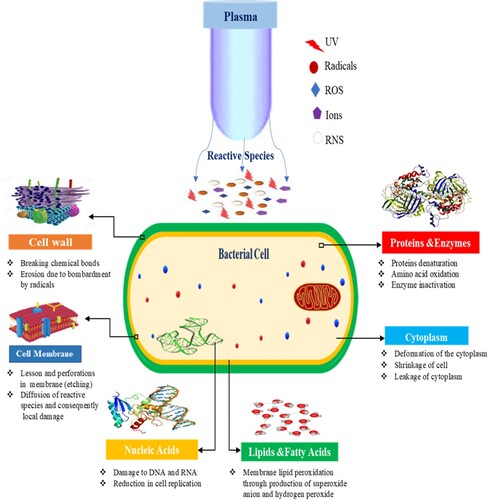
The lysis of microorganisms happens during plasma processing because the microorganism is subjected to a high-intensity radical bombarding on the cell surface. The bombarding of radicals causes damage on the cell surface that the microorganisms are unable to heal fast, resulting in the rapid destruction of the live cell. “Etching” is the name for this process (Pelletier, Citation1992). The formation of lesions is caused by the buildup of electrostatic forces on the live cell's outer surface. Non-thermal plasma efficiency is evaluated by two factors: substrate type and microbe parameters such as load, type, and physiological condition (Stratakos & Koidis, Citation2015). The action of reactive species and charged particles in non-thermal plasma destroys cell membranes and produces denaturation of DNA and chemical bonds, which leads the cell to become inviable. Although the mechanism behind the interaction between plasma species and microorganisms is still unknown, some processes such as oxidation and peroxidation occur both outside and within the cell and are mostly catalyzed by plasma ions (Dobrynin et al., Citation2009). The details of cold plasma action on the microorganism are schematically shown in . Furthermore, numerous attempts have been made to understand the mechanism of the inactivation of microorganisms in CP, but the detailed mechanism is unknown so far. The majority of studies on microbial destruction mechanisms in non-meat applications are available. CP has been proven to decontaminate successfully with low influence on the quality characteristics of meat and meat products. (Lee et al., Citation2018; Huang et al., Citation2019;Yoo et al., Citation2021; Yadav et al., Citation2020; Aboubakr et al., Citation2020; Gök et al., Citation2019). An increasing quantity of data on the antibacterial efficacy of CP has been published, as developed and used by numerous researchers in various applications of the technology. The cell membranes chemically interact with reactive molecules (O2, O3, NO, etc.) or radicals (O, OH, etc.), excited or charged particles are the most investigated of the several suggested mechanisms (electrons, and atomic or molecular ions). Microbial inactivation is aided by reactive species such as O3, atomic oxygen, superoxide, peroxides, and hydroxyl radicals, which are generated when air is broken down (Deng, Shi, Shama, & Kong, Citation2005), Whereas, nitrogen dioxide NO2 and NO, play a significant role in the inactivation of microorganisms by destroying chemical components such as proteins, lipids, and nucleic acids (Mai-Prochnow et al., Citation2014; Stoica et al., Citation2014).
3 Effect on meat and its products
3.1 Effect on colour
Color is a significant marker of freshness and pleasantness in meats. It is also one main important trait factors that consumers consider when purchasing meats (Mancini & Hunt, Citation2005; Luño et al., Citation2000; Kennedy et al., Citation2004). Color improvement of meats has been achieved using the cold plasma technique. The interaction of plasma and liquid causes the liquid to become acidic, as well as the nitrogen species and reactive oxygen species (ROS) formations, such as nitrate (NO3) and nitrite (NO2) (Oehmigen et al., Citation2010). For cured meat and products, it can utilize water that contains nitrite produced by DBD plasma treatment as a provider of nitrite. Nitrite was utilized as a curing ingredient to give cured meat its distinctive pinkish color and to avoid the growth of pathogens like Clostridium botulinum. According to Jung et al. (Citation2015a), the color of sausages prepared using treated water by plasma was comparable to sausages cured with traditional sodium nitrite. a similar method was developed to create nitrite supplies in a chamber, which led to the pink color distinctive of cured meats, employing continuous gas-phase plasma treatment rather than water throughout manufacturing phases including mixing, chopping, and cutting (Lim et al., Citation2015). However, Zhuang et al. (Citation2019b) reported only yellowness (b*) was substantially impacted by storage time among the 3 color assessments. There were almost no significant differences in the redness (a*) and yellowness (b*) of a chicken cutlet HVDBD-treated samples between treatment voltages parameter when compared to pre-packaging with post-treatment values. in other directions, the voltages at 85,70, and 55 kV, led to considerable increments in lightness (L*). Increasing voltage to at least 70 kV considerably enhanced L when compared to controls (0 s + 5 d storage). HVDBD treatment did not affect redness (a*) and yellowness (b*) assessments. These findings suggest that HVDBD treatment in-package may alter the appearance of skinless breast meat by turning the raw chicken meat look paler. Wang et al. (Citation2016) found that after 3 days of storage, L* values in HVDBD-treated breast meat packaged in the air were higher than pre-package values. Wang et al. (Citation2018) evaluated color parameters of ACP-treated raw chicken fillets such as a*, L*and b* at different lengths of time. Irrespective of treatment time, were there no variations in L* values between samples tested before treatments and those evaluated after treatments and storage after 3-day. The a* values of chicken fillets treated with ACP for 6 and 9 mins were considerably lower than those evaluated before packing; however, were there no variations between the control and the 3-min treatment. Chicken fillets treated with ACP (80 kV for 3, 6, or 9 min) and kept for 3 days had lower b* values than those before packing. The no difference in a* b* L*values of control samples before and after storage were found. These findings showed that after 3-day of storage, the treatment time of ACP does not affect L* values of packed raw chicken fillets. ACP treatment may have a considerable impact on the a* and b* values, or the yellowness and redness in raw chicken breast fillets. Furthermore, recently, it has been reported by Moutiq et al. (Citation2020) that over the 24-day storage time, the difference in values of green-red (a*) and blue–yellow (b*) indicators between the control and chicken breast (CB) treated by plasma groups was determined to be non-significant. On day 15, however, the b* value was determined to be considerably different. This is most likely caused by natural variation in the color of those individual chicken breast samples, as the b* values for the following days were determined to be insignificant. The lightness variable was shown to be considerably different for CB control and CB treated samples after 9th-day storage, unlike the b*and a* results. The difference was determined to be insignificant on the 24th day. However, Jung et al. (Citation2017) reported that after cooking of meat batter samples, these samples were treated with AP plasma which produced a cured pink color in all treated samples, whereas the control samples without AP plasma treatment developed a brown color. Treatment of plasma for cooked meat batter (CMB) samples had no impact on L* values, suggesting that nitrite had no impact on L* values of CMB samples. With extending the time of plasma treatment, a* values of the CMB sample increased greatly. The rise in nitrite values with extending time of plasma treatment was attributable to this finding. The b* values of the CMB sample significantly decreased as the time of plasma treatment extended. The formation of cured color in CMB samples was proportionate to this finding. In addition, Yadav et al. (Citation2019) reported that the plasma treatment effect on color parameters of one percent NaCl treated RTE ham (without or with rosemary). The study demonstrated that the b* (yellow–blue) and L* (brightness) values in the RTE ham (without or with rosemary) did not differ considerably from the untreated control RTE ham, after plasma treatment. However, RTE ham (without or with rosemary) had considerably lower a* values (red-green) compared to untreated RTE ham. Ulbin-Figlewicz et al., Citation2015 reported that after pork meat exposure to helium, nitrogen, and argon gas vacuum CP for 5 and 10 min, there were no considerable differences in parameters of color a*, b*, and L* values of pork meat. However, for pork jerky, L*, a*, and chroma values progressively increased with increasing time of APP plasma treatment, but b* value reduced. In other ways, the extended time of APP treatment resulted in the brighter color of jerky and more strongly red, but much less yellow. Among the procedures, jerky prepared using APP for 60 min had the greatest a* value, whereas jerky prepared using APP for 40 min had a* value equivalent to (i.e. insignificance difference from) jerky prepared using sodium nitrite (Yong et al., Citation2019). In addition, Wang et al. (Citation2021) reported from 0 days to 8 days, a change in color of DBD-CP treated beef patties at voltage levels and frequencies. On day 0, L* values for beef patties treated at 60 kV as well as at 70 kV, were considerably lower compared to the control sample, but those in 50 kV samples had not been found different significantly. The voltage used was determined to be proportionate to the decrease in L* values. The L* values of treated samples were relatively constant throughout storage. It could appear to suggest that the DBD-CP procedure had a stronger influence on the L* value than the storage treatment, particularly at higher voltages (70 kV). However, throughout storage, for treated beef patties, the b* values were found to be closely associated with those of control samples, with no significant difference between groups (P > 0.05). Additionally, when pork meat was treated with AP plasma, Moon et al. (Citation2009) found no noticeable variations in b* values. According to Bae et al. (Citation2015), the b* values of chicken breasts, beef loins, and pork meat were found to be not significantly different and it’s affected by AP plasma jet. However, Wang et al. (Citation2021) also reported that beef patties treated using DBD-CP at voltages of 50, 60, and 70 kV, the a* values were reduced significantly up to approximately 2.43, 4.56, and 4.43, respectively, compared to control samples, whereas, it was found that no difference significantly between that of samples treated at 60 kV and 70 kV. From 0 d to 08 days, the a* value of both samples’ groups without and with the treatment of DBD-CP, demonstrated a progressive declining trend. After treatment of DBD-CP, beef patties treated at 50, 60, and 70 kV, the a* values were reduced up to 4.86, 5.74, and 6.80, respectively, at the end storage, compared to the control samples. These findings suggested that treatment of a high voltage DBDCP might have a negative influence on the redness of raw meat. Moreover, the results indicated that control and treated samples had significantly lower a* values, although, during storage, there were no considerable differences in a* values between groups treated under different frequencies. Albertos et al. (Citation2019) found that herring fillets samples treated using DBD plasma under 70 kV, had considerably lower a* values than that of control samples. Moreover, the changes in meat lightness were seldom linked with the appearance of raw beef after storage, Over the storage time, there was no considerable variation in L* values between various frequencies of CP treatment (Wang et al., Citation2021; Jeong et al., Citation2009). Jayasena et al. (Citation2015) reported the values of L* for pork and beef were not significantly changed by CP treatment. During storage, all treated beef patties samples had the same L* value as that of the Control samples. However, Qian et al. (Citation2022) reported that in the beef patties after different CP treatments, there was no considerable variation in the L* values (p > 0.05). The a* value of treated beef patties samples was reduced significantly by 0.15–1.00 after successful treatment, compared to the control samples, but this variation was not statistically significant (p > 0.05). The a* value of all-beef patties samples reduced significantly on 2nd-day storage, and the films used were more helpful in retaining the redness in meat throughout the storage than the Control and Control-DBD samples. Similarly, according to Huang et al. (Citation2019), treatment of plasma technology did not affect the a* value of pork. Qian et al. (Citation2022) reported that there was no considerable variation in the b* value of samples after different treatments. Oxymyoglobin was quickly oxidized to metmyoglobin when beef patties samples were reacted with air or plasma directly (without being covered in films), resulting in a reduction in the redness in beef patties samples. In contrast to the mentioned color attributes, variations in the b* value of meat patties were more consistent throughout storage. However, Bae et al., Citation2015 claimed that plasma treatment for fresh meat at 28.5 kHz, 3.5 kV, N2 used as the working gas, for 5 min, did not influence the b* value. Fröhling et al. (Citation2012) observed that longer effectively indirect treatment of plasma resulted in a lighter color of meat samples. In respect to control untreated samples, there was a rise in a* values and a reduction values of b* for pork meat after treatment. Jayasena et al. (Citation2015) used a flexible thin-layer DBD plasma setup to treat both beef loin and pork butt and found that the b*-value for pork butt samples did not alter considerably. Moreover, the b*-value of pork butt samples treated by DBD plasma did not differ substantially from that of control untreated samples; nevertheless, the b*-value for beef loin samples subjected to 10 min under treatment of DBD plasma differed considerably from that of control untreated samples. however, Increased exposure length to the same treatment, reduced the a*-values (redness) of beef loin and pork butt samples.
3.2 Effect on pH
The pH is considered a crucial measure to assess the quality of meat and meat products following cold plasma treatment because it is a reliable indicator of meat quality. The pH value of meat was determined, which may also be used to determine the freshness of meat. (Qian et al., Citation2021). Qian et al. (Citation2022) reported that on Day 0, the values of pH for the beef patties samples following various plasma treatments varied from minimum (6.16) to maximum (6.32). Only the pH of the Control-DBD samples was substantially lower than the Control samples among these samples. the pH value of meat was reasonably constant over the first 4 storage period. After the 4-day storage, the pH value in all samples demonstrated a significantly lowered pattern, that may be attributed to the impact of the microbiota in the beef patties, proteins were gradually decomposed by microorganisms into organic acids, resulting in a slight reduction of pH value. According to Jung et al., Citation2017, the pH in meat batter was not significantly altered until 20 min of plasma treatment. But in another case, the pH value of the meat batter (treated with plasma for 25 and 30 min) was considerably lower than that of the control samples. The plasma treatment reduced the value of pH (from 6 00–5. 92) in the meat batter and the reduction in pH was small when compared to the decrease in deionized water. This outcome may be due to the meat batter's inherent buffering capacity. Jung et al. (Citation2015) reported that after 2 h of treatment with AC plasma, the pH of deionized water was reduced from 7 to 2–3. The formation of acids such as nitric and nitrous acids during plasma-liquid interaction leads to a fall in pH in plasma-treated liquid (Oehmigen et al., Citation2010). However, Moutiq et al. (Citation2020) observed that the pH value for the control chicken meat sample was found to differ from 5.6 and 6.1 values. All plasma exposure times led to a reduction of the pH value for chicken samples. Nevertheless, the pH decline between 1 and 3 mins of CP treatment was statistically significant but not between 3 and 5 mins of treatment. This is most probably due to plasma chemistry since much of the NxOy forms within the first 1–3 min of treatment before being dominated by the formation of or transformation to other reactive species. Moreover, several investigations have found a reduction in pH values for chicken and other muscle foods during the treatment of plasma (Kim et al., Citation2013a; Misra et al., Citation2015; Rothrock et al., Citation2017). Nevertheless, in some investigations, it was reported that no change in pH in meat samples under CP treatment including bacon (Kim et al., Citation2011) and beef loin (Bauer et al., Citation2017). These changes are most probably connected to plasma chemistry, skin moisture content, gas humidity, and muscle type buffering capabilities. Ulbin-Figlewicz et al., Citation2015 observed that in all plasma treatments using inert gases, the pH values of pork were not considerably affected. Similar observations were obtained on the surface of beef longissimus (Bauer et al., Citation2017) and dry-cured beef (Gök et al., Citation2019) was treated using oxygen or argon plasma. Additionally, Kim et al. (Citation2011) found no significant variations of the pH in bacon using plasma treatment under atmospheric pressure with helium gas as the product gas. Nevertheless, Kim, Yong, Park, Kim, et al. (Citation2013a) and Kim, Lee, et al. (Citation2013c) discovered that after pork loin was treated with DBD plasma, the pH was considerably reduced to around 5.3 and it was comparable to the pH of cured meat (ranging from 4.6–5.3). Furthermore, under indirect air plasma treatment, the pH of the pork loin muscle was significantly lowered (Fröhling et al. Citation2012). Similar outcomes were observed by Wang et al. (Citation2018) in chicken meat. The pH value of chicken fillets was unaffected by different plasma treatment voltages and treatment times. These two CP treatment variables had no impact on the pH of surface chicken fillets under refrigerated storage for 3 days. However, after treatment, the pH in chicken breast patties was considerably reduced than control. However, adding rosemary considerably raised the pH value of cold plasma-treated patties after 5 days storage period (Gao et al., Citation2019).
3.3 Effect on lipids
Reactive oxygen species such as hydrogen peroxide, hydroxyl radicals, and superoxide anions have been found in plasma and play a very effective role in the decontamination of microbial numbers (Afshari & Hosseini, Citation2014; Oehmigen et al., Citation2010; Attri et al., Citation2015). Nevertheless, by removing hydrogen ions from lipid molecules, reactive species (especially free radicals) can start lipid oxidation (Min & Ahn, Citation2005; Shahidi & Zhong, Citation2010). The TBARS analysis revealed that the difference in average MDA value, between chicken breast plasma treated and control samples, was found non-significant (p>0.05) at all treatment times (Moutiq et al., Citation2020). Oxidation of lipid by the influence of cold plasma induced in meat and meat products. The chicken meat breast is more stable against oxidation by plasma as compared to red meat. The low fat in lean chicken breast compared to pork or beef meat accounts for these variations (Gavahian et al., Citation2018; Lee et al., Citation2016). It has been reported by Jung et al. (Citation2017) that the cold plasma had almost no influence on the MDA value of meat batters. This finding is comparable to Kim et al. (Citation2011), who observed that treatment of cold plasma had almost no impact on the oxidation of lipid in bacon. Nevertheless, according to Jayasena et al. (Citation2015), both beef loin and pork butt samples were treated with cold plasma, resulting in increased lipid oxidation with treatment time increased. This difference might be due to the adoption of a distinct plasma treatment technology and the short plasma lifetime of reactive oxygen species (Attri et al., Citation2015). Applying the cold plasma on food surfaces may cause enhanced lipid oxidation, resulting in poor organoleptic qualities and decreased storage stability (Dirks et al., Citation2012). Malondialdehyde is a polyunsaturated fatty acid oxidation product, this product is used as a lipid oxidation indicator (Moutiq et al., Citation2020). However, the RONS produced by CAP can cause the oxidation of lipid on the surface of meats including pork, beef, poultry, and fish (Gavahian et al., Citation2018). Although plasma-treated samples showed a slightly greater lipid oxidation level than untreated RTE chicken, this difference was nonsignificant statistically (Georgescu, Citation2021). Additionally, the presence of a significant number of reactive species in plasma speeds up the oxidation of meat lipids (Kim et al. (Citation2013a). Meanwhile, treated beef patties (EO-MP film coating) did not significantly decrease the lipid oxidation than the control samples. Excluding the control beef patties samples, this difference statistically was not significant among the other treatments when the storage period was prolonged to three days. This finding suggested that, when wrapped in the meat by protein films, the lipid oxidation induced by plasma could significantly be decreased (Qian et al., Citation2022). Recently studied by Huang et al. (Citation2019), who reported that, when the concentration of oxygen increased, the TBARS values in DBD treated samples, rise somewhat, then remained unchanged until day 4 storage. For lipid oxidation, DBD and oxygen acted as synergists. The oxidation of lipid in pork meat treated using DBD plasma was accelerated during storage and greater than untreated controls after 4 days.
In several investigations, plasma was shown to speed up the lipid oxidation in meat. (Bae et al., Citation2015; Jayasena et al., Citation2015; Rød et al., Citation2012). However, Choi et al. (Citation2016) reported that when frozen pork was exposed to corona discharge plasma (CDP), the peroxides value (POV) increased significantly. These by-products were produced from lipid oxidation. Yong et al. (Citation2017) also reported that the POV in beef jerky was increased significantly under plasma treatment (flexible thin-layer) for 10 min. In addition, a considerable rise in TBARS was reported when fresh pork loin was subjected to APP containing oxygen and helium gases (Kim et al., Citation2013a). However, as the AP plasma treatment time increased, the TBARS levels decreased. The TBARS in jerky-containing sodium nitrite and treated with APP did not differ significantly. Whereas pork jerky treated with AP plasma, had the lowest TBARS value at maximum treatment time (Yong et al., Citation2019). Ripoll et al. (Citation2011) indicated that the TBARS threshold value (more than 2.5 mg/kg) which tends to rancidity flavor. TBARS value was increased and reached up to 3.97, 3.01, 1.91 mg MDA/kg at 70, 60, and 50 kV, respectively, on day 8 in treated samples under DBD with increased storage. Moreover, in beef patties, increasing the treatment frequency had little significant influence on the TBARS levels. Radicals produced during DBD treatments can promote peroxides formation and lipid oxidation, leading to a rise in TBARS in treated samples by DBD plasma (Kim et al., Citation2013b; Wang et al., Citation2021). However, Wang et al. (Citation2021) reported a significant difference in MDA values between plasma treated and control samples. This finding revealed that increased treatment voltage caused more significant lipid oxidation in the meat. Likewise, because of the increased treatment power, the TBARS levels in RTE meat treated by plasma were higher (Rod and others, 2012). Furthermore, Jayasena et al. (Citation2015) observed that both beef loin and pork butt samples treated using DBD Plasma at 40 kV, had higher TBARS levels.
An overview of many investigations on the effect of cold plasma on lipid oxidation in meat and its products are presented in .
Table 1. A overview of investigations on the effect of cold plasma on lipid oxidation in meat and its products
3.4 Effect on microbial quality
Cold plasma could be used as a suitable way to inhibit the microbe's growth to increase the meat and meat product's shelf-life. The microorganisms such as L. monocytogenes, C. jejuni, Salmonella sp., and E. coli contaminate the meat and can cause serious gastrointestinal diseases in consumers. To fulfill the increasing market for safe and qualitative meat products, the meat process industry is being developed and adopting technological advancements (Mor-Mur & Yuste, Citation2010). Kim et al. (Citation2013b) used an AP plasma technique and it operating at radiofrequency, utilizing argon gas to inactivate C. jejuni in chicken ham, they had seen a reduction in the growth of bacteria of up to 1.5 log CFU/cm2, and 3 logs CFU/cm2 after treatment for 10 and 6 min, respectively. Ulbin-Figlewicz et al., Citation2015 evaluated the CP influence on microbial decontamination on meat surfaces using three gases argon, helium, and nitrogen. The psychrotrophic proportion and total bacteria decreased by 2 and 3 logs CFU/cm2 after 10 min time of plasma treatment using argon and helium, respectively. Although there was no interaction with nitrogen treatment in these bacterial groups, after time 10 min, declined the yeasts and molds up to 1 log CFU/cm2. Noriega et al. (Citation2011) found that using the CP technology for sterilizing chicken meat and skin infected with Listeria innocua at various time intervals resulted in 3 log decreases. They also suggested that the CP technology be combined with commercial food applications and utilized for L. innocua sterilization. In addition, using air plasma DBD setup by Jayasena et al. (Citation2015) and they successfully decontaminated L. monocytogenes (2.04 log10 CFU/g), E. coli (2.54log10 CFU/g), and S. Typhimurium (2.68 log10 CFU/g) on the pork butt, and L. monocytogenes up to 1.90 log10 CFU/g, E. coli up to 2.57log10 CFU/g, and S. Typhimurium up to 2.58 log10 CFU/g, on the beef loin. Undertreatment time of 10 min with a flexible DBD plasma, Lee et al. (Citation2016) found a significant decline in the L. monocytogenes, total aerobic bacteria, S. Typhimurium, E. coli, up to 2.14, 3.36, 2.73, and 2.71 log10 CFU/g, respectively, on chicken breast. Using the same adaptable plasma source as before, Yong et al. (Citation2017) observed that, a decrease in the population of microorganisms namely E. coli, A. flavus, L. monocytogenes, and S. Typhimurium by 2.7,3.2, 2.4, and 3.0 log10, respectively, in AP plasma beef jerky treated sample for 10 min. In addition, Lee et al. (Citation2011) explored the jet plasma treatment time effect (2 min) on L. monocytogenes-contaminated cooked chicken breast. They applied the carrier gases such as N2, He, O2+ He, and N2+ He and found that all these plasma treatments reduced bacteria number up to 1.37 - 4.73 log CFU/g, depending on the type of carrier gas. The results showed that the most successful carrier gas against the microorganism was a combination of oxygen and nitrogen. Furthermore, using APJ treatment, the decrease in S. Typhimurium count on chicken breast, up to 4.41, 5.66-5.14log CFU/g after 10 and 5 min, respectively (Kim, et al., Citation2013a). According to Wang and others (2016), who used a MA for plasma production in a volumetric DBD system, the self-life of up to 14 days of chicken meat could be prolonged. When compared to the air control, significantly decreased the background microflora on chicken breast up to 4 logs. Moreover, Dirks and others (2012) found that CP treatment reduced the bacterial population on fresh poultry muscle and skins, including background microflora and pathogens. These results showed that cold plasma treatment-based DBD (in-package) may be utilized to decrease background microflora and increase in self-life stability of fresh chicken meat. However, Gao et al. (Citation2019), reported the CP treatment influenced microorganism numbers. Without CP treatment, microorganism numbers increased by more than 1.70 log cycles, but only by 1.14 log cycles after CP treatment. Furthermore, the storage period impacted the influence of CP treatment on the microbiota. In 0day patties, there was also no variation in microbiota between the CP and non-CP treated group. However, after storage for 5 days, the microbiota in the CP treated group was 0.78 log less than in the non-CP treated group. The inclusion of rosemary extract reduced total plate counts in chicken patties considerably in both CP and non-CP treated samples, independent of storage. However, Ulbin-Figlewicz et al., Citation2015 discovered that helium plasma and argon treatment reduced the proportion of meat microbiota. The logarithmic declines were influenced by the length of exposure and the type of gas used. Helium plasma treatment had the maximum inactivation efficiency. After 10 min of exposure to psychrotrophic microorganisms, yeasts and molds, and TPC from pork muscle, significantly reduced by approximately 1.60, 1.90, and 1.14 log cycles, respectively. The used argon plasma treatment resulted in a smaller decrease of pork microbiota about 0.41log, 0.77, and 1.20 log under the same treatment time. After 5 and 10 min, the TPC in beef was decreased by approximately 1.00 and 2.10 log, respectively. However, Bauer et al. (Citation2017) reported that the four different strains were significantly reduced when inoculated PCA plates were exposed to ACP, with low power producing a greater decrease than high power. However, no considerable decrease in microbial populations was found when contaminated PCA plates were enclosed with a 90 m polyamide-polyethylene barrier before treatment, demonstrating that ACP proved incapable of permeating the packing barrier. When bacteria suspended in 0.85% saltwater were put on packing material and subjected to AC plasma before drying, a significant decline in microorganisms was found. The above case resulted in a >1.5 log decrease at ‘high’ power and a >2 log decrease at low power, simulating ‘fresh’ contamination that may occur during packaging. Low power again outperformed high power in terms of bacterial elimination. In a recent study, Roh et al. (Citation2020) reported that RTE chicken cubes treated with DBD CP (in package) at a 39 kV, for the time of 3.5 min., resulted in 3.9 log CFU for E. coli and 3.7 log CFU for salmonella, respectively, in cube per RTE chicken. In addition, González-González et al. (Citation2021) reported that A piezoelectric direct discharge producer had been used to create cold non-equilibrium plasma utilizing ambient air in the current investigation. the impact of CA plasma on the surface of RTE chicken meat at distinct short exposure times (0–5 min) against E. coli and Salmonella. On RTE chicken breast, CAP's antibacterial action was time-dependent, with an equal decrease in both pathogens. After 1 min of treatment, decreases of about 1-log CFU/g were reported, with the greatest decreases of 1.8 log and 1.47 CFU/g for Salmonella and E. coli, respectively, after treatment of 5 min. At 2 min or longer treatments, there was a non-significant change in the population of microbial agents. Additionally, Timmermann et al. (Citation2020) observed that the ACP produced O3, NO, and NO2 as the temperature for generated plasma rises. However, Zhuang et al. (Citation2019a) reported that after five days of samples stored under refrigeration conditions, the microbial populations on CP-treated chicken breast meat were considerably lower than those on untreated fillets, independent of treatment time or bacterial species. In comparison to nontreated samples, cold plasma treatment for 1 min reduced psychrophiles, Salmonella, and Campylobacter by more than about 0.5, 0.4, and 0.7 logs, respectively. Increasing the CP-treatment period above 1 min did not affect the Campylobacter or Salmonella populations; however, prolonging the CP-treatment duration from 1 min to 3 min which decreased psychrophilic population numbers by 0.6 logs. Extending the timeframe of CP treatment had no impact on the number of psychrophilic organisms on the breast muscle. Zhuang et al. (Citation2019b) reported that irrespective of treatment voltage or microbial species, microbial populations on HVDBD treated chicken breast meat were substantially lower than those on untreated control fillets after 5 days of refrigerated storage. HVDBD treatment at 55 kV, resulted in about 1.1, 0.8, and 0.3-log decrease in Campylobacter jejuni, psychrophiles, and Salmonella Typhimurium, respectively, compared to the untreated sample. Increasing the HVDBD-treatment voltage above 55 kV did not lead to additional S. Typhimurium or psychrophilic numbers decreases. Nevertheless, extending the HVDBD treatment voltage from 55 to 85 kV led to a 0.5 log decrease in Campylobacter jejuni counts. Cold Plasma effects on the microorganisms of different meat and meat products are presented in .
Future Prospective
Table 2: Cold Plasma effect on microorganisms of different meat and its products
Cold plasma processing is a unique and promising technology that may be used to disinfect meats and its products. The retention of meat visual quality during storage has been improved by cold plasma. The use of cold plasma to increase the storability of meats and its products is interesting. Various intrinsically and extrinsically variables influence the efficiency of cold plasma processing which include surface characteristics, type of meats, type and characteristics of microbes, the potential of antimicrobial organisms to distribute and disperse on interfaces, their life period both during and after cp processing, process conditions, cost-effectiveness, etc. Since CP is dependent on the properties of the airflow and environmental conditions including temperature and humidity, a detailed understanding of the gaseous phase chemical reactions of air plasma is needed. Since Cold plasma is dependent on the properties of the air inflow and environmental conditions (temperature and humidity), a detailed understanding of the gaseous phase chemical reactions of air plasma is required. In general, treating meat products with cold plasma has the ability to improve their quality and extend their shelf life. Nevertheless, there is currently a limited investigation on the impacts of cold plasma on meat quality changes. Considering cold plasma's strong oxidative reactivity, its impact on fresh meat and processed meats should be investigated properly in order to reveal the nature of gas plasma's effect on meat quality. According to the overview of the research published, Cold plasma has the potential to be used in the meat industry, Cold plasma has demonstrated excellent anti-microbiology efficacy against food-borne microbes. The anti-microbiology performance of cold plasma is affected by many variables, including the type of plasma device, voltage, treatment exposure time, types of gas, and electric system. The important factor in enhancing anti-microbiology performance is controlling the variables of cold plasma. As consumer demand for meats and its products has increased, the demand for non-thermal processing technology has also increased. Cold plasma has gained extensive attention in the meat industry as a promising non-thermal processing technique. How to control process variables and other affecting variables of cold plasma in relation to different meats and its products is an important stage for its use, and it will need more coordinated efforts in the future from researchers, manufacturers, organizations, and consumers. The lab-scale study currently leads cold plasma investigation in the meat sector, while broad research in the industry is infrequently published. Despite a design concept of an industrial consistent processing facility in meat processing has indeed been revealed, a cold plasma device is still to be effectively developed and used in the food sector. Plasma has a range of affecting variables, making device development and evaluation more challenging. CP device improvement needs more scientific information assistance and takes a longer time to develop and manufacture. As a result, greater investigation is necessary on the cold plasma used in the meat sector, as well as the cooperation of different experts in the fields of meat, materials engineering, equipment, chemistry, and physics. CP technology provides a scientific opportunity to investigate the molecular influences of CP technology on the quality aspects of meat and meat products. To minimize adverse impacts on the quality of meats, such as color variation, rapid lipid oxidation, and pH changes, optimization of cold plasma processing parameters studies is recommended. For CP technology to obtain its full potential at the mass production target in the meat industry, comprehensive knowledge of the process and precise control over Meat quality characteristics would be needed. The bulk quantities of meat and meat products should be treated by cold plasma applications. As a result, research into the possibility of batch or continuous plasma devices for processing huge quantities of meat and its products is needed.
Conclusion
CP technology’s popularity has been increasing due to its special characteristics, such as treatment at a low or ambient temperature for a short time, which assists in the preservation of meat product quality. Cold plasma is an innovative non-thermal technique that has shown promise in the decontamination of meats and its products. The impact of CP on color, pH, oxidation of lipid, and microbial quality of meat products is dependent on the time exposure and energy. However, the majority of the investigators have mostly concentrated on the study of CP effect on meat quality in terms of microorganisms, and color with a limited focus on pH. CP treatment has shown to be successful at inactivating food-borne microorganisms from raw meat and its products. Additionally, since cold plasma contains a variety of reactive species with oxidizing abilities, most reported papers evaluate lipid oxidation in meats and meat products. The quality attributes of meat and its products are influenced by CP processing. Thus, cold plasma will be a boosting technology for meat processing in the near future.
Acknowledgment
We Wish to Express Our Sincere Gratitude to the Er. Teklay Abay (Dean), College of Engineering and Technology, Adigrat University, Adigrat, Tigray, Ethiopia for The Assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aboubakr, H. A., Nisar, M., Nayak, G., Nagaraja, K. V., Collins, J., Bruggeman, P. J., & Goyal, S. M. (2020). Bactericidal efficacy of a two-dimensional array of integrated, coaxial, micro hollow, dielectric barrier discharge plasma against Salmonella enterica serovar Heidelberg. Foodborne Pathogens and Disease, 17(3), 157–165. doi:10.1089/fpd.2019.2698
- Aboubakr, H. A., Williams, P., Gangal, U., Youssef, M. M., El-Sohaimy, S. A. A., Bruggeman, P. J., & Goyal, S. M. (2015). Virucidal effect of cold atmospheric gaseou plasma on feline calicivirus, a surrogate for human norovirus. Applied and Environmental Microbiology, 81(11), 3612–3622. doi:10.1128/AEM.00054-15
- Afshari, R., & Hosseini, H. (2014). Non-thermal plasma as a new food preservation method, its present and prospect. Archives of Advances in Biosciences, 5(1). doi:10.22037/jps.v5i1.5348
- Albertos, I., Martin-Diana, A. B., Cullen, P. J., Tiwari, B. K., Ojha, K. S., Bourke, P., & Rico, D. (2019). Shelf-life extension of herring (clupea harengus) using in-package atmospheric plasma technology. Innovative Food Science & Emerging Technologies, 53, 85–91. doi:10.1016/j.ifset.2017.09.010
- Alp, E., & Aksu, M. I. (2010). Effects of water extract of urtica dioica L. And modified atmosphere packaging on the shelf life of ground beef. Meat Science, 86(2), 468–473. doi:10.1016/j.meatsci.2010.05.036
- Arjunan, K. P., Sharma, V. K., & Ptasinska, S. (2015). Effects of atmospheric pressure plasmas on isolated and cellular DNA—a review. International Journal of Molecular Sciences, 16(2), 2971–3016. doi:10.3390/ijms16022971
- Attri, P., Kim, Y. H., Park, D. H., Park, J. H., Hong, Y. J., Uhm, H. S., & Choi, … , & H, E. (2015). Generation mechanism of hydroxyl radical species and its lifetime prediction during the plasma-initiated ultraviolet (UV) photolysis. Scientific Reports, 5(1), 1–8. doi:10.9734/JSRR/2015/14076
- Bae, S. C., Park, S. Y., Choe, W., & Ha, S. D. (2015). Inactivation of murine norovirus-1 and hepatitis A virus on fresh meats by atmospheric pressure plasma jets. Food Research International, 76, 342–347. doi:10.1016/j.foodres.2015.06.039
- Banu, M. S., Sasikala, P., Dhanapal, A., Kavitha, V., Yazhini, G., & Rajamani, L. (2012). Cold plasma as a novel food processing technology. IJETED, 4(2), 803–818.
- Bauer, A., Ni, Y., Bauer, S., Paulsen, P., Modic, M., Walsh, J. L., & Smulders, F. J. M. (2017). The effects of atmospheric pressure cold plasma treatment on microbiological, physical-chemical and sensory characteristics of vacuum packaged beef loin. Meat Science, 128, 77–87. doi:10.1016/j.meatsci.2017.02.003
- Bourke, P., Ziuzina, D., Han, L., Cullen, P. J., & Gilmore, B. F. (2017). Microbiological interactions with cold plasma. Journal of Applied Microbiology, 123(2), 308–324. doi:10.1111/jam.13429
- Chaplot, S., Yadav, B., Jeon, B., & Roopesh, M. (2019). Atmospheric cold plasma and peracetic acid–based hurdle intervention to reduce Salmonella on raw poultry meat. Journal of Food Protection, 82(5), 878–888. doi:10.4315/0362-028X.JFP-18-377
- Choi, M. S., Jeon, E. B., Kim, J. Y., Choi, E. H., Lim, J. S., Choi, J., & Park, S. Y. (2020). Impact of non-thermal dielectric barrier discharge plasma on Staphylococcus aureus and Bacillus cereus and quality of dried blackmouth angler (lophiomus setigerus). Journal of Food Engineering, 278, 1–9. doi:10.1016/j.jfoodeng.2020.109952
- Choi, S., Puligundla, P., & Mok, C. (2016). Corona discharge plasma jet for inactivation of Escherichia coli O157: H7 and Listeria monocytogenes on inoculated pork and its impact on meat quality attributes. Annals of Microbiology, 66(2), 685–694. doi:10.1007/s13213-015-1147-5
- Clydesdale, F. M., & Ahmed, E. M. (1978). Colorimetry — methodology and applications. C R C Critical Reviews in Food Technology, 10(3), 243–301. doi:10.1080/10408397809527252
- Cui, H., Wu, J., Li, C., & Lin, L. (2017). Promoting anti-listeria activity of lemongrass oil on pork loin by cold nitrogen plasma assist. Journal of Food Safety, 37(2), 1–10. doi:10.1111/jfs.12316
- Dave, D., & Ghaly, A. E. (2011). Meat spoilage mechanisms and preservation techniques: A critical review. American Journal of Agricultural & Biological Science, 6(4), 486–510. doi:10.3844/ajabssp.2011.486.510
- De Smet, S., & Vossen, E. (2016). Meat: The balance between nutrition and health. A review. Meat Science, 120, 145–156. doi:10.1016/j.meatsci.2016.04.008
- Deng, X. T., Shi, J. J., Shama, G., & Kong, M. G. (2005). Effects of microbial loading and sporulation temperature on atmospheric plasma inactivation of Bacillus subtilis spores. Applied Physics Letters, 87(15), 153901. doi:10.1063/1.2103394
- Dirks, B. P., Dobrynin, D., Fridman, G., Mukhin, Y., Fridman, A., & Quinlan, J. J. (2012). Treatment of raw poultry with nonthermal dielectric barrier discharge plasma to reduce campylobacter jejuni and Salmonella enterica. Journal of Food Protection, 75(1), 22–28. doi:10.4315/0362-028X.JFP-11-153
- Dobrynin, D., Fridman, G., Friedman, G., & Fridman, A. (2009). Physical and biological mechanisms of direct plasma interaction with living tissue. New Journal of Physics, 11(11), 115020. doi:10.1088/1367-2630/11/11/115020
- Frohling, A., Durek, J., Schnabel, U., Ehlbeck, J., Bolling, J., & Schluter, O. (2012). Indirect plasma treatment of fresh pork: Decontamination efficiency and effects on quality attributes. Innovative Food Science & Emerging Technologies, 16, 381–390. doi:10.1016/j.ifset.2012.09.001
- Gaens, W., & Bogaerts, A. (2013). Kinetic modelling for an atmospheric pressure argon plasma jet in humid air. Journal of Physics D: Applied Physics, 46(27), 275201. doi:10.1088/0022-3727/46/27/275201
- Gao, Y., Zhuang, H., Yeh, H. Y., Bowker, B., & Zhang, J. (2019). Effect of rosemary extract on microbial growth, pH, color, and lipid oxidation in cold plasma-processed ground chicken patties. Innovative Food Science & Emerging Technologies, 57, 1–6. doi:10.1016/j.ifset.2019.05.007
- Gavahian, M., Chu, Y. H., Khaneghah, A. M., Barba, F. J., & Misra, N. N. (2018). A critical analysis of the cold plasma induced lipid oxidation in foods. Trends in Food Science & Technology, 77, 32–41. doi:10.1016/j.tifs.2018.04.009
- Georgescu, N. (2015). Dielectric barrier discharges for egg decontamination with cold atmospheric plasma: Physical and chemical characteristics. Romanian Journal of Physics, 60(9-10), 1561–1573.
- González-González, C. R., Labo-Popoola, O., Delgado-Pando, G., Theodoridou, K., Doran, O., & Stratakos, A. C. (2021). The effect of cold atmospheric plasma and linalool nanoemulsions against Escherichia coli O157: H7 and Salmonella on ready-to-eat chicken meat. Lwt, 149, 1–10. doi:10.1016/j.lwt.2021.111898
- Gök, V., Aktop, S., Özkan, M., & Tomar, O. (2019). The effects of atmospheric cold plasma on inactivation of Listeria monocytogenes and Staphylococcus aureus and some quality characteristics of pastırma—a dry-cured beef product. Innovative Food Science & Emerging Technologies, 56, 1–8. doi:10.1016/j.ifset.2019.102188
- Han, L., Ziuzina, D., Heslin, C., Boehm, D., Patange, A., Sango, D. M., … Bourke, P. (2016). Controlling microbial safety challenges of meat using high voltage atmospheric cold plasma. Frontiers in Microbiology, 7, 977–988. doi:10.3389/fmicb.2016.00977
- Huang, M. M., Wang, J. M., Zhuang, H., Yan, W. J., Zhao, J. Y., & Zhang, J. H. (2019). Effect of in-package high voltage dielectric barrier discharge on microbiological, color and oxidation properties of pork in modified atmosphere packaging during storage. Meat Science, 149, 107–113. doi:10.1016/j.meatsci.2018.11.016
- Jayasena, D. D., Kim, H. J., Yong, H. I., Park, S., Kim, K., Choe, W., & Jo, C. (2015). Flexible thin-layer dielectric barrier discharge plasma treatment of pork butt and beef loin: Effects on pathogen inactivation and meat-quality attributes. Food Microbiology, 46, 51–57. doi:10.1016/j.fm.2014.07.009
- Jeong, J. Y., Hur, S. J., Yang, H. S., Moon, S. H., Hwang, Y. H., Park, G. B., & Joo, S. T. (2009). Discoloration characteristics of 3 major muscles from cattle during cold storage. Journal of Food Science, 74(1), C1–C5. doi:10.1111/j.1750-3841.2008.00983.x
- Jung, S., Kim, H. J., Park, S., Yong, H. I., Choe, J. H., Jeon, H. J., … Jo, C. (2015). The use of atmospheric pressure plasma-treated water as a source of nitrite for emulsion-type sausage. Meat Science, 108, 132–137. doi:10.1016/j.meatsci.2015.06.009
- Jung, S., Kim, H. J., Park, S., Yong, H. I., Choe, J. H., Jeon, H. J., … Jo, C. (2015a). Color developing capacity of plasma-treated water as a source of nitrite for meat curing. Korean Journal for Food Science of Animal Resources, 35(5), 703–706. doi:10.5851/kosfa.2015.35.5.703
- Jung, S., Lee, J., Lim, Y., Choe, W., Yong, H. I., & Jo, C. (2017). Direct infusion of nitrite into meat batter by atmospheric pressure plasma treatment. Innovative Food Science & Emerging Technologies, 39, 113–118. doi:10.1016/j.ifset.2016.11.010
- Kennedy, C., Buckley, D. J., & Kerry, J. P. (2004). Display life of sheep meats retail packaged under atmospheres of various volumes and compositions. Meat Science, 68(4), 649–658. doi:10.1016/j.meatsci.2004.05.018
- Kim, B., Yun, H., Jung, S., Jung, Y., Jung, H., Choe, W., & Jo, C. (2011). Effect of atmospheric pressure plasma on inactivation of pathogens inoculated onto bacon using two different gas compositions. Food Microbiology, 28(1), 9–13. doi:10.1016/j.fm.2010.07.022
- Kim, H. J., Yong, H. I., Park, S., Choe, W., & Jo, C. (2013a). Effects of dielectric barrier discharge plasma on pathogen inactivation and the physicochemical and sensory characteristics of pork loin. Current Applied Physics, 13(7), 1420–1425. doi:10.1016/j.cap.2013.04.021
- Kim, H. J., Yong, H. I., Park, S., Kim, K., Bae, Y. S., Choe, W., … Jo, C. (2013b). Effect of inactivating Salmonella Typhimurium in raw chicken breast and pork loin using an atmospheric pressure plasma jet. Journal of Animal Science and Technology, 55(6), 545–549. doi:10.5187/JAST.2013.55.6.545
- Kim, J. S., Lee, E. J., Cho, E. A., & Kim, Y. J. (2013c). Inactivation of Campylobacter jejuni using radio-frequency atmospheric pressure plasma on agar plates and chicken hams. Korean Journal for Food Science of Animal Resources, 33(3), 317–324. doi:10.5851/kosfa.2013.33.3.317
- Kim, J. S., Lee, E. J., Choi, E. H., & Kim, Y. J. (2014). Inactivation of Staphylococcus aureus on the beef jerky by radio-frequency atmospheric pressure plasma discharge treatment. Innovative Food Science & Emerging Technologies, 22, 124–130. doi:10.1016/j.ifset.2013.12.012
- Kronn, T. G., Lawrence, K. C., Zhuang, H., Hiett, K. L., Rothrock, M. J., Huang, Y. W., … Abdo, Z. (2015). Nonthermal plasma system for extending shelf life of raw broiler breast fillets. Transactions of the ASABE, 58(2), 493–500. doi:10.13031/trans.58.10887
- Langmuir, I. (1928). Oscillations in ionized gases. Proceedings of the National Academy of Science of the USA, 14(8), 627–637. doi:10.1073/pnas.14.8.627
- Lee, H. J., Jung, H., Choe, W., Ham, J. S., Lee, J. H., & Jo, C. (2011). Inactivation of Listeria monocytogenes on agar and processed meat surfaces by atmospheric pressure plasma jets. Food Microbiology, 28(8), 1468–1471. doi:10.1016/j.fm.2011.08.002
- Lee, H., Yong, H. I., Kim, H. J., Choe, W., Yoo, S. J., Jang, E. J., & Jo, C. (2016). Evaluation of the microbiological safety, quality changes, and genotoxicity of chicken breast treated with flexible thin-layer dielectric barrier discharge plasma. Food Science and Biotechnology, 25(4), 1189–1195. doi:10.1007/s10068-016-0189-1
- Lee, S., Lee, H., Kim, S., Lee, J., Ha, J., Choi, Y., … Yoon, Y. (2018). Microbiological safety of processed meat products formulated with low nitrite concentration—A review. Asian-Australasian Journal of Animal Sciences, 31(8), 1073–1077. doi:10.5713/ajas.17.0675
- Lim, Y. B., Park, S., Kim, H. R., Yong, H. I., Kim, S. H., & Lee, H. J. (2015). Plasma treatment process for processed meat and plasma treatment apparatus for processed meat. Korea Patent Submission, 10-2015, 0029641.
- Luño, M., Roncalés, P., Djenane, D., & Beltrán, J. A. (2000). Beef shelf life in low O2 and high CO2 atmospheres containing different low CO concentrations. Meat Science, 55(4), 413–419. doi:10.1016/S0309-1740(99)00170-9
- Mai-Prochnow, A., Murphy, A. B., McLean, M., Kong, M. G., & Ostrikov, K. (2014). Atmospheric pressure plasmas: Infection control and bacterial responses. International Journal of Antimicrobial Agents, 43(6), 508–517. doi:10.1016/j.ijantimicag.2014.01.025
- Mancini, R. A., & Hunt, M. (2005). Current research in meat color. Meat Science, 71(1), 100–121. doi:10.1016/j.meatsci.2005.03.003
- Min, B., & Ahn, D. U. (2005). Mechanism of lipid peroxidation in meat and meat products-A review. Food Science and Biotechnology, 14(1), 152–163.
- Misra, N. N., & Jo, C. (2017). Applications of cold plasma technology for microbiological safety in meat industry. Trends in Food Science & Technology, 64, 74–86. doi:10.1016/j.tifs.2017.04.005
- Misra, N. N., Keener, K. M., Bourke, P., & Cullen, P. J. (2015). Generation of in-package cold plasma and efficacy assessment using methylene blue. Plasma Chemistry and Plasma Processing, 35(6), 1043–1056. doi:10.1007/s11090-015-9638-5
- Misra, N. N., Schlüter, O., & Cullen, P. J. (2016). Cold plasma in Food and agriculture:Fundamentals and applications. (1st ed.). Academic press, Elsevier Ltd.
- Moon, S. Y., Kim, D. B., Gweon, B., Choe, W., Song, H. P., & Jo, C. (2009). Feasibility study of the sterilization of pork and human skin surfaces by atmospheric pressure plasmas. Thin Solid Films, 517(14), 4272–4275. doi:10.1016/j.tsf.2009.02.018
- Mor-Mur, M., & Yuste, J. (2010). Emerging bacterial pathogens in meat and poultry: An overview. Food and Bioprocess Technology, 3(1), 24–35. doi:10.1007/s11947-009-0189-8
- Moreau, M., Orange, N., & Feuilloley, M. G. J. (2008). Non-thermal plasma technologies: New tools for bio-decontamination. Biotechnology Advances, 26(6), 610–617. doi:10.1016/j.biotechadv.2008.08.001
- Moutiq, R., Misra, N. N., Mendonca, A., & Keener, K. (2020). In-package decontamination of chicken breast using cold plasma technology: Microbial, quality and storage studies. Meat Science, 159, 1–9. doi:10.1016/j.meatsci.2019.107942
- Noriega, E., Shama, G., Laca, A., Díaz, M., & Kong, M. G. (2011). Cold atmospheric gas plasma disinfection of chicken meat and chicken skin contaminated with Listeria innocua. Food Microbiology, 28(7), 1293–1300. doi:10.1016/j.fm.2011.05.007
- Oehmigen, K., Hähnel, M., Brandenburg, R., Wilke, C., Weltmann, K. D., & Von Woedtke, T. (2010). The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Processes and Polymers, 7(3-4), 250–257. doi:10.1002/ppap.200900077
- Patange, A., Boehm, D., Bueno-Ferrer, C., Cullen, P. J., & Bourke, P. (2017). Controlling Brochothrix thermosphacta as a spoilage risk using in-package atmospheric cold plasma. Food Microbiology, 66, 48–54. doi:10.1016/j.fm.2017.04.002
- Pelletier, J. (1992). Sterilization by plasma processing. Aggressologie, 33, 457–477.
- Qian, J., Wang, C., Zhuang, H., Nasiru, M. M., Zhang, J., & Yan, W. (2021). Evaluation of meat-quality and myofibrillar protein of chicken drumsticks treated with plasma-activated lactic acid as a novel sanitizer. LWT, 138, 110642. doi:10.1016/j.lwt.2020.110642
- Qian, J., Zhao, Y., Yan, L., Luo, J., Yan, W., & Zhang, J. (2022). Improving the lipid oxidation of beef patties by plasma-modified essential oil/protein edible composite films. LWT, 154, 112662. doi:10.1016/j.lwt.2021.112662
- Qian, J., Zhuang, H., Nasiru, M. M., Muhammad, U., Zhang, J., & Yan, W. (2019). Action of plasma-activated lactic acid on the inactivation of inoculated Salmonella enteritidis and quality of beef. Innovative Food Science & Emerging Technologies, 57, 102196. doi:10.1016/j.ifset.2019.102196
- Ripoll, G., Alcalde, M. J., Horcada, A., & Panea, B. (2011). Suckling kid breed and slaughter weight discrimination using muscle colour and visible reflectance. Meat Science, 87(2), 151–156. doi:10.1016/j.meatsci.2010.10.006
- Roh, S. H., Lee, S. Y., Park, H. H., Lee, E. S., & Min, S. C. (2019). Effects of the treatment parameters on the efficacy of the inactivation of Salmonella contaminating boiled chicken breast by in-package atmospheric cold plasma treatment. International Journal of Food Microbiology, 293, 24–33. doi:10.1016/j.ijfoodmicro.2018.12.016
- Roh, S. H., Oh, Y. J., Lee, S. Y., Kang, J. H., & Min, S. C. (2020). Inactivation of Escherichia coli O157: H7, salmonella, Listeria monocytogenes, and tulane virus in processed chicken breast via atmospheric in-package cold plasma treatment. Lwt, 127, 109429. doi:10.1016/j.lwt.2020.109429
- Rossow, M., Ludewig, M., & Braun, P. G. (2018). Effect of cold atmospheric pressure plasma treatment on inactivation of Campylobacter jejuni on chicken skin and breast fillet. LWT, 91, 265–270. doi:10.1016/j.lwt.2018.01.052
- Rothrock, M. J., Zhuang, H., Lawrence, K. C., Bowker, B. C., Gamble, G. R., & Hiett, K. L. (2017). In-package inactivation of pathogenic and spoilage bacteria associated with poultry using dielectric barrier discharge-cold plasma treatments. Current Microbiology, 74(2), 149–158. doi:10.1007/s00284-016-1158-x
- Royintarat, T., Choi, E. H., Boonyawan, D., Seesuriyachan, P., & Wattanutchariya, W. (2020). Chemical-free and synergistic interaction of ultrasound combined with plasma-activated water (PAW) to enhance microbial inactivation in chicken meat and skin. Scientific Reports, 10(1), 1–14. doi:10.1038/s41598-020-58199-w
- Rød, S. K., Hansen, F., Leipold, F., & Knøchel, S. (2012). Cold atmospheric pressure plasma treatment of ready-to-eat meat: Inactivation of Listeria innocua and changes in product quality. Food Microbiology, 30(1), 233–238. doi:10.1016/j.fm.2011.12.018
- Sahebkar, A., Hosseini, M., & Sharifan, A. (2020). Plasma-assisted preservation of breast chicken fillets in essential oils-containing marinades. LWT, 131, 109759. doi:10.1016/j.lwt.2020.109759
- Scholtz, V., Pazlarova, J., Souskova, H., Khun, J., & Julak, J. (2015). Nonthermal plasma – a tool for decontamination and disinfection. Biotechnology Advances, 33(6), 1108–1119. doi:10.1016/j.biotechadv.2015.01.002
- Shahidi, F., & Zhong, Y. (2010). Lipid oxidation and improving the oxidative stability. Chemical Society Reviews, 39(11), 4067–4079. doi:10.1039/b922183m
- Shi, Y., Wang, X., Borhan, M. S., Young, J., Newman, D., Berg, E., & Sun, X. (2021). A review on meat quality Evaluation methods based on Non-destructive computer vision and artificial intelligence technologies. Food Science of Animal Resources, 41(4), 563–588. doi:10.5851/kosfa.2021.e25
- Stoica, M., Alexe, P., & Mihalcea, L. (2014). Atmospheric cold plasma as new strategy for foods processing-an overview. Innovative Romanian Food Biotechnology, 15, 1–8.
- Stratakos, A. C., & Grant, I. R. (2018). Evaluation of the efficacy of multiple physical, biological and natural antimicrobial interventions for control of pathogenic Escherichia coli on beef. Food Microbiology, 76, 209–218. doi:10.1016/j.fm.2018.05.011
- Stratakos, A. C., & Koidis, A. (2015). Suitability, efficiency and microbiological safety of novel physical technologies for the processing of ready-to-eat meats and pumpable products. International Journal of Food Science and Technology, 50(6), 1283–1302. doi:10.1111/ijfs.12781
- Timmermann, E., Bansemer, R., Gerling, T., Hahn, V., Weltmann, K. D., Nettesheim, S., & Puff, M. (2020). Piezoelectric-driven plasma pen with multiple nozzles used as a medical device: Risk estimation and antimicrobial efficacy. Journal of Physics D: Applied Physics, 54(2), 025201. doi:10.1088/1361-6463/abb900
- Ulbin-Figlewicz, N., Brychcy, E., & Jarmoluk, A. (2015a). Effect of low-pressure cold plasma on surface microflora of meat and quality attributes. Journal of Food Science and Technology, 52(2), 1228–1232. doi:10.1007/s13197-013-1108-6
- Ulbin-Figlewicz, N., Jarmoluk, A., & Marycz, K. (2015b). Antimicrobial activity of low-pressure plasma treatment against selected foodborne bacteria and meat microbiota. Annals of Microbiology, 65(3), 1537–1546. doi:10.1007/s13213-014-0992-y
- Van Boekel, M., Fogliano, V., Pellegrini, N., Stanton, C., Scholz, G., Lalljie, S., Somoza, V., Knorr, D., Jasti, P. R., & Eisenbrand, G. (2010). A review on the beneficial aspects of food processing. Molecular Nutrition and Food Research, 54(9), 1215–1247. doi:10.1002/mnfr.200900608
- Wang, J. M., Zhuang, H., Lawrence, K., & Zhang, J. H. (2018). Disinfection of chicken fillets in packages with atmospheric cold plasma: Effects of treatment voltage and time. Journal of Applied Microbiology, 124(5), 1212–1219. doi:10.1111/jam.13637
- Wang, J., Zhuang, H., Hinton Jr, A., & Zhang, J. (2016). Influence of in-package cold plasma treatment on microbiological shelf life and appearance of fresh chicken breast fillets. Food Microbiology, 60, 142–146. doi:10.1016/j.fm.2016.07.007
- Wang, X., Wang, Z., Zhuang, H., Nasiru, M. M., Yuan, Y., Zhang, J., & Yan, W. (2021). Changes in color, myoglobin, and lipid oxidation in beef patties treated by dielectric barrier discharge cold plasma during storage. Meat Science, 176, 1–9. doi:10.1016/j.meatsci.2021.108456
- Yadav, B., Spinelli, A. C., Govindan, B. N., Tsui, Y. Y., McMullen, L. M., & Roopesh, M. S. (2019). Cold plasma treatment of ready-to-eat ham: Influence of process conditions and storage on inactivation of Listeria innocua. Food Research International, 123, 276–285. doi:10.1016/j.foodres.2019.04.065
- Yadav, B., Spinelli, A. C., Misra, N. N., Tsui, Y. Y., McMullen, L. M., & Roopesh, M. S. (2020). Effect of in-package atmospheric cold plasma discharge on microbial safety and quality of ready-to-eat ham in modified atmospheric packaging during storage. Journal of Food Science, 85(4), 1203–1212. doi:10.1111/1750-3841.15072
- Yong, H. I., Lee, H., Park, S., Park, J., Choe, W., Jung, S., & Jo, C. (2017). Flexible thin-layer plasma inactivation of bacteria and mold survival in beef jerky packaging and its effects on the meat's physicochemical properties. Meat Science, 123, 151–156. doi:10.1016/j.meatsci.2016.09.016
- Yong, H. I., Lee, S. H., Kim, S. Y., Park, S., Park, J., Choe, W., & Jo, C. (2019). Color development, physiochemical properties, and microbiological safety of pork jerky processed with atmospheric pressure plasma. Innovative Food Science & Emerging Technologies, 53, 78–84. doi:10.1016/j.ifset.2017.09.005
- Yoo, J. H., Baek, K. H., Heo, Y. S., Yong, H. I., & Jo, C. (2021). Synergistic bactericidal effect of clove oil and encapsulated atmospheric pressure plasma against Escherichia coli O157: H7 and Staphylococcus aureus and its mechanism of action. Food Microbiology, 93, 1–8. doi:10.1016/j.fm.2020.103611
- Zhang, M., Oh, J. K., Cisneros-Zevallos, L., & Akbulut, M. (2013). Bactericidal effects of nonthermal low-pressure oxygen plasma on S. Typhimurium LT2 attached to fresh produce surfaces. Journal of Food Engineering, 119(3), 425–432. doi:10.1016/j.jfoodeng.2013.05.045
- Zhang, X., Song, L., Gao, T., Zhang, L., Jiang, Y., Li, J. L., … Zhou, G. H. (2018). Effect of ultrasonic thawing method on quality characteristics of chicken breast meat. Food Sci, 39, 135–140.
- Zhao, W., Zhifei, H., Xiao, G., & Hongjun, L. (2018). Interrelationship among ferrous myoglobin, lipid and protein oxidations in rabbit meat during refrigerated and superchilled storage. Meat Science, 146, 131–139. doi:10.1016/j.meatsci.2018.08.006
- Zhuang, H., Rothrock Jr, M. J., Hiett, K. L., Lawrence, K. C., Gamble, G. R., Bowker, B. C., & Keener, K. M. (2019). In-package antimicrobial treatment of chicken breast meat with high voltage dielectric barrier discharge–electric voltage Effect1. Journal of Applied Poultry Research, 28(4), 801–807. doi:10.3382/japr/pfz036
- Zhuang, H., Rothrock Jr, M. J., Hiett, K. L., Lawrence, K. C., Gamble, G. R., Bowker, B. C., & Keener, K. M. (2019a). In-package air cold plasma treatment of chicken breast meat: Treatment time effect. Journal of Food Quality, 2019, 1–7. doi:10.1155/2019/1837351