ABSTRACT
This study was conducted to test the expression profiles of miRNAs in the liver in piglets after the LPS challenge using sequencing technology. Twelve barrows were assigned to two groups, including the saline group and the LPS group. Six small RNA (sRNA) libraries were constructed, and novel miRNAs were identified using mirevo and mirdeep2 software and validated by quantitative real-time PCR (qRT-PCR). The results showed the mRNA expression of IL-1β, IL-6 and TNF-α in the liver increased after the LPS challenge (P < .05). A total of 29 differentially expressed miRNAs were identified in the liver after the LPS challenge. And 11 miRNAs were validated by qRT-PCR. In addition, the results of statistics of pathway enrichment showed that these miRNAs could be related to lipid metabolism response. This study provides the first miRNA expression profiles of LPS-mediated changes in the liver, which might provide potential insights into miRNAs involved in regulating lipid metabolism in pigs challenged by LPS.
KEYWORDS:
Introduction
More risk of disease incidence occurs as the size of pig farms expands. Innate immunity serves as the first line of host defence against pathogenic microorganisms and maintains liver regeneration after the loss of liver mass. The liver is a very important organ with innate immune features (Dong et al., Citation2007). Its function includes detoxification, nutrient metabolism, glycogen storage (Pan et al., Citation2010), and immunity (Racanelli & Rehermann, Citation2006). Consequently, liver health is critical to animals.
MicroRNAs (miRNAs) are a class of short noncoding RNAs that are expressed in most organisms. Many acute and chronic liver diseases relate to miRNAs, which influence numerous signalling and metabolic pathways in the liver (Klieser et al., Citation2019). Increasing evidence has shown that miRNAs are involved in liver diseases, regeneration, and nutrient metabolism. For example, miR-122 regulates iron (Castoldi et al., Citation2011) and lipid metabolism (Hsu et al., Citation2012; Tsai et al., Citation2012), acts as a tumor suppressor and promotes liver development (Laudadio et al., Citation2012; Xu et al., Citation2010). However, besides these miRNA functions, it may be essential to consider gross miRNA function in light of the pathogenesis of the liver disease. Therefore, expression patterns of miRNAs can potentially serve as possible therapeutic targets in liver disease.
Virtually all liver diseases are related to inflammation (Guillot & Tacke, Citation2019). Our previous studies have found that the LPS challenge could elevate plasma concentrations of pro-inflammatory cytokines, such as tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6, which then cause liver injury in piglets (Li et al., Citation2012). Therefore, this study was conducted to test the expression profiles of miRNAs in the liver in piglets after the LPS challenge using sequencing technology. In addition, because of the same anatomy, physiology, genetics, and many genes in the immune system between pigs and humans, pigs are also good immune system models for studying humans. Collectively, these results will help to understand the role of miRNAs in the injured livers of humans.
Materials and methods
Animal and experimental design
Twelve crossbred castrated barrows (Duroc × Large White × Landrace, 7.02 ± 0.21 kg, weaned at 28 ± 3 day of age) were selected and assigned randomly to two groups, including the saline group (slaughtered at 4 h after saline injection) and the LPS (Escherichia coli serotype 055: B5; Sigma Chemical, St. Louis, MO) group (slaughtered at 4 h after LPS challenge). Each group had six replicated pens, and each pen had one pig. The living environment for piglets was in accordance with our previous studies during a 14-day adaption period (Kang et al., Citation2020). Signs of diarrhoea, sickness, and abnormal behaviour were not observed throughout the adaption period. On day 15, the piglets were slaughtered at 4 h after 100 μg/kg body weight (BW) LPS treatment, or the same volume of 0.9% sterile saline solution. The animal use protocol for this research was approved by the Animal Care and Use Committee of Wuhan Polytechnic University.
Sample collection
At 4 h after saline or LPS injection, pigs were slaughtered under anesthesia with an intramuscular injection of pentobarbital sodium (50 mg/kg BW), and a portion of the liver (approximately 3 g) was fixed in fresh 4% paraformaldehyde/phosphate-buffered saline for 24 h for histological analysis. The remaining liver tissues were removed and frozen in liquid nitrogen immediately, and then stored at −80°C for further analysis.
Liver histological analysis
The histology protocol used was adapted from a published method (Xu et al., Citation2021). Paraffin-embedded tissue slides were stained with haematoxylin and eosin (H&E). A histological analysis was conducted in a blinded manner using morphometric software (BioScan Optimetric, BioScan, Edmonds, WA, USA).
mRNA and miRNA abundance analysis by quantitative real-time PCR (qRT-PCR)
The genes of pro-inflammatory cytokine mRNA expression were measured, as previously described (Kang et al., Citation2020), and the mRNA expression relative to the housekeeping gene (GAPDH) was calculated according to the 2−△△CT method (Livak & Schmittgen, Citation2001). Mature miRNAs were detected and quantified using primers obtained from the mirVanaTM qRT-PCR Primer Set (Gene Pharma, Shanghai, China). The miRNA expression was normalized to the level of U6 and analyzed by the 2−△△CT method (Livak & Schmittgen, Citation2001).
miRNA library construction
Six small RNA (sRNA) libraries were constructed from pigs after the saline treatment (C1, C2, C3), and after the LPS challenge (L1, L2, L3). Total RNA was prepared and purified by electrophoretic separation on a 15% urea denaturing polyacrylamide gel electrophoresis (PAGE) gel, and gel slices of 18–30 nt were excised and recovered (14–30 ssRNA Ladder Marker, TAKARA). Then these 18–30 nt small RNAs were ligated to a 5′-adaptor and a 3′-adaptor and transcribed into cDNA by SuperScript II Reverse Transcriptase (Invitrogen, USA). Subsequently, these cDNA fragments were enriched using PCR primer Cocktail and PCR Mix. The PCR products in the range 100–120 bp were selected by 4% agarose gel electrophoresis and used for sequencing on the BGISEQ-500 platform (BGI-Shenzhen, China). In order to predict novel miRNAs, clean reads were obtained by removing low-quality reads, and reads shorter than 18 nt were compared against sRNAs deposited in GenBank and Rfam databases, as previously described (Wang et al., Citation2018). The hairpin structure, characteristic of miRNA precursors, can be used to predict novel miRNAs. miRNA prediction software of mirevo (Wen et al., Citation2012) and mirdeep2 (Friedländer et al., Citation2011) was integrated to analyze new miRNAs.
Differential expression analysis of miRNAs
miRNA expression levels compared between the saline treatment and the LPS challenge were the same as in the previous studies (Wang et al., Citation2018; Zhang et al., Citation2015). The expression of each miRNA was normalized by the following formula: Normalized expression = (actual miRNA read count/total clean read count) ×1,000,000. The fold change in miRNA reads was presented as log 2 transformation and calculated by the following formula: Fold change = log 2 (the LPS group/the saline group). A P-value less than .01 and log2 (fold change) > 1 is considered significant.
Target gene prediction
RNA hybrid and TargetScan were used to predict miRNA candidates. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were performed to reveal the potential biological functions of miRNA candidates and the main pathways targeted by the miRNA candidates. The GO enrichment analysis of the DEGs was performed using the GOseq (Young et al., Citation2010). KEGG pathway enrichment analysis was performed using a hypergeometric test and a corrected P-value (≤.05) was considered significantly enriched.
Statistical analysis
The data of the mRNA expression of target genes were analyzed by ANOVA with SPSS 17.0 software. Differences between means were determined by T-tests. All data were expressed as means ± SE. The statistical significance level for all analyses was set at P < .05.
Results
Liver histological analysis
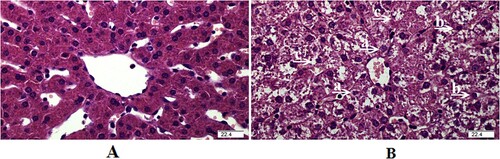
The changes in liver histology at 4 h after the LPS challenge are shown in . Compared with the saline group, the liver in pigs treated with LPS wase damaged evidenced by the caryolysis and karyopycnosis of hepatocytes, as well as the disordered hepatic cell cord arrangement.
The mRNA expression of pro-inflammatory cytokines in the liver
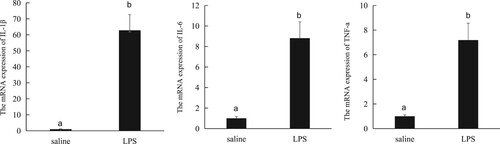
As shown in , compared with the saline injection, the LPS challenge increased the mRNA expression of IL-1β, IL-6 and TNF-α in the liver (P < .05).
Sequence analysis of short RNAs
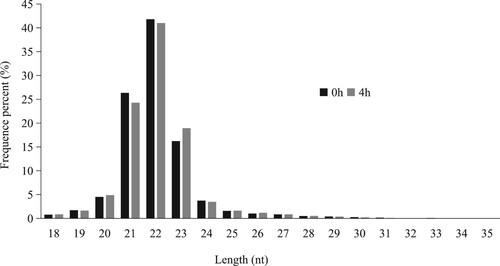
Six sRNA libraries were built from the saline (n = 3) group and the LPS (n = 3) group. The most size distribution of clean reads was 20–24 nt (). The total reads, novel-miRNA number and other basic data are shown in .
Table 1. Basic data of sequencing in LPS-stimulated and unstimulated liver.
miRNA target gene prediction, GO enrichment, and KEGG pathway analysis
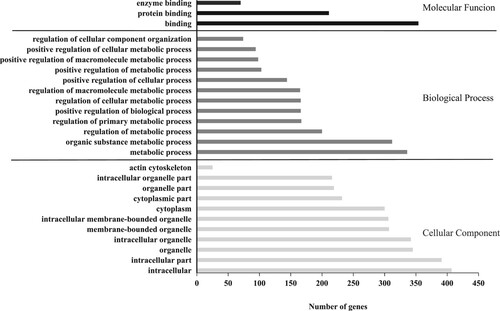
The results of the GO enrichment analysis are shown in . Genes related to intracellular, cytoplasm, and organelle were identified in the cellular component category, the biological process category contained genes involved in metabolic process, and the molecular function category contained genes mainly involved in binding and enzyme activity.
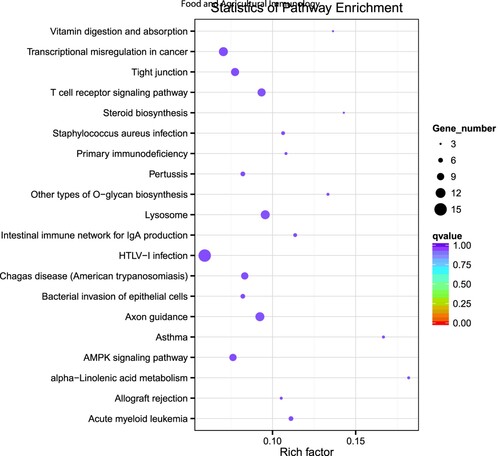
Target genes of differential miRNAs were classified to identify pathways according to KEGG functional annotation. And the scatter plot of candidate target gene KEGG enrichment was a graphical display of KEGG enrichment analysis results. The top 20 signalling pathways were enriched (), and some target genes were annotated to immune response pathways, including “AMPK signalling pathway,” “Intestinal immune network for IgA production,” “Primary immunodeficiency” and “T cell receptor signalling pathway.” However, they all had not been significantly enriched (P > .05). In addition, according to the rich factor, the α-Linolenic acid metabolism pathway had the highest enrichment degree (P > .05).
Differentially expressed miRNAs between the saline and LPS groups
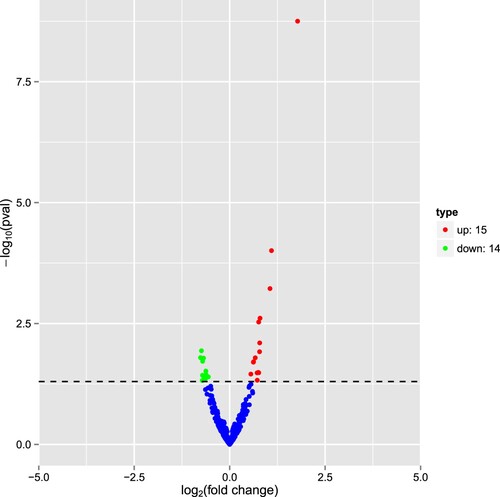
Using the miRBase database, we identified 307 known miRNAs in six libraries. In addition, 173 novel miRNAs were identified, with 29 differentially expressed novel miRNAs in the saline vs. the LPS group, including 15 up-regulating miRNAs and 14 down-regulating miRNAs ().
RT-qPCR validation of the liver miRNAs in pigs
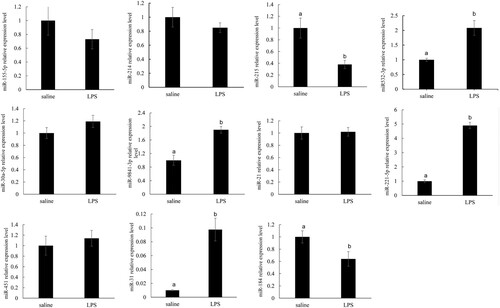
Eleven microRNAs were selected to validate the reliability of microRNA sequencing. Consistent with the high-throughput sequencing results, miR-221-5p and miR-9841-3p expressions were up-regulated, miR-184 and miR-215 expression were down-regulated (P < .05). However, RT-qPCR validation results showed that miR-31 and miR-3p expressions decreased (P < .05), which were not in line with the high-throughput sequencing results ().
Discussion
The liver is a very important organ with innate immune features (Dong et al., Citation2007). Innate immunity serves as the first line of host defence against pathogenic microorganisms by inducing a variety of inflammatory and antimicrobial responses. Virtually all liver diseases are related to inflammation (Guillot & Tacke, Citation2019). In this experiment, the LPS challenge induced the inflammation in liver evidenced by the elevated mRNA expression of IL-1β, IL-6 and TNF-α. In addition, the LPS challenge induced liver injury, which was identified by the changes in histology.
Previous studies reported that miRNAs could be a new therapeutic frontier for the management of cancer and other diseases in a new era (Rupaimoole & Slack, Citation2017) and a potentially novel diagnostic biomarker in drug-induced liver injury (Sanjay & Girish, Citation2017). miRNAs influence numerous signalling and metabolic pathways in the liver (Klieser et al., Citation2019). Most studies focus on the individual miRNA function; however, it is essential to consider gross miRNA function in light of the pathogenesis of the liver disease. This study found that LPS treatment could induce liver injury at 4 h after the LPS challenge. On this basis, we further analyzed DEG patterns, important pathways, and miRNAs in the liver from piglets treated with LPS. Our studies might be useful to identify new mechanisms involved in liver injury.
According to the miRBase database, we identified 29 differentially expressed novel miRNAs after the LPS challenge, including 15 up-regulating miRNAs and 14 down-regulating miRNAs. And 11 miRNA expressions were tested based on the P-value. Through RT-qPCR validation of the liver miRNAs in pigs, we found miR-31, miR-221-5p, miR-532-3p, and miR-9841-3P expressions were significantly increased; however, miR-184 and miR-215 expressions were significantly decreased. The results exhibited that the trend of expression level for the selected miRNAs showed a little discrepancy to that of the RNA-Seq analysis, which might be due to the differences in the sensitivity, specificity, and algorithm between the two techniques.
Previous studies have shown that miR-31 could attenuate the inflammatory response in the colon epithelium of mice (Tian et al., Citation2019), and also be a therapeutic potential in skin injury repair (Shi et al., Citation2018). Zheng et al. (Citation2021) found the miR-31 expression was highly increased in the injured liver and thought miR-31 could be a therapeutic potential in liver injury repair (Zheng et al., Citation2021). Consistent with these results, in the present study, we found that miR-31 expression was increased, which indicated that it could play a positive role in the liver after the LPS challenge. Spahn et al. (Citation2010) found that miR-221 down-regulation could stimulate tumour progression and increase the risk of prostate cancer cohort (Spahn et al., Citation2010). In addition, miR-221-5p could decrease the synthesis of triglyceride and cholesterol in the chicken liver (Zhang et al., Citation2020). Conversely, miR-184 expression was significantly increased in the goose fatty liver to stimulate fat synthesis (Fan et al., Citation2021). Therefore, the increased miR-221-5p expression and the decreased miR-184 expression could stimulate lipolysis, which could supply more energy to the liver to alleviate the liver stress induced by LPS.
In the present study, according to the rich factor and q value results, the enriched signalling pathway obtained was “α-Linolenic acid metabolism” (lipid metabolism pathway). However, the TLR4 and NOD signalling pathways relating to inflammation after LPS were not found in the top 20 signalling pathways. The liver plays an essential role in whole-body energy utilization (McBride & Kelly, Citation1990). Alipour et al. (Citation2007) reported that the LPS challenge caused insufficient energy supply in the liver (Alipour et al., Citation2007). Our previous studies have shown flaxseed oil, rich in ɑ-linolenic acid, could attenuate liver injury by improving carbohydrate metabolism to produce more energy for relieving stress induced by LPS (Kang et al., Citation2020). Some miRNAs could have a correlation with ɑ-linolenic acid metabolisms, such as miR-30b and miR-21 (Kim et al., Citation2016; Mandal et al., Citation2012). Collectively, our results indicated that these significantly increased miRNAs could mainly stimulate lipid metabolism to supply more energy to the liver in order to alleviate liver stress induced by LPS.
In summary, the LPS challenge induced inflammation and injury in the liver. Twenty-nine significant differentially expressed known miRNAs (15 up-regulated and 14 down-regulated) were identified using Illumina sequencing. The target gene expression and pathway enrichment analyses indicated that these DEMs might influence the liver after the LPS challenge by regulating pathways related to lipid metabolism. This study provides a basis for future studies to identify target mRNAs regulated by abundant miRNAs in the liver, which will be critical to uncovering their exact biological functions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alipour, M., Omri, A., Smith, M. G., & Suntres, Z. E. (2007). Prophylactic effect of liposomal N-acetylcysteine against LPS-induced liver injuries. Journal of Endotoxin Research, 13(5), 297–304. https://doi.org/10.1177/0968051907085062
- Castoldi, M., Vujic Spasic, M., Altamura, S., Elmén, J., Lindow, M., Kiss, J., Stolte, J., Sparla, R., D'Alessandro, L. A., Klingmüller, U., Fleming, R. E., Longerich, T., Gröne, H. J., Benes, V., Kauppinen, S., Hentze, M. W., & Muckenthaler, M. U. (2011). The liver-specific microRNA miR-122 controls systemic iron homeostasis in mice. Journal of Clinical Investigation, 121(4), 1386–1396. https://doi.org/10.1172/JCI44883
- Dong, Z., Wei, H., Sun, R., & Tian, Z. (2007). The roles of innate immune cells in liver injury and regeneration. Cellular & Molecular Immunology, 4(4), 241–252.
- Fan, X., Xing, Y., Liu, L., Zhao, C., Chen, Z., Khogali, M. K., Zhao, M., Hu, X., Cui, H., Geng, T., & Gong, D. (2021). Screening of MicroRNAs with potential systemic effects released from goose fatty liver. Journal of Poultry Science, 58(4), 263–269. https://doi.org/10.2141/jpsa.0200097
- Friedländer, M. R., Mackowiak, S. D., Li, N., Chen, W., & Rajewsky, N. (2011). miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Research, 40(1), 37–52. https://doi.org/10.1093/nar/gkr688
- Guillot, A., & Tacke, F. (2019). Liver macrophages: Old dogmas and new insights. Hepatology Communications, 3(6), 730–743. https://doi.org/10.1002/hep4.1356
- Hsu, S. H., Wang, B., Kota, J., Yu, J., Costinean, S., Kutay, H., Yu, L., Bai, S., La Perle, K., Chivukula, R. R., Mao, H., Wei, M., Clark, K. R., Mendell, J. R., Caligiuri, M. A., Jacob, S. T., Mendell, J. T., & Ghoshal, K. (2012). Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. Journal of Clinical Investigation, 122(8), 2871–2883. https://doi.org/10.1172/JCI63539
- Kang, P., Wang, Y., Li, X., Wan, Z., Wang, X., Zhu, H., Wang, C., Zhao, S., Chen, H., & Liu, Y. (2020). Effect of flaxseed oil on muscle protein loss and carbohydrate oxidation impairment in a pig model after lipopolysaccharide challenge. British Journal of Nutrition, 123(8), 859–869. https://doi.org/10.1017/S0007114519002393
- Kim, J., Okla, M., Erickson, A., Carr, T., Natarajan, S. K., & Chung, S. (2016). Eicosapentaenoic acid potentiates brown thermogenesis through FFAR4-dependent upregulation of miR-30b and miR-378. Journal of Biological Chemistry, 291(39), 20551–20562. https://doi.org/10.1074/jbc.M116.721480
- Klieser, E., Mayr, C., Kiesslich, T., Wissniowski, T., Fazio, P. D., Neureiter, D., & Ocker, M. (2019). The crosstalk of miRNA and oxidative stress in the liver: From physiology to pathology and clinical implications. International Journal of Molecular Sciences, 20(21), 5266–5289. https://doi.org/10.3390/ijms20215266
- Laudadio, I., Manfroid, I., Achouri, Y., Schmidt, D., Wilson, M. D., Cordi, S., Thorrez, L., Knoops, L., Jacquemin, P., Schuit, F., Pierreux, C. E., Odom, D. T., Peers, B., & Lemaigre, F. P. (2012). A feedback loop between the liver-enriched transcription factor network and miR-122 controls hepatocyte differentiation. Gastroenterology, 142(1), 119–129. https://doi.org/10.1053/j.gastro.2011.09.001
- Li, Q., Liu, Y., Che, Z., Zhu, H., Meng, G., Hou, Y., Ding, B., Yin, Y., & Chen, F. (2012). Dietary L-arginine supplementation alleviates liver injury caused by Escherichia coli LPS in weaned pigs. Innate Immunity, 18(6), 804–814. https://doi.org/10.1177/1753425912441955
- Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and 2-ΔΔCT method. Methods, 25(4), 402–408. https://doi.org/10.1006/meth.2001.1262
- Mandal, C. C., Ghosh-Choudhury, T., Dey, N., Choudhury, G. G., & Ghosh-Choudhury, N. (2012). miR-21 is targeted by omega-3 polyunsaturated fatty acid to regulate breast tumor CSF-1 expression. Carcinogenesis, 33(10), 1897–1908. https://doi.org/10.1093/carcin/bgs198
- McBride, B. W., & Kelly, J. M. (1990). Energy cost of absorption and metabolism in the ruminant gastrointestinal tract and liver: A review. Journal of Animal Science, 68(9), 2997–3010. https://doi.org/10.2527/1990.6892997x
- Pan, C. C., Kavanagh, B. D., Dawson, L. A., Li, X. A., Das, S. K., Miften, M., & Ten Haken, R. (2010). K radiation-associated liver injury. International Journal of Radiation Oncology, Biology, Physics, 76(3), S94–S100. https://doi.org/10.1016/j.ijrobp.2009.06.092
- Racanelli, V., & Rehermann, B. (2006). The liver as an immunological organ. Hepatology, 43(S1), S54–S62. https://doi.org/10.1002/hep.21060
- Rupaimoole, R., & Slack, F. J. (2017). MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nature Reviews Drug Discovery, 16(3), 203–222. https://doi.org/10.1038/nrd.2016.246
- Sanjay, S., & Girish, C. (2017). Role of miRNA and its potential as a novel diagnostic biomarker in drug-induced liver injury. European Journal of Pharmacology, 73(4), 399–407.
- Shi, J., Ma, X., Su, Y., Song, Y., Tian, Y., Yuan, S., Zhang, X., Yang, D., Zhang, H., Shuai, J., Cui, W., Ren, F., Plikus, M. V., Chen, Y., Luo, J., & Yu, Z. (2018). MiR-31 mediates inflammatory signaling to promote re-epithelialization during skin wound healing. Journal of Investigative Dermatology, 138(10), 2253–2263. https://doi.org/10.1016/j.jid.2018.03.1521
- Spahn, M., Kneitz, S., Scholz, C. J., Stenger, N., Rüdiger, T., Ströbel, P., Riedmiller, H., & Kneitz, B. (2010). Expression of microRNA-221 is progressively reduced in aggressive prostate cancer and metastasis and predicts clinical recurrence. International Journal of Cancer, 127(2), 394–403.
- Tian, Y., Xu, J., Li, Y., Zhao, R., Du, S., Lv, C., Wu, W., Liu, R., Sheng, X., Song, Y., Bi, X., Li, G., Li, M., Wu, X., Lou, P., You, H., Cui, W., Sun, J., Shuai, J., … Yu, Z. (2019). MicroRNA-31 reduces inflammatory signaling and promotes regeneration in colon epithelium, and delivery of mimics in microspheres reduces colitis in mice. Gastroenterology, 156(8), 2281–2296. https://doi.org/10.1053/j.gastro.2019.02.023
- Tsai, W. C., Hsu, S. D., Hsu, C. S., Lai, T. C., Chen, S. J., Shen, R., Huang, Y., Chen, H. C., Lee, C. H., Tsai, T. F., Hsu, M. T., Wu, J. C., Huang, H. D., Shiao, M. S., Hsiao, M., & Tsou, A. P. (2012). MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. Journal Of Clinical Investigation, 122(8), 2884–2897. https://doi.org/10.1172/JCI63455
- Wang, Y., Yang, H. M., Cao, W., Li, Y. B., & Wang, Z. Y. (2018). Deep sequencing identification of miRNAs in pigeon ovaries illuminated with monochromatic light. BMC Genomics, 19(1), 446–454. https://doi.org/10.1186/s12864-018-4831-6
- Wen, M., Shen, Y., Shi, S., & Tang, T. (2012). miREvo: An integrative microRNA evolutionary analysis platform for next-generation sequencing experiments. BMC Bioinformatics, 13(1), 140–150. https://doi.org/10.1186/1471-2105-13-140
- Xu, H., He, J. H., Xiao, Z. D., Zhang, Q. Q., Chen, Y. Q., Zhou, H., & Qu, L. H. (2010). Liver-enriched transcription factors regulate microRNA-122 that targets CUTL1 during liver development. Hepatology, 52(4), 1431–1442. https://doi.org/10.1002/hep.23818
- Xu, Q., Guo, J., Li, X., Wang, Y., Wang, D., Xiao, K., Zhu, H., Wang, X., Hu, C. A., Zhang, G., & Liu, Y. (2021). Necroptosis underlies hepatic damage in a piglet model of lipopolysaccharide-induced sepsis. Frontiers in Immunology, 12, Article 633830. https://doi.org/10.3389/fimmu.2021.633830.eCollection2021
- Young, M. D., Wakefield, M. J., Smyth, G. K., & Oshlack, A. (2010). Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biology, 11(R14), https://doi.org/10.1186/gb-2010-11-2-r14,2010.
- Zhang, D. D., Wang, D. D., Wang, Z., Wang, Y. B., Li, G. X., Sun, G. R., Tian, Y. D., Han, R. L., Li, Z. J., Jiang, R. R., Liu, X. J., Kang, X. T., & Li, H. (2020). Estrogen abolishes the repression role of gga-miR-221-5p targeting ELOVL6 and SQLE to promote lipid synthesis in chicken liver. International Journal of Molecular Sciences, 21(5), 1624. https://doi.org/10.3390/ijms21051624
- Zhang, J., Fu, S. L., Liu, Y., Liu, Y. L., & Wang, W. J. (2015). Analysis of microRNA expression profiles in weaned pig skeletal muscle after lipopolysaccharide challenge. International Journal of Molecular Sciences, 16(9), 22438–22455. https://doi.org/10.3390/ijms160922438
- Zheng, J., Zhou, H., Yang, T., Liu, J., Qin, T., Gu, X., Wu, J., Zhang, Y., Wang, H., Tang, Y., Xue, F., Mao, Y., & Xia, Q. (2021). Protective role of microrna-31 in Acetaminophen-induced liver injury: A negative regulator of c-jun n-terminal kinase (jnk) signaling pathway. Cellular and Molecular Gastroenterology and Hepatology, 12(5), 1789–1807. https://doi.org/10.1016/j.jcmgh.2021.07.011