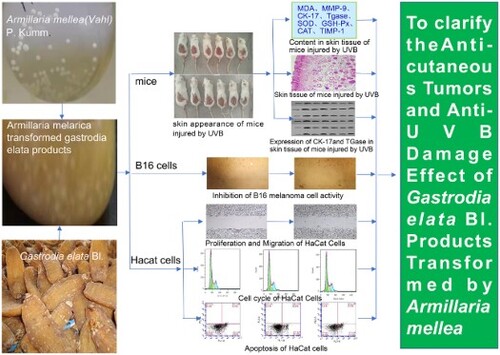
ABSTRACT
Skin cancer is a malignant tumour that can spread in the skin and is a serious threat to human health. Excessive ultraviolet radiation is one of the most common factors that leads to skin cancer. The purpose of this study was to evaluate the anti-skin tumour and anti-UVB damage effects of Armillaria mellea (Vahl) P. Kumm transformed Gastrodia elata Bl products (ATGP). Gastrodia elata Blume f.glauca S. Chows from Changbai Mountain and 4 Armillaria mellea (Vahl) P. Kumm strains were used as experimental materials. First, the optimum fungal strains of Armillaria mellea used to transform gastrodin were screened. Then, the MTT assay and scratch test proved that ATGP had anti-B16 melanoma cell activity in vitro. Furthermore, the anti-UVB damage effect of ATGP was proven by a UVB damage mouse skin model and HaCat photoaging model, and its mechanism was preliminarily discussed.
GRAPHICAL ABSTRACT
1. Introduction
Skin, as the largest organ of the human body, is in direct contact with the outside world and is the first barrier that protects the body from the environment. Skin is also one of the main targeted organs of ultraviolet (UV) radiation (Lan et al., Citation2019). UVB radiation is the main band of UV rays that reaches the skin to cause damage, and most of this radiation is absorbed by keratinocytes causing oxidative stress, which results in cell damage and, subsequently, cell death (Perde-Schrepler et al., Citation2013). UV irradiation has the effect of sterilization and promotes the synthesis of vitamin D while protecting against rickets and osteoporosis. However, excessive UV radiation penetrates the skin and produces reactive oxygen species (ROS) (Ahn et al., Citation2020) in cells. ROS causes cellular oxidative stress and prolonged cutaneous inflammation (Subedi et al., Citation2017). Light-damaged skin is characterized by loss of tone and elasticity, roughness, dryness, hyperpigmentation, red spots, and the formation of deep skin wrinkles (Jang & Kim, Citation2021). Skin cancer, mostly occurring on the face and on skin of limbs exposed to the sun for a long time, is one of the most common cancers seriously affecting public health and increasing the burden of medical care costs, and it is still on the rise worldwide. Through a large number of experimental studies at home and abroad, the International Agency for Research on Cancer has confirmed that UVR is a complete carcinogen (Gallagher & Lee, Citation2006; Guerra et al., Citation2021). It has been proven that excessive UV radiation is one of the most common factors which induces skin cancer, so preventing and delaying skin photosenescence caused by UV radiation has become a hot spot in medical research.
At present, most of the drugs applied in the clinical treatment of skin photoaging are antioxidant and anti-inflammatory drugs, such as all-trans retinoic acid, which can improve the skin roughness caused by long-term UV radiation (Griffiths, Citation2001); it can further reduce damage to skin and cutaneous inflammation (Lowe et al., Citation2004) but confers moderately serious side effects and comes with a risk of irritation. In addition, local application of nonsterol drugs has been reported to alleviate the damage caused by skin photoaging (Antoniou et al., Citation2010). Using aliphatic hydroxy acid and aspirin can treat the thickening of skin keratin, epidermal hyperplasia, and epidermal pigment deposition (Phillips, Citation2004; Röck et al., Citation2012). Although these drugs prevent wrinkles to a certain extent and have a certain anti-photoaging effect, these chemosynthetic drugs have extensive side effects and cause strong irritation, as well as addiction. The toxic and side effects of traditional Chinese medicine were small. Compared with synthetic compounds, the chemical components extracted from plants are much less harmful to the human body (Ahn et al., Citation2021; Wang et al., Citation2020). Therefore, the study of the antitumour and anti-UV effects of Chinese herbal medicine has become a hot topic (Kim et al., Citation2021).
Regarding plant-based, antitumour drugs, Xu Ruiqi, Hu Yi, Cenariu Diana et al. found that they could inhibit the occurrence of melanoma through the study of Cornus officinalis leaves, licorice, and lycium barbarum (Cenariu et al., Citation2021; Hu et al., Citation2022; Xu et al., Citation2021). Coumarin extracted from Cachrys species by Marrelli Mariangela has been shown to have photocytotoxic properties, and oleic acid, high-vanillin, and hydroxytyroalcohol from olive oil compounds by Brito Cheila have cytotoxic potential on melanoma cells (Brito et al., Citation2021; Marrelli et al., Citation2021). Zeyuan Liu et al. found that gastrodin in Gastrodia elata can improve the immunological response mediated by CD8+ T cells and improve the protection against tumour-attacking animals; moreover, it is a potential adjuvant in the immunological regulation of anti-melanoma cells (Liu et al., Citation2019). Hou Jiguang et al. found that curcumin in turmeric blocked the effect of UVB by reducing ROS and apoptosis and reversing the expressional changes of UVB-induced, apoptotic proteins (Hou et al., Citation2020). Traditionally, Chinese medicine has been proven to have antiradiation, antitumour, and anti-UV effects.
Gastrodia elata is a valuable traditional Chinese medicine with many pharmacological effects (Wang et al., Citation2021; Zhou et al., Citation2021) and high medicinal and nutritional value. Relevant studies have shown that Gastrodia elata cannot grow without the assistance of microorganisms, including Armillaria mellea and germinating bacteria. Compared with plant cell culture or plant culture, microbial transformation is more suitable for industrial production of gastrodin because of its shorter fermentation cycle, simple medium, and easy-to-master fermentation technology. Song Shengnan, Ji Qing, Gao Yugang et al. studied the transformation of ginseng by Bacillus polymyxis. The content of effective components of ginseng increased significantly. In addition, pharmacodynamic experiments showed that the transformed products had anti-fatigue effects and enhanced immunity and antitumour activity (Ji et al., Citation2015). However, content changes in medicinal components in Gastrodia elata are transformed by different Armillaria mellea. The content of Gastrodia elata products transformed by Armillaria mellea and its anti-skin tumour and anti-UVB damage effects has not been reported.
Therefore, in this study, Gastrodia elata and four kinds of Armillaria mellea were used as experimental materials. By HPLC technology, the contents of gastrodin and p-hydroxybenzyl alcohol of the four kinds of Armillaria-mellea transformed products were used as evaluative indices to screen out the optimal transformative bacterial strains. MTT assay and scratch testing were used to evaluate the anti-B16 melanoma cell activity of ATGP in vitro. In vitro cell and UVB damage HaCat light-aging model mice experiments were performed with HaCat cell proliferation, IL 6, TNF alpha, cell cycle, and cell apoptosis as evaluative indices. ATGP antitumour and anti-UVB damage effects were evaluated, and a preliminary study of the mechanisms was performed to find effective antitumour and anti-UVR drugs. Resource development and utilization on Gastrodia elata were performed to provide a theoretical basis and reference in improving its efficacy for the promotion of human health.
2 Materials and method
2.1. Gastrodia elata and Armillaria
Gastrodia elata Bl. f. glauca of Changbai Mountain is a manufactured product of origin provided by Jingzhen Gastrodia Development Co., LTD. Four Armillaria gallica Marxm.& Romagn. DNA ITS sequences (accession numbers: MW404994, MW404995, MW404996, MW404997) were registered in the NCBI database. One of the strains was isolated, identified, preserved in our laboratory and identified as Armillaria Gaul by the China General Microbial Conservation and Management Center. The strain was stored in the Microbial Conservation and Management Center NO. AM-JY-GYG1.
2.2. Primary instrument
Full-wavelength microplate reader (Infinite Pro 200) TECAN, medical X-ray film (X-OMAT BT) Kodak, flow cytometry (Guava@easyCyte) Merck, inverted microscope (XSB-1A) FACTORY ADJUSTMENT.
2.3. Mice
Healthy Kunming (ICR) male mice weighing 18–22 g were purchased from Changchun YI Si Experimental Animal Technology Co., Ltd. (animal license number: SCIXK(JI)-2020-0002). All rats were fed by conventional methods, monitored, and documented. All experiments were carried out in accordance with the Guide for Animal Experimentation of Jilin Agricultural University. The agreement was approved by the Institutional Animal Ethics and Use Committee of Jilin Agricultural University.
2.4. Cell culture
B16 melanoma cells (batch number: BNCC101685) were obtained from Beina Chuanglian Biotechnology Co., Ltd. Human skin keratinocyte HaCaT (batch number: BNCC102169) BeiNa Chuanglian Biotechnology Co, Ltd.
2.5. Preparation of ATGP
The four cryopreserved Armillaria strains were inoculated into PDA solid medium and cultured in a constant temperature incubator at 25°C for two weeks. Armillaria blocks with good growth status were selected and inoculated into PDB liquid medium and subcultured once every 7 days in a shaker at 25°C and 110 r/min. Through microplate reader detection, Armillaria with an OD value of approximately 0.5 was used as the seed solution. Gastrodia powder was obtained by grinding 720 g of gastrodia through a 60 mesh (0.25 mm) sieve and mixing. The transformational process of each Armillaria was as follows: three parts of gastrodia powder, each 40 g, were accurately weighed and wrapped in aluminum foil and subsequently put into an oven at 80°C for dry heat sterilization for 3 h; the sterilized gastrodia powder was then placed into a 500 ml triangular bottle on a sterile operating table. A sterile pipette was used to add 160 ml of four kinds of Armillaria seed solution with an OD value of 0.5 into a triangular bottle, which was then placed in a shaker at 25°C and 110 r/min for coculture. After 7 days of culture, the products were dried at 60°C and ground into powder to form ATGP. The sterile transformed control group was transformed by the same method as Armillaria, but without Armillaria inoculation, which was replaced by sterile PDB medium.
2.6. Optimization of the strains transformed by Armillaria into Gastrodin and p-hydroxybenzyl alcohol
For the sterile transformed control group, the ATGP of four Armillaria strains and Gastrodia powder in the control group were 2 g each. Gastrodin and p-hydroxybenzyl alcohol were measured, and each group was repeated three times. Using HPLC technology, the best transformed bacterial strains were screened out using the effective components of gastrodin and p-hydroxybenzyl alcohol in the products of the four Armillaria mellea-transformed Gastrodia elata as evaluative indicators.
2.7. Effect of ATGP on the inhibition of B16 melanoma cell activity
Set of Dacarbazine group, Gastrodia elata group, JMGL-3 ATGP group and control group. ATGP with 50% diluted ethanol extraction and preparation of samples. Dacarbazine and ATGP were dissolved in DMEM and diluted to 50, 100, 200, 400, and 800 µg/mL for culture drug cultivation of B16 melanoma cells and counting.
2.7.1. MTT assay
Viability was measured by the 3-[4,5-dimethyltiazol2yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. The optical density (OD) value of each well at 490 nm was then evaluated using a microplate reader (TECAN Infinite Pro 200).
2.7.2. Scratch test
B16 melanoma cells were seeded into a 96-well plate at 1 × 105 cells/well. Upon achieving approximately 70% confluence, the cells were vertically and linearly scratched with a 10 μL micropipette in a 96-well plate. The cells were then washed with PBS solution to remove floating cells. After rinsing each well with PBS, 1 mL of 1% fetal bovine serum culture solution was added to each well, and the cells were incubated at 37°C and 5% CO2 in an incubator. After 24 h, the cells were observed and photographed with three replicates in each group.
2.8. Study on the anti-ultraviolet effect of ATGP
2.8.1. Experimental, animal grouping
Female Kunming mice aged 5 weeks were randomly divided into 6 groups (n = 6/group) as follows: I: control group, II: model group, III: dacarbazine group, IV, V, VI: JMGL-3 ATGP group (high, medium, and low doses), and VII: Gastrodia elata group (medium dose). Mice in the control group and model group were daubed with distilled water (0.4 mL/d). The dacarbazine group was daubed with dacarbazine (2 mg//0.4 ml/d); the JMGL-3 ATGP groups included a low-dose group (2.88 mg/0.4 mL/d), medium-dose group (8.64 mg/0.4 mL/d), and high-dose group (25.95 mg/0.4 mL/d); and Gastrodia elata was the medium-dose group (8.64 mg//0.4 ml/d). Except for the control group, animals in all light groups were placed in cages, and each group was given medicine 15 min in advance according to the above dose of UV irradiation. The dorsal hairs of the mice were shaved 3 days before UVB exposure. The source of UVB was a UVB Display Lamp (HR40 W, Nanjing Huaqiang Electronics Co, LTD), and UVB irradiation exposure was applied at 0.56 w/cm2 for 2 h for 14 days.
2.8.2. Skin histology
Throughout the experimental period, the body weight of the mice was measured every two days, and changes in the dorsal skin were observed and recorded. At the end of 14 days, the mice were sacrificed by cervical dislocation. The full-thickness skin of the depilated area was cut from the lower back by UVB irradiation, and part of the sample was fixed with 4% paraformaldehyde solution. The other part of the sample was frozen and stored in a refrigerator at – 20°C for measurement. First, 0.8 cm × 0.8 cm tissue blocks were removed from the fixation solution for embedding and cut into 5 μm paraffin sections. The sections were stained with hematoxylin and eosin and dehydrated with serial ethanol. Finally, the stained sections were sealed with neutral resin to observe the changes in pathological tissue structure in each group under a light microscope, and the changes were photographed and marked.
2.8.3. Assessment of the oxidative stress parameters
In this study, antioxidant components in skin tissue samples were analyzed. The skin tissue was homogenized by an automatic homogenizer. During the preparation, a part of the skin tissue was homogenized in ninefold (w/v) cold normal saline and centrifuged at 3000 rpm at 4°C for 15 min. The supernatant was used for the malonaldehyde (MDA), glutathione (GSH), catalase (CAT), and superoxide dismutase (SOD) assays, which were reflected as common indices of the antioxidant status of tissues; all of these indices were measured by using commercial, reagent kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer’s instructions.
2.8.4. Determination of TIMP-1 and MMP-9 in skin tissue
The skin tissue homogenate centrifugal supernatant was used for the TIMP-1 and MMP-9 assays, and all of these indices were measured by using TIMP-1 (CUSABIO) and MMP-9 (CUSABIO) reagent kits according to the manufacturer’s instructions.
2.8.5. The degree of CK-17 and TGase expression in each tissue
Cells were washed with PBS, harvested, and then lysed for total protein extraction. The extracted proteins were electrophoresed using an SDS polyacrylamide gel and then transferred onto a PVDF membrane (IPVH00010, Millipore, US). The membranes were blocked with 2% BSA for 1 hr and then incubated with primary antibodies against CK 17 (ab109725; Abcam 1:1000) and TGase (bs-6690r; Beijing Bo Olsen 1:200) at 4°C overnight. After washing with PBST, the membrane was incubated with secondary antibody (zb-2301; Beijing Chinese fir 1:3000). The membrane was gently stirred for 60 min at room temperature without light. Then, TBST was used to remove the working solution of the secondary antibody. Finally, ImageJ software was used to analyze the gray value after exposure and washing. The method of TGase was the same except that the primary antibody was different.
2.9. Study on the effect of ATGP on the proliferation of HaCaT cells damaged by UVB irradiation
2.9.1. Sample solution preparation and grouping
The dacarbazine group, Gastrodia elata group, and JMGL-3 ATGP were dissolved in DMEM to prepare a culture medium containing each drug at a final concentration of 100 µg/mL. They were divided into 5 groups: the control group, model group, dacarbazine group, Gastrodia elata group, and JMGL-3 ATGP. The peak value of the UVB tube was 312 nm, 9 W, 8 pieces, parallel to the top of HaCat cells, and the vertical distance between the cells and the light source was 15 cm after the lamp was turned on for 10 min. Except for the normal control group, all other groups were irradiated with UVB at a dose of 30 mJ/cm2, radiation dose (mJ/cm2) = radiation intensity (mW/cm2) × radiation time (S).
2.9.2. MTT assay
Viability was measured by the 3-[4,5-dimethyltiazol2yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. The OD value of each well at 490 nm was then evaluated using a microplate reader (TECAN Infinite Pro 200).
2.9.3. Scratch test
HaCat cells were seeded into a 96-well plate at 1 × 105 cells/well. Upon achieving approximately 70% confluence, the cells were vertically and linearly scratched with a 10 μL micropipette in a 96-well plate. The cells were then washed with PBS solution to remove floating cells. After rinsing each well with PBS, medium with a working concentration in each treatment group was added after imaging. After 24 h, the cells were observed and photographed with three replicates in each group.
2.9.4. Determination of associated inflammatory molecules
The cell culture medium of each sample was collected and centrifuged at 3000 rpm at 4°C for approximately 20 min, and the supernatant was collected. The supernatant levels of IL-6 (built in Nanjing) and TNF-α (Shanghai Xinran Biotechnology Co., Ltd.) were measured by ELISA kits according to the manufacturer’s instructions.
2.9.5. Flow cytometry
Two milliliters of HaCaT cell suspension (1 × 106 cells/mL) was inoculated into a 6-well plate and cultured at 37°C in a CO2 incubator. After 70% HaCaT cells adhered to the wall, 2 mL medium containing the final concentration of (100 µg/mL) dacarbazine, JMGL-3 ATGP, and Gastrodia elata solution were added, and the blank control group was a complete medium without drug solution. This process was repeated three times for each group. Cells were analyzed by flow cytometry for apoptosis/cycle assays.
2.10. Statistical analysis
IBM SPSS Statistics 23 was used for one-way analysis of variance. Methodology includes inputting the obtained data into “factors” and “dependent variables” according to groups. Click Compare Means in the menu bar Analysis, and from there, click Single Factor ANOVA. Add the different variables to the List of Dependent Variables and Factors areas, and then click the Compare pairwise button. In the pairwise comparison window, the method of “S‒N-K (S), LSD(L), Duncan(D), significance level is 0.05” was selected to analyze the significance. Select descriptive, statistical means and standard deviations from the options.
3. Results
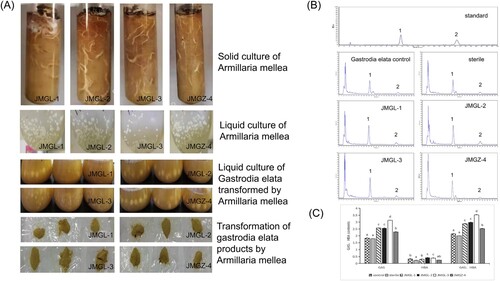
3.1. Transformation of Armillaria-mellea products from Gastrodia elata
Four strains of Armelaria mellea could grow well in the same liquid medium and solid medium, and there was no significant difference among the strains. They could all grow well in the liquid medium of transformed Gastrodia elata, which ensured the feasibility of the Armellea mellea-transformation experiment. (A) The four strains had significant effects on the contents of gastrodin and p-hydroxybenzyl alcohol in the products of Gastrodia elata transformed by them (P < 0.05) among which strain JMGL-3 had the strongest transformative effect, and the additive value of gastrodin and p-hydroxybenzyl alcohol in transformed Gastrodia elata products was as high as 0.35% (P < 0.05) making it an ideal transformative bacterium (B and C).
Figure 1. (A) Culture of Armillaria mellea and transformation of Gastrodia elata Bl. Products. (B) HPLC chromatogram of gastrodin in gastrodia elata products transformed by Armillaria mellea (1) gastrodin (GAS). (2) p-hydroxybenzyl alcohol (HBA). (C) Different letters indicate significant differences (P < 0.05).
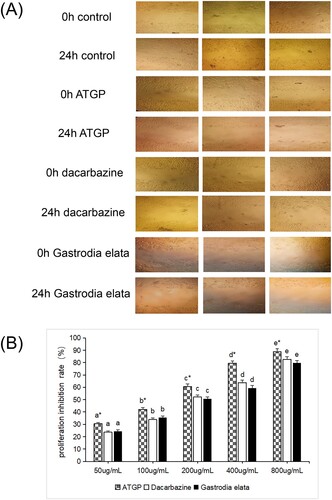
3.2. Effects of ATGP on proliferation and migration of B16 cells
A scratch test was conducted to elucidate the effects of ATGP on migration in B16 melanoma cells. The results showed that the ATGP group, dacarbazine group, and gastrodia elata group showed a significant decline in B16 melanoma cell migration. After 24 h of culture, compared with the control group, cell migration and invasion were significantly inhibited, and the scratch healing rate was significantly lower than that of the control group. The migration ability of B16 melanoma cells was inhibited most obviously in the ATGP group. Meanwhile, the MTT assay was performed to further investigate the viability of B16 melanoma cells. The proliferation of B16 melanoma cells was significantly inhibited in a dose-dependent manner in the ATGP group, dacarbazine group, and Gastrodia elata group (P < 0.05). Under the same concentration conditions, the anti-B16 melanoma cell effect of the ATGP group was significantly stronger than that of the dacarbazine and Gastrodia elata groups (P < 0.05), and the Gastrodia elata group and dacarbazine group had the same effect against B16 melanoma cells (P > 0.05). The results showed that the anti-B16 melanoma cell effect of the ATGP group was significantly stronger than that of the dacarbazine group and the Gastrodia elata group (P < 0.05) (B).
Figure 2. (A) Effect of ATGP on the scratch healing of B16 melanoma cells (400×). (B) Effect of ATGP on the proliferation inhibition rate of B16 melanoma cells (%) The values are presented as the mean ± SEM (n = 3). The IC50 values of dacarbazine, ATGP and gastrodia elata on the proliferation inhibition of B16 melanoma cells were 189.01, 125.62 and 200.51 g/mL, respectively. In the same drug treatment group, the difference in letters between different concentrations was significant (P < 0.05), and there was no significant difference in the same letter (P > 0.05). * indicates that there was a significant difference between the same concentration and different drug treatment groups (P < 0.05).
3.3. Effects of ATGP on UVB-damaged mice
3.3.1. Effects of ATGP on skin appearance and skin tissue of UVB-damaged mice
The ATGP group (high dose, medium dose) and Gastrodia elata group (medium dose) protected the skin of UVB ray-injured mice and significantly improved the appearance of the UVB light injury mouse aging state. UVB damage in mouse skin roughness, desquamation, and wrinkling symptoms improved markedly. There was no significant difference in the body weight of UVB-injured mice at the same time among all groups (P < 0.05). Dacarbazine group, ATGP group, and Gastrodia elata group (medium dose) skin structure was relatively intact; the corneous layer was slightly thickened; and each cell layer boundary was analyzed. ATGP group (high dose, medium dose) and Gastrodia elata group (medium dose) skin structure, compared with the model group and dacarbazine group, had different degrees of repair and improvement. The corneous layer thickened, Angle phenomenon all had different degrees of improvement, epidermis thickness decreased, and the dermis fibrous tissue distribution had uniformity. Inflammatory cell infiltration was significantly improved (B).
Figure 3. (A) Effect of ATGP on the skin appearance of mice injured by UV radiation. (B) Effects of ATGP on the skin tissue of mice injured by UV radiation (100×). (C) Effect of ATGP on MDA, GSH-Px, CAT, and SOD activity in skin tissue of mice injured by UV radiation. (D) Effect of ATGP on the expression of TIMP-1 and MMP-9 in the skin of mice injured by UV radiation. (E) Effect of ATGP on the expression of CK-17 and TGase in skin tissue of mice injured by UV radiation. The values are presented as the mean ± SEM (n = 6). Different letters indicate significant differences (P < 0.05); the same letters indicate no significant difference (P > 0.05); a was the control group; b was the model group; c was the dacarzine group; d e f was the ATGP (low-dose group, medium-dose group, high-dose group); and g was the Gastrodia elata group.
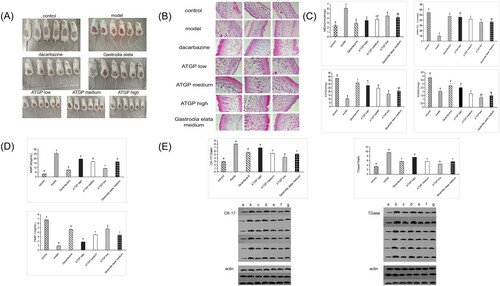
3.3.2. Effects of ATGP on MDA, SOD, GSH-Px, CAT, TIMP-1, MMP-9, CK-17, and TGase in mouse skin tissue
Both ATGP and Gastrodia elata significantly decreased MDA content, MMP-9 content, CK-17 expression, and TGase expression in UVB-damaged mouse skin tissues (P < 0.05). The activity of SOD, GSH-Px, and CAT and the expression of TIMP-1 in UVB-irradiated mice were significantly increased (P < 0.05). A high dose of ATGP had a better anti-UVB damage effect than Gastrodia elata (C–E).
3.4. Effects of ATGP on UVB-irradiated HaCaT cells
3.4.1. Effect of ATGP on the proliferation and migration of HaCaT cells damaged by UVB
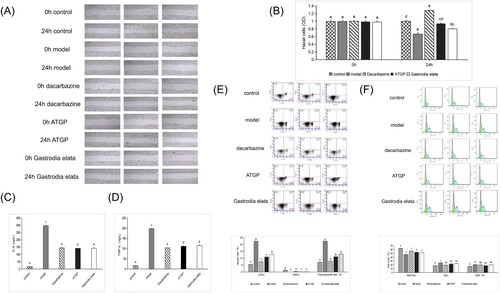
The effect of ATGP on the migration of UVB-damaged HaCaT cells was studied by scratch experiments. The results showed that, compared with the model group, the dacarbazine group, ATGP group, and Armellea elata group had obvious scratch healing, scratch narrowing, cell proliferation, and healing effects: ATGP group > dacarbazine group > Gastrodia elata group > model group. ATGP effectively promoted the healing of UVB-damaged HaCaT cell scratches. (A) Meanwhile, compared with the model group at 24 h, the OD value of cells in the dacarbazine group, ATGP group, and Gastrodia elata group increased significantly (P < 0.05).
Figure 4. (A) Effect of ATGP on the scratch healing of HaCaT cells damaged by UVB (400×). (B) Effect of ATGP on the OD of HaCaT cells damaged by UVB. (C) and (D) Effects of Gastrodia elata products transformed by Armillaria mellea on UVB-damaged HaCat cytokines IL-6 and TNF-α. The values are presented as the mean ± SEM (n = 5). (E) Effect of ATGP on apoptosis of HaCaT cells damaged by UVB. (F) Effects of ATGP on UVB-damaged HaCaT cell cycle (%). The values are presented as the mean ± SEM (n = 3). Different letters indicate significant differences (P < 0.05), and the same letters indicate no significant difference (P > 0.05).
3.4.2. Effects of ATGP on UV-induced damage to the HaCaT cytokines IL-6 and TNF-α
Compared with the model group, the levels of the HaCaT cytokines IL-6 and TNF-α were significantly decreased in the dacarbazine group, ATGP group, and Gastrodia elata group (P < 0.05) (C, D) indicating that both ATGP and Gastrodia elata could inhibit the release of the HaCaT cytokines IL-6 and TNF-α by UVB damage.
3.4.3. Effects of ATGP on the UVB-induced cell cycle and apoptosis in HaCaT cells
Compared with the damaged model group, the cell cycle of UVB-damaged HaCaT cells in the dacarbazine group, ATGP group, and Gastrodia elata group was significantly increased in the G0/G1 phase (P < 0.05); the cell proportion of S and G2/M phase was significantly decreased (P < 0.05); the early apoptotic rate and total apoptotic rate of cells in the dacarbazine group, ATGP group, and Gastrodia elata group were significantly decreased (P < 0.05) (E and F) indicating that the dacarbazine group, ATGP group, and Gastrodia elata group increased the ratio of G0/G1 phase of UVB damage to HaCaT cells. All of them were resistant to UVB damage to HaCaT cells. Both the ATGP group and Gastrodia elata group had protective effects on the apoptosis of UVB-damaged HaCaT cells, and ATGP group had a better effect on reducing the apoptosis of UVB-damaged cells.
4. Discussion
Gastrodia elata is a valuable medicinal herb in China that exerts antitumor, anti-insomnia, anti-radiation, antioxidant, and anti-inflammation effects mainly through gastrodin and p-hydroxybenzyl alcohol (Lin et al., Citation2021; Zhan et al., Citation2016). Because gastrodia elata has few side effects and definite efficacy, it is welcomed by the medical market. Studies have found that with traditional Chinese medicine as a substrate, Trichoderma harziae (Yue et al., Citation2014), Aspergillus niger (Kouipou Toghueo et al., Citation2021), Penicillium fermentans (Li et al., Citation2006), Lactobacillus fermentans (Her et al., Citation2020) and other bacteria can be used as transformative vectors to modify and transform traditional Chinese medicine substrates by using microbes or whole cells secreting enzymes and can obtain a higher content of targeted transformation products. Bai et al. constructed a heterologous pathway for gastrodin biosynthesis (Bai et al., Citation2016). In Escherichia coli, 4-hydroxybenzoic acid was converted to gastrodin using Nocardia carboxylic acid reductase, endogenous alcohol dehydrogenase, and salidrosea glycosyltransferase UGT73B6. Hongli ZHU et al., using Chinese Rhizopus SAITO AS3.1165, were able to convert p-hydroxybenzaldehyde to gastrodin (Zhu et al., Citation2010). Fan et al. screened Aspergillus foetidus ZU-G1 and Penicillium cyclopium AS 3.4513, which could synthesize gastrodin using a 4-hydroxybenzyl alcohol AS substrate (Fan et al., Citation2013). This study demonstrated that the transformation of Gastrodia elata by Armillaria increased the contents of gastrodin and p-hydroxybenzyl alcohol and selected Armillaria strains of transformed gastrodin and p-hydroxybenzyl alcohol, which provided a theoretical basis for the biotransformation of gastrodin and p-hydroxybenzyl alcohol. However, the specific conversion pathways of gastrodin and p-hydroxybenzyl alcohol by Armillaria are still unclear. The biosynthetic pathways and key enzymatic genes of gastrodin and p-hydroxybenzyl alcohol by Armillaria need to be further explored, and methods of improving the conversion efficiency and its regulation needs to be further studied.
UVB is the main band that causes light damage to skin. Under the induction of UVB, the skin secretes ROS, which leads to oxidative stress, DNA damage, and the release of a variety of inflammatory factors (Terazawa & Imokawa, Citation2018); collectively, these effects lead to the occurrence of an inflammatory response. This response leads to apoptosis and cell cycle disturbance and affects the production and breakdown of the extracellular matrix and collagen. When mice are exposed to UV light, the back skin exhibits erythema, pigmentation, roughness, darkening, traumatic scabbing, and other photoaging phenomena, which are mainly caused by the degeneration of elastic fibers and collagen fibers in the dermis after exposure to UV light. UV irradiation indirectly induces changes in the content of MMPs by affecting the release of inflammatory factors, and the decrease and increase in TIMP-1 and MMP-9 cause photoaging phenomena (Park et al., Citation2021; Wang & Bi, Citation2006). UV irradiation can cause intracellular lipid peroxidation to produce MDA, which damages cellular function and structure. Under normal physiological conditions, ROS produced by cells can be eliminated by superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) in vivo. UVB irradiation can cause cells to secrete ROS, inhibit the production of antioxidant enzymes, and disrupt the balance of ROS in the body (Yong & Ahn, Citation2018). In vivo, ATGP inhibited the production of matrix metalloproteinases and the degradation of collagen. Moreover, ATGP has a good antioxidant capacity and can effectively improve the antioxidant system. After treatment, the accumulation of ROS and the expression of keratin (CK-17) and transglutaminase (TGase) were decreased. This is an effective way to reduce skin damage caused by exposure to UVB. ATGP improved and repaired the skin tissue of UV-irradiated mice, which may be achieved by promoting skin cell proliferation, wound healing, and tissue regeneration and differentiation. In vitro, ATGP showed the best inhibitory effect on the proliferation of melanoma cells. At the same time, the ability of B16 melanoma cells to metastasize was inhibited. It can promote the proliferation of HaCaT cells, promote the wound healing of HaCaT cells damaged by UVB, inhibit the release of inflammatory factors, and inhibit apoptosis. We hypothesized that ATGP may achieve its anti-UVB effect by promoting cell proliferation and reducing the apoptotic rate and inflammatory response of UVB-damaged cells. This study demonstrated that ATGP has stronger anti-UVB damage and anti-melanoma effects than the original Rhizoma gastrodia and preliminarily elucidated its mechanistic action, which provides a theoretical basis for ATGP to be an effective antitumor and anti-UV drug. However, other mechanisms of ATGP’s action against UVB damage and its induced melanoma need further investigation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahn, J. H., Kim, D. W., Park, C. W., Kim, B., Sim, H., Kim, H. S., Lee, T. K., Lee, J. C., Yang, G. E., Her, Y., Park, J. H., Sim, T. H., Lee, H. S., & Won, M. H. (2020). Laminarin attenuates ultraviolet-induced skin damage by reducing superoxide anion levels and increasing endogenous antioxidants in the dorsal skin of mice. Marine Drugs, 18(7), 345. https://doi.org/10.3390/md18070345
- Ahn, S. G., Simu, S. Y., Yang, D. C., Jang, M., & Um, B. H. (2021). Effects of Ginsenoside Rf on dextran sodium sulfate-induced colitis in mice. Food and Agricultural Immunology, 32(1), 360–372. https://doi.org/10.1080/09540105.2021.1950128
- Antoniou, C., Kosmadaki, M. G., Stratigos, A. J., & Katsambas, A. D. (2010). Photoaging: prevention and topical treatments. American Journal of Clinical Dermatology, 11(2), 95–102. https://doi.org/10.2165/11530210-000000000-00000
- Bai, Y., Yin, H., Bi, H., Zhuang, Y., Liu, T., & Ma, Y. (2016). De novo biosynthesis of Gastrodin in Escherichia coli. Metabolic Engineering, 35, 138–147. https://doi.org/10.1016/j.ymben.2016.01.002
- Brito, C., Tomás, A., Silva, S., Bronze, M. R., Serra, A. T., & Pojo, M. (2021). The impact of olive oil compounds on the metabolic reprogramming of cutaneous melanoma cell models. Molecules, 26(2), 289. https://doi.org/10.3390/molecules26020289
- Cenariu, D., Fischer-Fodor, E., Țigu, A. B., Bunea, A., Virág, P., Perde-Schrepler, M., Toma, V. A., Mocan, A., Berindan-Neagoe, I., Pintea, A., Crișan, G., Cenariu, M., & Maniu, A. (2021). Zeaxanthin-rich extract from superfood Lycium barbarum selectively modulates the cellular adhesion and MAPK signaling in melanoma versus normal skin cells In Vitro. Molecules, 26(2), 333. https://doi.org/10.3390/molecules26020333
- Fan, L., Dong, Y., Xu, T., Zhang, H., & Chen, Q. (2013). Gastrodin production from p-2-hydroxybenzyl alcohol through biotransformation by cultured cells of Aspergillus foetidus and Penicillium cyclopium. Applied Biochemistry and Biotechnology, 170(1), 138–148. https://doi.org/10.1007/s12010-013-0166-6
- Gallagher, R. P., & Lee, T. K. (2006). Adverse effects of ultraviolet radiation: A brief review. Progress in Biophysics and Molecular Biology, 92(1), 119–131. https://doi.org/10.1016/j.pbiomolbio.2006.02.011
- Griffiths, C. E. (2001). The role of retinoids in the prevention and repair of aged and photoaged skin. Clinical and Experimental Dermatology, 26(7), 613–618. https://doi.org/10.1046/j.1365-2230.2001.00892.x
- Guerra, K. C., Zafar, N., & Crane, J. S. (2021). Skin cancer prevention. In StatPearls. StatPearls Publishing.
- Her, Y., Lee, T. K., Ahn, J. H., Lim, S. S., Kang, B. G., Park, J. S., Kim, B., Sim, H., Lee, J. C., Kim, H. S., Sim, T. H., Lee, H. S., & Won, M. H. (2020). Chemical composition of a novel distillate from fermented mixture of nine anti-inflammatory herbs and its UVB-protective efficacy in mouse dorsal skin via attenuating collagen disruption and inflammation. Molecules, 26(1), 124. https://doi.org/10.3390/molecules26010124
- Hou, J., Fang, F., Kang, S., Wang, Z., & Yang, Y. (2020). Curcumin from Jianghuang (Rhizoma Curcumae Longae) protects against exposure to ultraviolet B by antioxidation and attenuating mitochondrion-dependent apoptosis. Journal of Traditional Chinese medicine = Chung i tsa chih ying wen pan, 40(5), 782–791. doi:10.19852/j.cnki.jtcm.2020.05.008
- Hu, Y., Wu, Y., Jiang, C., Wang, Z., Shen, C., Zhu, Z., Li, H., Zeng, Q., Xue, Y., Wang, Y., Liu, L., Yi, Y., Zhu, H., & Liu, Q. (2022). Investigative on the molecular mechanism of licorice flavonoids anti-melanoma by network pharmacology, 3D/2D-QSAR, molecular docking, and molecular dynamics simulation. Frontiers in Chemistry, 10, 843970. https://doi.org/10.3389/fchem.2022.843970
- Jang, Y. A., & Kim, B. A. (2021). Protective effect of spirulina-derived C-Phycocyanin against ultraviolet B-induced damage in HaCaT cells. Medicina, 57(3), 273. https://doi.org/10.3390/medicina57030273
- Ji, Q., Gao, Y., Zhao, Y., He, Z., Zang, P., Zhu, H., Yang, H., Li, X., & Zhang, L. (2015). Determination of ginsenosides by bacillus polymyxa conversion and evaluation on pharmacological activities of the conversion products. Process Biochemistry, 50(6), 1016–1022. https://doi.org/10.1016/j.procbio.2015.03.013
- Kim, H., Park, S. Y., & Chung, D. K. (2021). Effect of the oral administration of common evening primrose sprout (Oenothera biennis L.) extract on skin function improvement in UVB-irradiated hairless mice. Pharmaceuticals (Basel, Switzerland), 14(3), 222. https://doi.org/10.3390/ph14030222
- Kouipou Toghueo, R. M., Kemgne, E., Sahal, D., Yadav, M., Kenou Kagho, D. U., Yang, B., Baker, B. J., & Boyom, F. F. (2021). Specialized antiplasmodial secondary metabolites from Aspergillus niger 58, an endophytic fungus from Terminalia catappa. Journal of Ethnopharmacology, 269, 113672. https://doi.org/10.1016/j.jep.2020.113672
- Lan, C. E., Hung, Y. T., Fang, A. H., & Ching-Shuang, W. (2019). Effects of irradiance on UVA-induced skin aging. Journal of Dermatological Science, 94(1), 220–228. https://doi.org/10.1016/j.jdermsci.2019.03.005
- Li, L., Liu, R., Min, Y., Hu, X., Qiao, W., Bi, K., & Guo, D. (2006). Microbial metabolism of evodiamine by penicillium janthinellum and its application for metabolite identification in rat urine. Enzyme and Microbial Technology, 39(4), 561–567. https://doi.org/10.1016/j.enzmictec.2005.10.029
- Lin, Y. E., Lin, C. H., Ho, E. P., Ke, Y. C., Petridi, S., Elliott, C. J., Sheen, L. Y., & Chien, C. T. (2021). Glial Nrf2 signaling mediates the neuroprotection exerted by Gastrodia elata Blume in Lrrk2-G2019S Parkinson’s disease. eLife, 10, e73753. https://doi.org/10.7554/eLife.73753
- Liu, Z., Wang, S., Zhang, J., Wang, Y., Wang, Y., Zhang, L., Zhang, L., Li, L., Dong, J., & Wang, B. (2019). Gastrodin, a traditional Chinese medicine monomer compound, can be used as adjuvant to enhance the immunogenicity of melanoma vaccines. International Immunopharmacology, 74, 105699. https://doi.org/10.1016/j.intimp.2019.105699
- Lowe, N., Gifford, M., Tanghetti, E., Poulin, Y., Goldman, M., Tse, Y., Yamauchi, P., Rosenzweig, H., & Kang, S. (2004). Tazarotene 0.1% cream versus tretinoin 0.05% emollient cream in the treatment of photodamaged facial skin: A multicenter, double-blind, randomized, parallel-group study. Journal of Cosmetic and Laser Therapy, 6(2), 79–85. https://doi.org/10.1080/14764170410032406
- Marrelli, M., Perri, M. R., Amodeo, V., Giordano, F., Statti, G. A., Panno, M. L., & Conforti, F. (2021). Assessment of photo-induced cytotoxic activity of cachrys sicula and cachrys libanotis enriched-coumarin extracts against human melanoma cells. Plants (Basel, Switzerland), 10(1), 123. doi:10.3390/plants10010123
- Park, S. H., Kim, J. G., Jang, Y. A., Bayazid, A. B., & Lim, B. O. (2021). Fermented black rice and blueberry with Lactobacillus plantarum MG4221 improve UVB-induced skin injury. Food and Agricultural Immunology, 32(1), 499–515. https://doi.org/10.1080/09540105.2021.1967300
- Perde-Schrepler, M., Chereches, G., Brie, I., Tatomir, C., Postescu, I. D., Soran, L., & Filip, A. (2013). Grape seed extract as photochemopreventive agent against UVB-induced skin cancer. Journal of Photochemistry and Photobiology B: Biology, 118, 16–21. https://doi.org/10.1016/j.jphotobiol.2012.10.008
- Phillips, T. J. (2004). Tazarotene 0.1% cream for the treatment of photodamage. Skin Therapy Letter, 9(4), 1–2. PMID:15146262.
- Röck, K., Meusch, M., Fuchs, N., Tigges, J., Zipper, P., Fritsche, E., Krutmann, J., Homey, B., Reifenberger, J., & Fischer, J. W. (2012). Estradiol protects dermal hyaluronan/versican matrix during photoaging by release of epidermal growth factor from keratinocytes. Journal of Biological Chemistry, 287(24), 20056–20069. https://doi.org/10.1074/jbc.M112.353151
- Subedi, L., Lee, T. H., Wahedi, H. M., Baek, S. H., & Kim, S. Y. (2017). Resveratrol-enriched rice attenuates UVB-ROS-induced skin aging via downregulation of inflammatory cascades. Oxidative Medicine and Cellular Longevity, 2017, 1. https://doi.org/10.1155/2017/8379539
- Terazawa, S., & Imokawa, G. (2018). Signaling cascades activated by UVB in human melanocytes lead to the increased expression of melanocyte receptors, Endothelin B receptor and c-KIT. Photochemistry and Photobiology, 94(3), 421–431. https://doi.org/10.1111/php.12848
- Wang, G. L., Luo, Y., Yang, J. Q., Hou, C., & Li, J. K. (2020). Inhibitory effects of polyphenols-enriched extracts from Debregeasia orientalis leaf against human cervical cancer in vitro & in vivo. Food and Agricultural Immunology, 31(1), 176–192. https://doi.org/10.1080/09540105.2020.1712330
- Wang, X. Y., & Bi, Z. G. (2006). UVB-irradiated human keratinocytes and interleukin-1α indirectly increase MAP kinase/AP-1 activation and MMP-1 production in UVA-irradiated dermal fibroblasts. Chinese Medical Journal, 119(10), 827–831. https://doi.org/10.1097/00029330-200605020-00006
- Wang, Y., Zhao, L., & Li, A. Y. (2021). Gastrodin – A potential drug used for the treatment of Tourette Syndrome. Journal of Pharmacological Sciences, 145(3), 289–295. https://doi.org/10.1016/j.jphs.2021.01.005
- Xu, R., Zeng, M., Wu, Y., Wang, S., Zhang, B., Zhang, J., Kan, Y., Li, B., Cao, B., Zheng, X., & Feng, W. (2021). Acetone extract of cornus officinalis leaves exerts anti-melanoma effects via inhibiting STAT3 signaling. OncoTargets and therapy, 14, 3487–3501. https://doi.org/10.2147/OTT.S308371
- Yong, H. J., & Ahn, J. J. (2018). Anti-inflammatory effects of prunin on UVB-irradiated human keratinocytes. Biomedical Dermatology, 2(1), 14–20. https://doi.org/10.1186/s41702-018-0024-9
- Yue, S. D., Le, P. L., Yong, M. B., Ai, Y. H., Ying, Q., Zu, J. W., & Zhi, L. X. (2014). Biotransformation of geniposide in Gardenia jasminoides to genipin by Trichoderma harzianum CGMCC 2979. Chinese Journal of Catalysis, 35(9), 1534–1546. https://doi.org/10.1016/S1872-2067(14)60134-0
- Zhan, H. D., Zhou, H. Y., Sui, Y. P., Du, X. L., Wang, W. H., Dai, L., Sui, F., Huo, H. R., & Jiang, T. L. (2016). The rhizome of Gastrodia elata Blume – An ethnopharmacological review. Journal of Ethnopharmacology, 189, 361–385. https://doi.org/10.1016/j.jep.2016.06.057
- Zhou, Y., Li, M., Lv, T., Huang, M., Cheng, B., Zhang, Y., & Zhu, J. (2021). Gastrodin inhibits virus infection by promoting the production of Type I interferon. Frontiers in Pharmacology, 11, 608707. https://doi.org/10.3389/fphar.2020.608707
- Zhu, H., Dai, P., Zhang, W., Chen, E., Han, W., Chen, C., & Cui, Y. (2010). Enzymic synthesis of gastrodin through microbial transformation and purification of gastrodin biosynthesis enzyme. Biological and Pharmaceutical Bulletin, 33(10), 1680–1684. https://doi.org/10.1248/bpb.33.1680
