ABSTRACT
To study the antioxidant and immune protection activity of velvet antler (VA) in cyclophosphamide-induced immunocompromised mice, BALB/c mice were intragastrically administered 100, 300, or 500 mg/(kg·d) VA for 30 days, and were intraabdominally injected with cyclophosphamide to create a hypoimmune murine model. Cellular immunity, macrophage phagocytic activity, natural killer (NK) cell activity, white blood cell count, splenic lymphocyte subsets and the mRNA expression levels of serum cytokines were analysed. We found that the IC50 for removing 2,2'-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS), OH, and 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals of VA were 0.476, 4.33, and 37.92 mg/mL, respectively. VA could enhance innate and adaptive immunity of mice by improving cellular immunity, macrophage phagocytic ability, and NK cell activity; promoting the production of IL-4, IFN-γ, TNF-α and other cytokines; increasing the content and ratio of lymphocyte subsets. These results indicate that VA has good antioxidant activity and can improve the immune function of cyclophosphamide-induced immunodeficient mice.
The content of the article
Velvet antler has good antioxidant activity
Velvet antler can improve the immune function of cyclophosphamide-induced immunodeficient mice
Velvet antler can stimulate the production and the mRNA expression of immune factors
1. Background
As a precious Chinese medicine, velvet antler (VA) has been used for more than 2000 years to treat medical illnesses (Fraser et al., Citation2010; Ran & Li, Citation2009). According to the pharmacopoeias of China, Japan, Korea, and other countries and regions, the VA of sika deer and red deer is considered as a traditional medicine (Sui et al., Citation2014). In addition, VA has been used as a functional food in China, Japan, South Korea, Canada, and other countries and regions (Kawtikwar et al., Citation2010; Yang, Citation1999). Existing studies have shown that VA is rich in many ingredients, such as amino acids, collagen, minerals, proteins, peptides, and polysaccharides (Liu et al., Citation2020; Sui et al., Citation2014). It has various pharmacological activities, such as anti-inflammatory (Kim et al., Citation2012), anti-ageing (Kim et al., Citation2016) and anti-oxidation (Ding et al., Citation2019).
In addition, VA plays an important role in the immune system. For example, VA increases the proportion of CD4+ T cells, CD8+ T cells and NK cells and reduces myeloid-derived suppressive cells in mouse peripheral blood which suggests that VA can inhibit the growth of mouse 4T1 tumours through modulating immune system of the mouse (Zheng et al., Citation2018); In vitro tests show that VA can significantly stimulate splenocytes proliferation and enhance the NK-cell cytotoxicity and CD4+/CD8+ cell subpopulations which indicates that VA has immunomodulatory effects on the immune system (Zha et al., Citation2016); VA extracts significantly promote chondrocyte viability, keep chondrocytes proliferating continuously and block maturation and further differentiation which suggests that VA extracts might serve as a potent anti-oxidant, anti-inflammatory agent, and immune modulator (Yao et al., Citation2018).
However, the current research on the immunomodulatory effects of VA mostly focuses on in vitro tests, leading to the incomplete evaluation. In order to study the immunomodulatory effect of VA in immunocompromised mice, the cellular immunity, macrophage phagocytic activity, NK cell activity, splenic lymphocyte subsets, serum cytokine content, and antioxidant activity were determined of cyclophosphamide (CTX)-induced immunocompromised BALB/c mice in the paper.
2 Material and methods
2.1 Material
RPMI-1640 medium (1996980), fetal bovine serum (42A0378K, FBS), benzylpenicillin and streptomycin (P1400) were offered by Gibco (Grand Island, NY, USA); Dimethyl sulfoxide (EB26BA0030, DMSO), ConA (C8110), 5-diphenyl-tetrazolium bromide, 3-(4,5-dimethylthiazol-2-yl)-2 (MKBX0151V, MTT) were purchased from Sigma Aldrich (St. Louis, MO, USA); ABTS (F1719012) were purchased from Aladdin (Shanghai, China); Sheep red blood cells (SRBC) was purchased from Sbjbio (Nanjing, China); Vitamin C (VC) were purchased from Tianjin Guangfu Fine Chemical Research Institute; Levamisole hydrochloride tablets (IHT) were obtained from Renhetang (Shandong, China); DPPH (W09A7E12633) was purchased from Yuanye (Shanghai, China); YAC-1 Cell was provided by Institute of Cell Research, Chinese Academy of Sciences; CTX was purchased from Shengdi (Jiangsu, China); Anti-mouse CD3 (100220), CD4 (100509), CD8a (100712), CD25 (100904) were provided by BioLegend (San Diego, CA, USA); IL-4 (MS2165A), IL-12 (MS2173A), IFN-γ (#MS2182A), TNF-α (MS2144A) Elisa kit were provided by Maisha Industrial (Jiangsu, China); GoTaq® Green Master Mix (0000410912), Transcriptor Hifi Cdna Synth. Kit (3805) and Eastep Super Total RNA Extraction Kit (0000301230) were purchased from Promega (Madison, WI, USA).
2.2 Antioxidant
2.2.1 ABTS free-radical scavenging rate
Ten grams of VA powder was mixed with 300 mL water. The mixture was left undisturbed overnight. Then, it was heated to boil the water for 1 h twice. The resulting extracts were combined, filtered, and freeze-dried, and the extract of VA was obtained. The VA water extract was reconstituted with water, and 0.1 mL VA extract (water (A2)) of different concentrations was combined with 1 mL ABTS (absolute ethanol (A3)). This was vortexed and allowed to react for 1 h in the dark. Then the absorbance was measured at 734 nm with a microplate reader as A1. The ability to scavenge ABTS free radicals was calculated as follows: [A2-(A1-A3)]/A2×100%.
2.2.2 OH radical scavenging rate
0.3 mL VA extract (water (A2)) of different concentrations was combined with 0.4 mL ferrous sulphate and 0.4 mL salicylic acid. 90 μL of the supernatant was obtained and added to 30 μL of hydrogen peroxide (without hydrogen peroxide (A3)). After 30 min, the absorbance was measured at 510 nm with a microplate reader as A1. The ability to scavenge OH radicals = [A2-(A1-A3)]/A2×100%.
2.2.3 DPPH free-radical scavenging rate
0.1 mL of different concentrations of VA water extract (water (A2)) and 0.4 mL DPPH (absolute ethanol (A3)) was taken, vortexed, and protected from light. The absorbance was measured at 517 nm with a microplate reader and determined as A1. The ability to scavenge DPPH free radicals = [A2-(A1-A3)]/A2×100%.
2.3 Animal experiment
48 Eight-week-old male BALB/c mice weighing 18–22 g were purchased from Liaoning Changsheng Biotechnology Co., and they were raised under a constant temperature (24 ± 2°C) and humidity (50 ± 10%) and a 12-h light–dark cycle. Five mice were kept in each cage with free access to food. All experiments were carried out in strict accordance with the “Guidelines for the Care and Use of Laboratory Animals”. After one week of acclimatization, the mice were randomly divided into six groups (n = 8): (1) control group, (2) CTX group (50 mg/kg), (3) LHT group (levamisole hydrochloride, 40 mg/kg), (4) VAL (100 mg/kg), (5) VAM (300 mg/kg), (6) VAH (500 mg/kg). The control group was given normal saline by intragastric administration, and the other five groups were given the corresponding drugs for 30 days.
2.3.1 Carbon clearance
Mice were injected intraperitoneally with 50 mg/kg CTX to induce immunosuppression from day 26 to 30 for 4 consecutive days. On day 31, the mice were injected intravenously into the coccygeal vein with India ink 0.1 mL/10 g. A 20-μL blood sample was collected and mixed with 2-mL Na2CO3(0.1%) solution at 2 and 10 min after the injection; the OD was measured at 600 nm by a multifunction microplate reader. After blood was collected, the mice were euthanized, and their spleen and liver were collected and weighed. Carbon clearance index (α, ΔOD/time) = weight×3√k/(liver weight + spleen weight); k = (lg OD1-lg OD2)/(T2-T1) (Chen et al., Citation2019).
2.3.2 Delayed-type hypersensitivity assay
On days 26–29, the mice were injected intraperitoneally with cyclophosphamide to induce immunosuppression. One hour after the injection of cyclophosphamide on day 26, the mice were injected intraperitoneally with 2% defibrillated SRBC (1×108 cells). On day 30, the thickness of the left hind foot of the mouse was measured, and 20 µL of SRBC was injected subcutaneously into the left hind foot. After 24 h, the thickness of the left hind foot plantar was remeasured.
2.3.3 Determination of lymphocyte proliferation and NK cell activity
The spleen cells concentration was adjusted to 3×106 cells/mL and was cultured with complete medium (control wells) or 75 µL ConA and incubated for 68 h. After incubation, 0.7 mL of FBS-free RPMI-1640 and 50 µL MTT were added. The plates were incubated for 4 h. After 4 h, acidic isopropanol solution was added to dissolve the purple precipitate. A microplate reader was used to measure the OD value at 570 nm.
100 μL target cells (YAC-1) and effector cells (splenocytes) were added to the reaction wells, 100 μL target cells and complete culture medium were added to the natural release wells of target cells, and target cells and 100 μL NP40 were added to the maximum release wells. All the above items were set with three replicate wells and cultured for 4 h. After 4 h, 100 μL of the LDH matrix solution was added, and the mixture was allowed to react for 3 min. Finally, 30 μL of HCl was added and the OD was measured at 490 nm.
2.3.4 Determination of splenic T lymphocyte subsets and determination of white blood cell count
On days 26–29, cyclophosphamide was injected intraperitoneally to mice. On day 31, a 20 µL blood sample was collected. The white blood cell count was measured. The spleen was aseptically obtained, and the spleen cell suspension was adjusted to 1×106/mL according to the above method. Then, the splenic lymphocyte subsets were determined by flow cytometry.
2.3.5 Determination of immune factors
A blood sample was obtained from the mouse ocular venous plexus and centrifuged at 4°C for 10 min, and the serum was extracted and stored at −80°C. The content of various immune factors in the blood was determined according to the manufacturer's instructions of the ELISA kit.
2.3.6 mRNA expression
Twenty milligrams of the mouse spleen was weighed, and the mRNA was extracted according to the kit method. The mRNA expression levels of IL-6, TNF-α, IFN-γ, IL-12, and IL-1β were measured by agarose gel electrophoresis, and the relative mRNA expression was calculated by the 2-ΔΔCt method. The PCR conditions were as follows: pre-denaturation at 95°C for 2 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min. IL-1β: CCTGTACGATCACTGAACTG (forward), TTGGGATCTACACTCTCCAG (reverse); IL-6: CCAGTTGCCTTCTTGGGACTG (forward), CAATCAGAATTGCCATTG CACAACTC (reverse); TNF-α: AGCCCCCAGTCTGTATCCTT (forward), CATTCGAGGCTCCAGTGA (reverse); GAPDH: ATGGTGAAGGTCGGTGTGAA (forward), CGCTCCTGGAAGATGGTGAT (reverse).
2.3.7 Statistical analysis
All data were shown as mean ± standard deviation (S.D.). Statistically significant differences were determined by one-way ANOVA. The significant difference was set at P value < 0.05.
3 Results
3.1 Antioxidant activity
3.1.1 ABTS free-radical inhibition rate
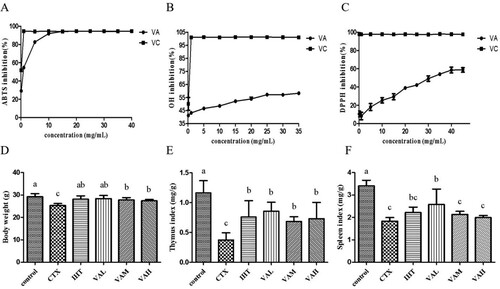
The ABTS free-radical scavenging rate increased with increasing extract concentration ((A)). When the concentration was higher than 15 mg/mL, the activity of VA in scavenging ABTS free radicals, which was 94.28%, was stable. The IC50 value of VA scavenging ABTS free radicals was 0.476 mg/mL.
3.1.2 OH radical inhibition rate
The OH radical scavenging rate increased as the extract concentration increased ((B)). When the concentration was higher than 25 mg/mL, the OH radical scavenging activity of VA water extract was basically stable at 57.17%. The IC50 values of VA scavenging OH radicals were 4.33 mg/mL.
3.1.3 DPPH free-radical inhibition rate
The DPPH free radical scavenging rate increased as the extract concentration increased ((C)). When the concentration was higher than 40 mg/mL, the extract had a stable DPPH free-radical scavenging activity at 58.66%. The IC50 value of antler scavenging DPPH free radicals was 37.92 mg/mL.
3.2 Effect of VA on the immune function of mice
3.2.1 Effect of VA on the body weight and immune organ index of mice
As we can see in (D–F), the body weight and thymus index of mice in the IHT, VAL, VAM, and VAH groups were significantly higher than the CTX group (P < 0.05). Compared with the CTX group, the spleen index in the VAL mice significantly increased (P < 0.05), whereas that in the IHT, VAM, and VAH mice did not change significantly.
3.2.2 Effect of VA on cellular immunity
3.2.2.1 Effect of VA on delayed-type hypersensitivity (DTH) of sheep red blood cells
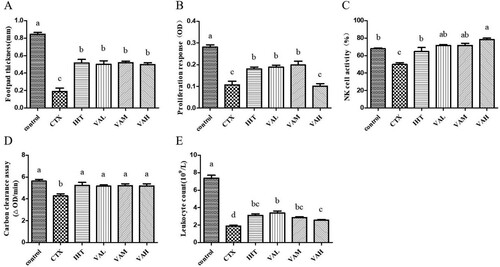
As shown in (A), the thickness of the left hind foot of the mice in the IHT, VAL, VAM, and VAH groups was significantly greater than the CTX group (P < 0.05), indicating that the degree of delayed-type allergy was significantly higher than the CTX group.
3.2.2.2 Effect of VA on the proliferation of splenocytes induced by ConA (ConA)
(B) shows that the proliferation of splenic lymphocytes induced by ConA in the IHT, VAL, and VAM groups was significantly increased (P < 0.05), which increased by approximately 64%, 73%, and 82% respectively, compared with CTX group. However, there was no significant difference in the proliferation of spleen cells induced by ConA between the VAH group and CTX group (P > 0.05).
3.2.3 Effect of VA on the activity of spleen NK cells
Compared with the control group, the NK cell activity of the CTX group was significantly reduced by 26% (P < 0.05), as shown in (C). Compared with the CTX group, IHT, VAL, VAM, and VAH significantly enhanced the activity of NK cells (P < 0.05), increasing by approximately 29%, 43%, 43%, and 57%, respectively.
3.2.4 Effect of VA on the removal rate of carbon particles
From (D), the carbon clearance ability of the IHT, VAL, VAM, and VAH groups was significantly increased compared with the CTX group (P < 0.05), increasing by approximately 22%, 21%, 22%, and 21%, respectively.
3.2.5 Effect of VA on the white blood cell count
As shown in (E), the number of white blood cells was significantly lower in the CTX group than that in the control group (P < 0.05). The number of white blood cells was significantly higher in the IHT, VAL, VAM, and VAH groups than the CTX group (P < 0.05), which increased by approximately 64%, 78%, 48%, and 36%, respectively.
3.2.6 Effect of VA on spleen T lymphocyte subsets
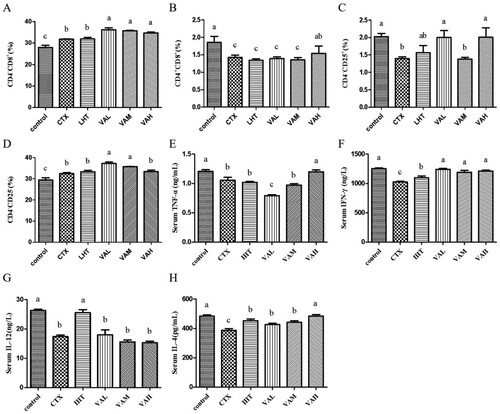
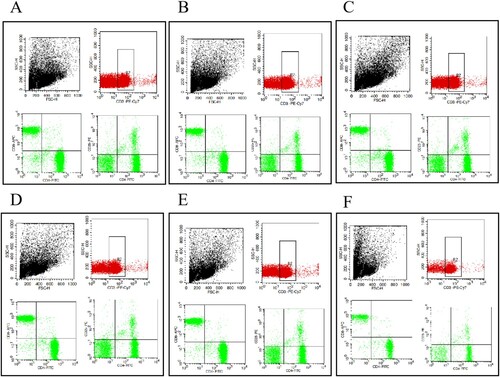
The pheno-typic analysis of T cell subsets can be seen in and . The CD4-CD8+ content in the control group was significantly lower than the CTX group (P < 0.05). Compared with the CTX group, the CD4-CD8+ content in LHT group was not significantly different, whereas the CD4-CD8+ content in the VAL, VAM, and VAH groups increased significantly (P < 0.05). Compared with the CTX group, the CD4 + CD8+ content in the control group and VAH significantly increased (P < 0.05), but there was no significant change in the LHT, VAL, VAM, and VAH groups. We found that CD4-CD25+ content in the control, VAL, and VAH groups increased by 44.90%, 43.11%, and 43.59% (P < 0.05) compared with that in the CTX group. The CD4-CD25- content in the control group was significantly lower than that in the CTX group (P < 0.05), and the CD4-CD25- content in VAL and VAM was significantly higher than that in the CTX group (P < 0.05).
3.2.7 Effect of VA on serum cytokines
As shown in , the TNF-α content was higher in mice of the VAH group than the CTX group (P < 0.05). Compared with that in the CTX group, the IFN-γ content of mice in the IHT, VAL, VAM, and VAH groups all increased significantly (P < 0.05). The IL-12 content of mice in the IHT group was higher than the CTX group (P < 0.05), while the IL-12 content of mice in the VAL, VAM, and VAH groups did not change significantly (P < 0.05). Compared with the CTX group, the IL-4 content of mice in the IHT, VAL, VAM, and VAH groups were significantly increased (P < 0.05).
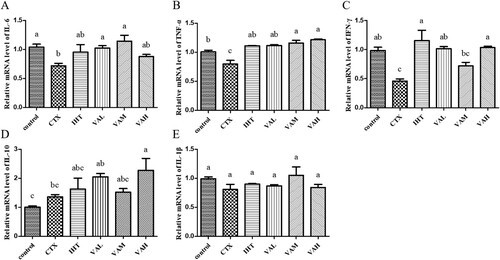
3.2.8 Effect of VA on mRNA expression
As shown in , the relative expression of IL-6 mRNA in the IHT and VAH groups was higher than that of the CTX group (P > 0.05). Compared with the CTX group, the relative expression of TNF-α mRNA in the IHT, VAL, VAM, and VAH groups was all increased significantly (P < 0.05). The relative expression of IFN-γ mRNA in the IHT, VAL, and VAH groups was significantly higher than that in the CTX group (P < 0.05). Only the relative expression of IL-10 mRNA in the VAH group was significantly higher than that in the CTX group (P < 0.05). The relative expression of IL-1β mRNA in the control, IHT, VAL, VAM, and VAH groups was not significantly different than that of the CTX group.
4 Discussion
CTX is an alkylating agent that is widely used in immunotherapy and tumour chemotherapy. Its adverse reactions include immunosuppression, bone marrow suppression, and leukopoenia (Deng et al., Citation2018; Manente et al., Citation2018; Sun et al., Citation2018). In order to develop drugs that antagonize CTX immunosuppression, CTX is often used to prepare animal immunosuppression models and evaluation of the immune effect of related drugs by studying the effects of target drugs against CTX-induced immunosuppressed (Liu et al., Citation2017; Yu et al., Citation2014). At the same time, immunity is an active regulatory mechanism for the body to inhibit the occurrence, growth and metastasis of tumours. At present, tumour immunotherapy has increasingly become an emerging anti-cancer therapy (Wei et al., Citation2015).
Previous studies have shown that VA had antitumour activity (Huo et al., Citation2014). In order to study the regulatory effect of VA in the immune system, the study focused on the effect of VA on CTX-induced immunosuppression in mice, and explored the possible mechanism of VA to improve immune function and anti-cancer effect. Our study showed that VA can resist the atrophy of immune organs induced by CTX. Specific immunity protects the human body from a microorganism or its products, including cellular and humoral immune responses. Cell-mediated immunity involves effector mechanisms performed by T lymphocytes and B lymphocytes and their products (Li et al., Citation2018). Lymphocyte proliferation is a response to stimuli induced by antigens or mitogens, and it has been widely used to assess cellular immune responses (Fu et al., Citation2013). The phagocytic index of mice with VA significantly increased after gavage, indicating that VA promoted the phagocytic activity of macrophages. CD4+ and CD8+ are two common T lymphocytes that play an important role in adaptive immunity (Yu et al., Citation2014). The percentage of CD4-CD8+, CD4 + CD8+, CD4-CD25+ and CD4-CD25- cells treated with VA increased significantly, indicating that VA can restore CTX-induced immunosuppression by increasing the percentage of splenic lymphocytes. In addition, VA increased the activity of NK cells, upregulated the mRNA expression of IL-6, TNF-α, IFN-γ, IL-12, IL-1β and stimulated the production of TNF-α, IFN-γ, IL-4. The research also showed that VA can effectively inhibit DPPH, OH, and ABTS free radicals, indicating that VA has certain antioxidant activities. In addition, CTX can generate free radicals in biological systems and cause oxidative stress (Gaté et al., Citation1999), indicating that VA may reduce the level of reactive oxygen species produced by CTX in the body which remains to be further studied.
In conclusion, it can be considered that VA is an immune enhancer, which can enhance the body's specific and non-specific immunity and reduce the side effects of CTX in the clinical treatment. In the later research, the tumour-bearing mouse model should be used to clarify the anti-tumour effect of the VA.
5 Conclusion
Taken together, the data presented in this study demonstrate that VA has good antioxidant activity and can improve the immune function of CTX-induced immunodeficient mice. VA enhances both innate and adaptive immune responses in mice, which is most likely due to increasing macrophage phagocytosis, NK cell activity and stimulating cytokine secretion. These results demonstrate the potential efficacy of VA in enhancing immune function in the body.
Availability of data
The data that support the findings of this study are available from the corresponding author, [SUN Yin-shi], upon reasonable request.
Ethics approval and consent to participate
All animal experiments were carried out in accordance with European regulations on animal experimentation (NO.ISAPSAEC-2021-40).
Authors’ contributions
Song-Xin Liu and Yin-shi Sun conceived the project and edited the manuscript. Song-Xin Liu, Zhi-Man Li, Zi-Jun Shao, Yun-shi Xia, Li-juan Zhao and Duo-duo Ren carried out the experiments in this study. Yin-shi Sun obtained fifinancial support. All authors read and approved the final manuscript.
Acknowledgements
We appreciate Mr. Cui Xuezhe for his excellent instruction and assistance in collecting the VA. We would like to thank Ms. Chen Lixue for her help in constructing the mice model, and also thank Editage (www.editage.cn) for editing the language of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chen, L. X., Qi, Y. L., Qi, Z., Gao, K., Gong, R. Z., Shao, Z. J., Liu, SX, Li , SS, Sun, YS, (2019). A comparative study on the effects of different parts of Panax ginseng on the immune activity of cyclophosphamide-induced immunosuppressed mice. Molecules, 24(6), 1096. https://doi.org/10.3390/molecules24061096
- Deng, J., Zhong, Y. F., Wu, Y. P., Luo, Z., Sun, Y. M., Wang, G. E., Kurihara, H., Li, Y-F., & He, R-R. (2018). Carnosine attenuates cyclophosphamide-induced bone marrow suppression by reducing oxidative DNA damage. Redox Biology, 14, 1–6. https://doi.org/10.1016/j.redox.2017.08.003
- Ding, Y., Ko, S. C., Moon, S. H., & Lee, S. H. (2019). Protective effects of novel antioxidant peptide purified from alcalase hydrolysate of velvet antler against oxidative stress in Chang liver cells in vitro and in a zebrafish model in vivo[J]. International Journal of Molecular Sciences, 20(20), 5187. https://doi.org/10.3390/ijms20205187
- Fraser, A., Haines, S. R., Stuart, E. C., Scandlyn, M. J., Alexander, A., Somers-Edgar, T. J., & Rosengren, R.J. (2010). Deer velvet supplementation decreases the grade and metastasis of azoxymethane-induced colon cancer in the male rat. Food and Chemical Toxicology, 48(5), 1288–1292. https://doi.org/10.1016/j.fct.2010.02.024
- Fu, L. H., Wang, Y. P., Wang, J. J., Yang, Y., & Hao, L. (2013). Evaluation of the antioxidant activity of extracellular polysaccharides from Morchella esculenta. Food & Function, 4(6), 871–879. https://doi.org/10.1039/c3fo60033e
- Gaté, L., Paul, J., Ba, G. N., Tew, K. D., & Tapiero, H. (1999). Oxidative stress induced in pathologies: The role of antioxidants. Biomedicine & Pharmacotherapy, 53(4), 169–180. https://doi.org/10.1016/S0753-3322(99)80086-9
- Huo, Y. S., Huo, H., & Zhang, J. (2014). The contribution of deer velvet antler research to the modern biological medicine. Chinese Journal of Integrative Medicine, 20(10), 723–728. https://doi.org/10.1007/s11655-014-1827-1
- Kawtikwar, P. S., Bhagwat, D. A., & Sakarkar, D. M. (2010). Deer antlers-traditional use and future perspectives[J]. Indian J Tradit Know, 9(2), 245–251. https://doi.org/10.1007/s11627-010-9286-7
- Kim, H. K., Kim, M. G., & Leem, K. H. (2016). Comparison of the effect of velvet antler from different sections on longitudinal bone growth of adolescent rats. Evid-Based Compl Alt, 1927534. https://doi.org/10.1155/2016/1927534
- Kim, J. H., Yang, Y. I., Ahn, J. H., Lee, J. G., Lee, K. T., & Choi, J. H. (2012). Deer (Cervus elaphus) antler extract suppresses adhesion and migration of endometriotic cells and regulates MMP-2 and MMP-9 expression. Journal of Ethnopharmacology, 140(2), 391–397. https://doi.org/10.1016/j.jep.2012.01.032
- Li, Q., Chen, G., Chen, H., Zhang, W., Ding, Y., Yu, P., Zhao, T., Mao, G., Feng, W., Yang, L., & Wu, X. (2018). Se-enriched G. frondosa polysaccharide protects against immunosuppression in cyclophosphamide-induced mice via MAPKs signal transduction pathway. Carbohydrate Polymers, 196, 445–456. https://doi.org/10.1016/j.carbpol.2018.05.046
- Liu, N., Dong, Z., Zhu, X., Xu, H., & Zhao, Z. (2017). Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice[J]. International Journal of Biological Macromolecules, 107, 796–802. https://doi.org/10.1016/j.ijbiomac.2017.09.051
- Liu, S. X., Gong, R. Z., Lu, Y. S., Wang, Z. S., Zhang, L., Liu, C., Li , SS, Sun , YS, (2020). Research progress on chemical constituents and pharmacological effects of different Cervi Cornu Pantotrichum. Chinese Traditional and Herbal Drugs, 51(3), 806–811. https://doi.org/10.7501/j.issn.0253-2670.2020.03.034
- Manente, F. A., Quinello, C., Ferreira, L. S., Andrade, C. R., Jellmayer, J. A., Portuondo, D. L., Batista-Duhart, A., Carlos, I. Z. (2018). Experimental sporotrichosis in a cyclophosphamide-induced immunosuppressed mice model. Medical Mycology, 56(6), 711–722. https://doi.org/10.1093/mmy/myx098
- Ran, Z., & Li, S. (2009). In vitro antioxidant analysis and characterisation of antler velvet extract. Food Chemistry, 114(4), 1321–1327. https://doi.org/10.1016/j.foodchem.2008.11.010
- Sui, Z., Zhang, L., Huo, Y., & Zhang, Y. (2014). Bioactive components of velvet antlers and their pharmacological properties. Journal of Pharmaceutical and Biomedical Analysis, 87, 229–240. https://doi.org/10.1016/j.jpba.2013.07.044
- Sun, C., Yang, J., Pan, L., Guo, N., Li, B., Yao, J., W, M., Qi, C., Zhang, G., & Liu, Z. (2018). Improvement of icaritin on hematopoietic function in cyclophosphamide-induced myelosuppression mice. Immunopharmacology and Immunotoxicology, 40(1), 25–34. https://doi.org/10.1080/08923973.2017.1392564
- Wei, T., Zhang, J., Qin, Y., Wu, Y., Shen, Q., (2015). Increased expression of immunosuppressive molecules on intratumoral and circulating regulatory T cells in non-small-cell lung cancer patients. American Journal of Cancer Research, 5(7), 2190–2201. https://doi.org/10.6090/jarq.43.173
- Yang, F. H. (1999). Development and countermeasures of deer industry in China. Special Economic Animals and Plants, 2, 3–5. https://doi.org/10.3969/j.issn.1001-4713.1999.01.001
- Yao, B., Zhang, M., Leng, X., Liu, M., Liu, Y., Hu, Y., Wang, M., Qi, C., Zhang, G., & Liu, Z. (2018). Antler extracts stimulate chondrocyte proliferation and possess potent anti-oxidative, anti-inflammatory, and immune-modulatory properties. In Vitro Cellular & Developmental Biology - Animal, 54(6), 439–448. https://doi.org/10.1007/s11626-018-0266-2
- Yu, Q., Nie, S. P., Wang, J. Q., Liu, X. Z., Yin, P. F., Huang, D. F., Li, W-J, Gong, D-M, Xie, M-Y. (2014). Chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced mice. International Journal of Biological Macromolecules, 64, 395–401. https://doi.org/10.1016/j.ijbiomac.2013.12.029
- Zha, E., Dandan, L., Bai, X., Zhou, T., Li, Y., Shenyang, G., & Yue, X. (2016). A recombinant polypeptide from velvet antler of Cervus nippon Temminck exhibits similar immunomodulatory effects as its natural counterpart. Immunopharmacology and Immunotoxicology, 38(6), 385–389. https://doi.org/10.1080/08923973.2016.1233978
- Zheng, K., Fu, C., Jiang, C., Yue, X., & Ma, S. (2018). Water extract of pilose antler can inhibit breast cancer progression of the mouse through modulating its immune system. Food and Agricultural Immunology, 29(1), 785–796. https://doi.org/10.1080/09540105.2018.1457012