ABSTRACT
Schizophyllum commune is a fungus with applications both in medicine and foods, which produces IPS and EPS. However, there is no comparative study on the activity comparison of IPS and EPS and its mechanism of antioxidant effect. In this study, the antioxidant activities of the two polysaccharides were measured in biochemical, cellular and molecular levels, and H2O2 induced oxidative damage model of HSFs was established to explore the protective effects of EPS and IPS. We found IPS and EPS both achieved a good antioxidant effect. They can also improve the cellular levels of SOD and reduce MDA. Insights regarding the molecular mechanism were studied, we found that both EPS and IPS could increase the expression of Nrf2 in Keap1-Nrf2/ARE signaling pathway. At the same time, they could inhibit the expression of the negative feedback gene Keap1 and promote the expression of downstream gene GCLC, Gstt-1, Nqo1 and Ho-1.
1. Introduction
The mycelium and fermentation broth of Schizophyllum commune (S. commune) – known as white ginseng fungi - contains a variety of proteins, polysaccharides, and trace elements, with biological activity. S. commune is an edible fungus, with good stability, often used in food and as a preservative. The polysaccharides produced by S. commune are divided into two types: intracellular polysaccharides (IPS) and extracellular polysaccharides (EPS). IPS are extracted from the microbial mycelia through physical or chemical methods. While EPS are obtained from the fermentation broth secreted by the microorganisms. Many nutritional and pharmacological studies have shown that some dietary supplements have potential chemoprophylactic activities, such as green tea (Katiyar et al., Citation2007), ferulic acid (Prasad et al., Citation2007), collagen polypeptide (Fan et al., Citation2013; Hou et al., Citation2012; Zhuang et al., Citation2009), and soy phosphatidylserine (Choi et al., Citation2013). Besides, functional polysaccharides are good immune enhancers, and can improve the resistance of immune cells to foreign bacteria, viruses, and abnormal cells in the body (Bohn & Bemiller, Citation1995). Komatsu et al. found that the fermentation product of S. commune fungus contained neutral extracellular polysaccharides, then confirmed the anti-tumour activity of the substance through a series of experiments (Komatsu et al., Citation1969). Additionally, S. commune polysaccharides also act as an oxygen-impermeable membrane protecting food from deterioration (Schulz et al., Citation1992). In addition to the substances produced by itself during the fermentation process, S. commune fermented grains (corn bran, soybeans, etc.) have also shown positive results in antioxidant and disease treatment studies (Abdullah et al., Citation2012; Wang et al., Citation2018). While limited information about the comparison of S. commune polysaccharides secreted in and out of cell membrane is collected.
Generally, free radicals in the body are in a dynamic balance between generation and elimination. Shifting the delicate balance between the production and elimination of oxygen radicals or inflammatory cytokines can result in cellular damage. Excessive free radicals will affect the body’s biological macromolecules and cause oxidative stress damage to cells, thereby accelerating the body’s aging and causing related diseases (Lephart, Citation2016; Poprac et al., Citation2017). Gutteridge J. M. et al. defined antioxidants as substances that can effectively inhibit the oxidation of substrates under conditions lower than in concentrations of easily oxidizable substrates (Gutteridge & Halliwell, Citation1992). Previous reports indicated that polysaccharides could attenuate the risk of skin damage induced by UV radiation (Ku et al., Citation2008; Moon et al., Citation2008; Moon et al., Citation2009). Polysaccharides from S. commune showed antioxidant activity by scavenging superoxide anions and hydroxyl radicals (Klaus et al., Citation2011). Thus, it is demanded and urgent to screen of protective substances against oxidation and explore their in-depth mechanisms.
The Keap1-Nrf2/ARE signalling has an important role in the regulation of the antioxidant system in the body. Normally, nuclear factor-erythroid 2-related factor 2 (Nrf2) and the inhibitor cytoplasmic protein Kelch-like epichlorohydrin-related protein-1 (Keap1) is bounded in the cytoplasm and remains in a low activity state (Sun et al., Citation2022a). When stimulated with Oxidative Stress, Nrf2 separates from Keap1 and translocates into the nucleus. Maf small protein binds first to Nrf2, then recognizes and binds to the antioxidant response element ARE, activating a series of detoxification enzymes and antioxidant enzymes genes, such as NAD (P) H quinone oxidoreductase 1 (Nqo1), haem oxygenase (Ho-1), glutathione S transferase (GSTs), peroxide hydrogenase (CAT), superoxide dismutase (SOD) (Baird & Dinkova, Citation2011;; Chen, Citation2018 Kobayashi & Yamamoto, Citation2005; Shih & Yen, Citation2007). These antioxidant enzymes scavenge excess oxygen free radicals, and finally lead to a dynamic balance of oxygen free radical production-scavenging. Previous studies reported the activation of the mitogen-activated protein kinases (MAPKs) related to cell growth and stress response. The three major MAPK pathway are represented by kinase cascades leading to activation of extracellular signal-regulated kinase (Erk), c-Jun N-terminal kinase (Jnk), and p38(Johnson & Lapadat, Citation2002). All three pathways appear to be involved to some extent in the up-regulation of Nrf2 expression in response to diverse stimuli (Xiang et al., Citation2017). Whether EPS and IPS could also exhibit antioxidant effects by MAPK/Nrf2 signalling, it’s need to be further studied.
In this study, we established H2O2 damage model of Human Skin Fibroblasts (HSFs), explored the antioxidant mechanisms of IPS and EPS in free radical scavenging activities, key biomarkers in Keap1-Nrf2/ARE signalling pathway, and its upstream and downstream genes expressions. We hope that the results obtained could have some referential significance for the in-depth development of IPS and EPS and their application in nutritional supplements, foods, cosmetics, and drugs.
2. Materials and methods
2.1. Materials
S. commune EPS and IPS were prepared in the laboratory according to literature with some modifications, and the detailed information could be seen in the Supplementary Materials; HSFs were purchased from the Cell Resource Center, Institute of Basic Medicine, Chinese Academy of Medical Sciences; Dulbecco’s Modified Eagle Medium(DMEM), Fibroblast Medium (FM), Fetal Bovine Serum (FBS), Phosphate Buffer Saline (PBS), 1 × 105 U/L penicillin, 100 mg/L streptomycin, and 0.25% trypsin (with EDTA) were all purchased from GIBCO Life Technologies(USA); Lipid oxidation MDA (Malondialdehyde) Assay Kit, total superoxide anion dismutase (SOD) activity detection kit (WST-8 method), ROS detection kits, total antioxidant capacity Detection kit (ABTS method) and Trizol were purchased from Beyotime Biotechnology Co., Ltd.; EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix reverse transcription kit, TransStart® Top Green qPCR SuperMix kit were obtained from Beijing TransGen Biotech Co., Ltd. Cell Counting Kit-8 (CCK-8) was purchased from Biorigin (Beijing) Inc. All other chemicals were of analytical grade or complied with the standards required for cell culture experiments.
2.2. Preparation of IPS and EPS
The contents of polysaccharide, reducing sugar, total sugar, and protein of active substances were determined according to our previous experimental methods (Chien et al., Citation2015; Sun et al., Citation2022b).
The crude IPS was obtained by hot water extraction. The mycelium was obtained by fermentation in rice medium (containing 1% rice) for 3 days and was used to extract IPS as follows. The dried mycelium was crushed into powder by a disintegrator and dissolved in distilled water at a ratio of 1:50(material to liquid, g/mL). After incubated at 65°C for one hour and centrifuged at 3600 rpm for 20 min, the supernatant was considered as crude IPS. The total sugar content of the crude IPS prepared under these conditions was 4.02 ± 0.14 mg/mL.
The crude EPS was obtained by fermentation. The working volume for fermentation was 300 mL. Three millilitreis (1% inoculation amount) of the starter culture were inoculated into the rice medium (containing 1% rice). The fermentation was carried out for 120 h at 28°C shaking at 150 rpm. The supernatant was considered as crude EPS. The total sugar content of the crude EPS prepared under these conditions was 16.50 ± 0.57 mg/mL.
Detailed information of IPS and EPS in single-factor experiments and orthogonal tests was shown in the Supplementary Material Table S1, Figure S1, Table S2, Table S3, Table S4, Table S5, Figure S2, Table S6, Table S7, and Table S8.
The crude IPS and EPS were freeze-dried. The Sevage method (Sevag et al., Citation1938; Zhang et al., Citation2018) was used to remove the residual protein. The parameter of removal times was examined, and twice was selected for IPS and EPS protein removal. Detailed information was shown in the Supplementary Material Figure S3. The final contents of EPS and IPS after protein removal were determined in the study.
2.3. UV-Vis spectrum and FT-IR spectrum analysis
The UV-Visible spectrum analysis within 200∼800 nm was performed using the solutions of IPS and EPS at concentrations of 1 mg/mL separately (Zhe & Chen, Citation2019).
The samples (IPS and EPS) were mixed with KBr (1:100) and grounded in an agate mortar, respectively. IR absorbance was measured with KBr disc as a blank using Fourier Transform Infrared Spectrometer within 4000∼400 cm−1(Maryam et al., Citation2016).
2.4. Determination of in vitro antioxidant activity
DPPH scavenging capacity determination in vitro was assessed according to the reference (Wu et al., Citation2019) with modifications. The reaction mixture contained 2 mL of sample solution and 2 mL DPPH• solution. After 30 min’ incubation in the dark, the absorbance was recorded at 517 nm against a blank consisting of deionized water instead of sample by using a UV-Vis spectrophotometer.
The hydroxyl radical (•OH) scavenging activity was measured as described with minor modifications (Zhao et al., Citation2016). Briefly, homogenized samples with different concentrations were added to the reaction solution [2 mL 6 mmol/L to the test tube sequentially FeSO4, 2 mL samples of different dilution multiples, 2 mL 6 mmol/L H2O2]. A salicylic acid solution was added and incubated in a 37°C water bath. After centrifugation, the supernatant OD was measured at 510 nm.
The method of chelating ability to Fe2+ was as follows. Samples at different concentrations, ferrous (II) chloride, and phenanthroline were mixed in turn (distilled water is used to replace phenanthroline in the blank test). After 10 min at room temperature, the absorbance was measured at 562 nm against a blank.
The Fe3+ reduction ability was determined by a standard method. Samples at different concentrations were mixed with phosphate-buffered saline (0.2 M, pH 6.6, PBS) and 0.1% potassium hexacyanoferrate solution followed by incubation at 50°C for 20 min. The reaction was terminated with 0.5 mL 10% TCA. 1 ml reaction mixture was diluted with 1 mL distilled water followed by the addition of 0.1 mL FeCl3 solution (0.01%). The absorbance was measured at 700 nm after 10 minutes incubation at room temperature.
2.5. Cell culture and CCK-8 assay
The HSFs were cultured in DMEM, supplemented with 10% fetal bovine serum, 1% fibroblast growth additives, and 1% penicillin (1 × 105 U/L) – streptomycin (100 mg/L). Cells were incubated at 37°C in a humidified atmosphere with 5% CO2 (Hseu et al., Citation2015; Li et al., Citation2020; Song et al., Citation2020).
Samples with different concentrations (0 mg/mL to 10 mg/mL) were added in HSFs for 24 h. The viability of HSFs was determined using CCK-8, according to the manufacturer’s instruction.
2.6. Model establishment of hydrogen peroxide-induced damage
The HSFs were seeded in 96-well plates at a cell number of 1 × 104 per well, and incubated at 37°C and 5% CO2 for 12 h. Then, 100 μL of different concentrations of H2O2 (0–2000 μmol/L) was added and then incubated for 2 h. The cell viabilities were detected. The cell survival rate is expressed as the proportion of living cells in the presence of a sample compared to the control group. The H2O2 condition with a survival rate of about 50% was selected as the modelling condition.
2.7. Determination of intracellular ROS, ABTS, SOD, and MDA
Intracellular ROS levels were measured using a ROS detection kit according to the instruction. Cells were incubated with 5,6-carboxy-2′,7′-dichlorofluoresceindiacetate (DCFH-DA) fluorescent probe for 20 min. After a wash, dichlofluorescein diacetate (DCF) fluorescence was measured with excitation at 485 nm and emission at 530 nm. The fluorescence intensity of ROS in HSF cells was observed under an inverted fluorescence microscope.
After the treatment, cell samples were collected, homogenized, and centrifuged. Then, ABTS, SOD, and MDA levels were detected using the kits according to the manufacturer’s instructions. The ABTS scavenging capacities were expressed as trolox equivalent antioxidant capacity (TEAC) values. The SOD vitality and MDA levels were expressed as U per mg protein, and μmol per mg protein, respectively.
2.8. Quantitative reverse transcriptional PCR
Total RNAs were extracted using Trizol (Beyotime, China) according to manufacturer’s instruction. The cDNA was synthesized with the reverse transcription kit. The reaction system was represented in Table S9. Gene sequences were retrieved from the NCBI database, and primers of Nqo-1 Ho-1, Gstm-1, Gstt-1, Erk2, Jnk, p38, Keap1, Nrf2, Gclm, and Gclc were designed using PrimerExpress software. β-actin was the housekeeping, and the internal reference gene. The sequence-specific primers were shown in Table S10. The cDNA was subjected to PCR amplification using the TransStart® Top Green qPCR SuperMix kit. The detailed reaction system was represented in Table S11. Cyclic parameters were: pre-denaturation at 94°C for 30 s, the reaction by PCR (45 cycles of 94°C for 15 s, 60°C for 15 s, 72°C for 10 s), and a final extension at 95°C for 15 s. Calculated relative genes expression were analysed using the 2−ΔΔCT method (Livak & Schmittgen, Citation2001).
2.9. Statistics analysis
All experiments were performed in triplicate and data are presented as means ± standard deviations. Data were analysed by SPSS 17.0 software. One-way analysis of variance was used for the significance of differences among groups (*, #, $ p < 0.05, **, ##, $$ p < 0.01).
3. Results
3.1. Content index and structure characterization of IPS and EPS
After protein removal, typical compounds of IPS and EPS were measured referring to the method of literature (Chien et al., Citation2015; Sun et al., Citation2022b). As shown in , the proportions of polysaccharide reached 69.05% in EPS and 63.59% in IPS. The protein proportions of IPS and EPS were both less than 0.5%, which were significantly lower than that of the crude samples. Detailed data could be seen in Supplementary Material (Figure S3).
Table 1. Contents of extracellular polysaccharides in intracellular samples.
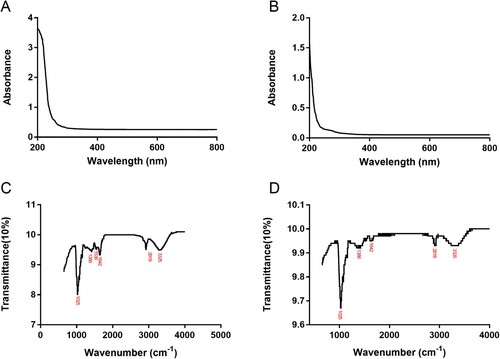
Preliminary structural characterization was then performed by UV and infrared Spectrometers. The UV spectra of IPS and EPS were shown in (A,B). There is no obvious peak value of IPS and EPS at 200–600 nm, indicating that the extraction is relatively successful and IPS and EPS are relatively pure. The infrared spectra of IPS and EPS were shown in (C,D). The absorption peak at 3325 cm−1 in IPS was caused by the stretching vibration of the hydroxyl group (O–H) (Kozarski et al., Citation2012). The absorption peak at 2919 cm−1 suggested the stretching vibration of the C–H bond (Shao et al., Citation2015). Besides, peaks at 1642 and 1536 cm−1 both belonged to carbonyl (C = O) stretching vibration (Cai et al., Citation2018), and a bond at 1386 cm−1 indicated the presence of the carboxyl group (COOH) (Muhammad et al., Citation2020). In addition, there was also an absorption peak at 1025 cm−1 in IPS, which belonged to the stretching vibration of the C–O bond (Shi et al., Citation2016). The appearance of this absorption peak led us to speculate that IPS may contain sugars in the form of pyranose or the existence of glycosidic bonds. The infrared spectrum of EPS was similar to those of IPS to some extent. The main difference was that the absorption peak area at 1025 cm−1 of EPS was stronger than that of IPS. It was speculated that EPS may have a greater number of glycosidic bonds than IPS. To sum up, the main functional groups in IPS and EPS were hydroxyl, carboxyl, and glycosidic bonds. The existence and number of these functional groups would determine the physical and chemical properties of IPS and EPS.
3.2. In vitro antioxidant capacities of IPS and EPS
The in vitro antioxidant capacities of IPS and EPS were shown in . The DPPH free radical scavenging test results ((A)) in the range of 0.20∼1.20 mg/mL showed that both the two polysaccharides and VC were in dose-dependent manners. Besides, at the same concentration, EPS and IPS were both lower than that of VC, which was consistent with our previous study (Fu et al., Citation2022). Meanwhile, the scavenging ability of IPS was stronger than EPS. The scavenging rate of IPS at the maximum concentration set in the experiment (1.2 mg/mL) could reach 66.72%.
Figure 2. Determination of antioxidant capacities of IPS, EPS and VC in vitro. A, DPPH• scavenging effects; B, •OH free radicals scavenging effects; C, Fe2+ chelating abilities; D, reduction capabilities. VC, namely ascorbic acid, was chosen as the positive control. Results were expressed as the mean ± SD(n = 3).
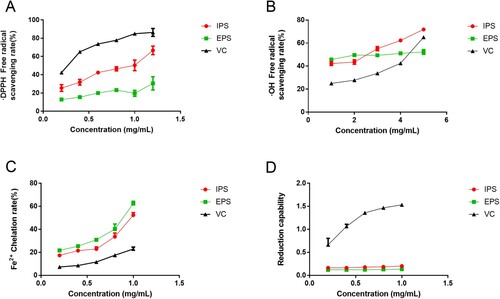
Because of the low abilities at small concentrations, the hydroxyl radical scavenging effects at the concentrations of 1 mg/mL to 5 mg/mL were shown in (B). The dose-dependent manner was observed, too. Among the samples, IPS above 3 mg/mL showed the strongest scavenging effects, with almost 70% clearance rate at 5 mg/mL. The positive control VC at 5 mg/mL showed a medium ability between them.
The results of Fe2+ chelation abilities were shown in (C). These effects were also dose-dependent. The experimental data showed that the chelating abilities of EPS and IPS to Fe2+ were significantly higher than that of VC at the same concentration. What’s more, EPS exhibited the greatest chelating ability.
The results of Fe3+ reducing abilities were shown in (D). The results showed that the reducing abilities of IPS and EPS were obviously weaker than that of VC at the same concentration. Besides, the data did not suggest the dose-dependent relationship between the concentrations and the reducing ability of IPS and EPS to Fe3+.
Summarizing the above experimental results, we concluded that IPS and EPS have excellent free radical scavenging ability, can effectively scavenge oxygen free radicals, and also have good Fe2+ chelating ability. Although poor reducing ability was obtained, the overall antioxidant activities still drew our attention.
3.3. IPS and EPS toxicities and modelling
The cell viabilities of HSFs treated by IPS, EPS, and VC for 24 h were shown in A-C. There could be seen the dose-dependent relationship between the samples (EPS and IPS) and their cell viabilities ((A,B)). A similar tendency could be found in the positive control at the concentrations of 0∼200 mg/mL, with the cell viabilities ranging from 5% to 99.36% ((C)). IPS at the concentrations of 0.08–10 mg/mL was used to find out the effects to HSFs, and the cell viabilities were ranging from 72.19% to 104.82% ((A)). When the concentration of EPS was less than, or equal to 2.5 mg/mL, cell viability reached more than 80%. Therefore, we chose 2.5 mg/mL for 24 h as the condition of IPS and EPS for the follow-up experiments.
Figure 3. Cytotoxicity determination. A, cell viabilities of IPS; B, cell viabilities of EPS; D, cell viabilities of VC; D, cell viabilities of H2O2; E, the status of human fibroblasts on different concentrations of H2O2. VC was chosen as the positive control. ##p < 0.01, #p < 0.05, in A∼ D, compared with the sample at the concentration of 0.
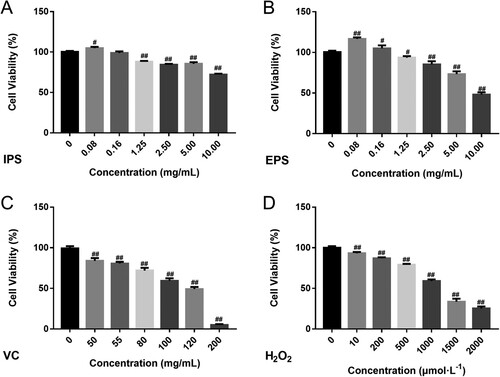
What’s more, hydrogen peroxide was used to establish the damage model. According to (D), there was a decreasing tendency in cell viability as the increase of H2O2 concentration. Therefore, 1000 μmol/L H2O2 (IC50) for 2 h was used as the modelling condition.
3.4. ROS and antioxidant enzyme levels
The intracellular antioxidant levels were also determined in our study. The increase of ROS can lead to DNA damage in cells and even promote apoptosis. ROS fluorescence intensity photographs of HSF cells treated with DCFH-DA probe were taken under an inverted fluorescence microscope, as shown in (A). ROS fluorescence intensity represents the change of ROS level. The ROS fluorescence intensity increased significantly after H2O2 treatment of HSFs, while decreased significantly after IPS, EPS, and VC treatment ((B)). Compared with VC, significant decreases of fluorescence intensity could be seen when cells were treated by IPS and EPS, indicating their excellent ROS-reducing capacities.
Figure 4. The fluorescence intensity photo of ROS (A, B) and in HSF cells. The Intracellular antioxidant activities of different samples on ABTS scavenging capacities(C), the total SOD activities (D) and MDA contents(E). The control group did not do any treatment, and the model group was stimulated with H2O2. IPS and EPS represent the cells treated respectively by the intracellular and extracellular polysaccharides at the concentration of 2.5 mg/mL. VC at the concentration of 55μg/mL is chosen as the positive control. ##p < 0.01, compared with Control group; *p < 0.05, **p < 0.01, compared with Model group, $p < 0.05, $$p < 0.01, compared with VC.
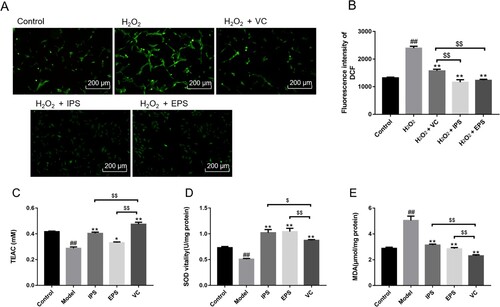
The total antioxidant capacity of HSFs in the Model group was significantly reduced ((C), p < 0.01) compared with that in the Control group, indicating that the modelling was successful. IPS and EPS could improve the total antioxidant capacity of cells to some extent, and the effect of IPS was extremely significant, compared with control. It was shown that both IPS and EPS could significantly increase the total antioxidant capacity decreased by H2O2 (p < 0.05 and p < 0.01). While VC showed the greatest total antioxidant capacity.
(D.E) showed the effects of IPS and EPS on the cellular SOD vitalities and MDA levels. H2O2 stimulation can inhibit the content of intracellular SOD ((D), p < 0.01), and significantly increased the MDA content in fibroblasts ((E), p < 0.01). IPS and EPS can effectively increase the expression levels of SOD and significantly reduce the MDA content elevated by H2O2 (p < 0.01). More importantly, significant increases in SOD levels could be observed by IPS and EPS, compared with VC (p < 0.01). Although IPS and EPS exhibited relatively greater scavenging abilities than VC both in SOD levels, and in free radical scavenging abilities in section 3.2, the MDA content of VC was still lower than the other polysaccharides. We can suspect that other antioxidant factors may be accelerated after VC treatment. Several studies have shown the antioxidant effects of VC (Fu et al., Citation2022; Shi et al., Citation2021; Su et al., Citation2022; Zhang et al., Citation2022).
3.5. Expression of Keap1-Nrf2 /ARE signalling pathway-related genes
The Keap1-Nrf2/ARE pathway is one of the important regulatory pathways in the body. These antioxidant enzymes scavenge excess oxygen-free radicals, and finally lead to a dynamic balance of oxygen-free radical production-scavenging.
The effects of IPS and EPS on the expressions of genes related to the Keap1-Nrf2/ARE pathway were shown in (A–J). H2O2 stimulation to HSFs resulted in a significant increase in the expression level of Keap1 (p < 0.01. (A)) and a significant decrease in the expression level of Nrf2 (p < 0.01, (B)). Highly expressed Keap1 inhibited the activation and nuclear transfer of Nrf2, and could not achieve the activation and regulation of downstream antioxidant genes. The effect of IPS and EPS can inhibit the increase of Keap1 expression level caused by H2O2, thus effectively inhibit its binding to Nrf2, and the EPS group can even reduce the content of Keap1 to the unstimulated state. The effect of IPS and EPS can significantly increase the expression level of Nrf2 mRNA and make it return to the undamaged state, especially. These results indicated that IPS and EPS can effectively regulate cells’ response to oxidative stress injury through the Keap1-Nrf2/ARE pathway, and promote the transfer of Nrf2 into the nucleus to activate downstream regulatory genes.
Figure 5. The relative expression of anti-aging related genes. Keap1(A), Nrf2(B), Gstm-1(C), Gstt-1(D), Gclc(E), Ho-1(F), Nqo1(G), Erk2(H), p38(I) and Jnk1(J). IPS and EPS represent the cells treated respectively by the intracellular and extracellular polysaccharides at the concentration of 2.5 mg/mL, ##p < 0.01, compared with Control group; **p < 0.01, compared with Model group.
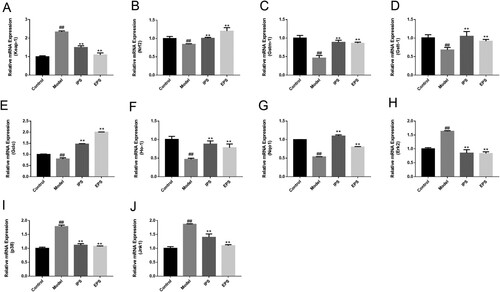
The effects of IPS and EPS on the gene expressions of the downstream of Keap1-Nrf2/ARE signalling pathway were shown in (C–G). GSTs are the major phase II metabolic enzymes in the body and can bind to oxidative metabolites to facilitate their excretion from the body, while Gstm-1 and Gstt1 are the main gene forms of Gsts. Ho-1 and Nqo1 play important roles in peroxide scavenging and reduction of ROS production (Chen et al., Citation2022). Besides, Kaspar and Jaiswal (Citation2010) confirmed that Nqo1 is a downstream product of Nrf2, and the activation of Nrf2 can stimulate the expression of Nqo1. Glutathione peroxidase (GSH) is the most abundant antioxidant enzyme in cells, and glutamate cysteine ligase (GCLC) can catalyse the first step of glutathione synthesis by linking cysteine and glutamate. The first step is to determine whether the antioxidant activity of intracellular GSH can be improved (Yang et al., Citation2022). IPS and EPS can promote the body to inhibit and scavenge excessive ROS through downstream antioxidant proteins encoded by Gstm-1, Gstt-1, Gclc, Ho-1, and Nqo1, resulting in the reduction of the damage induced by H2O2 on HSFs.
It has been reported in the literature that promoting the translocation of Nrf2 into the nucleus can be achieved by inhibiting the activation of upstream genes including p38 and Jnk (Boo, Citation2020; Kang et al., Citation2020; Saha et al., Citation2020). The experimental results showed that H2O2 injury led to the activation of p38 and Jnk1 expression in cells, which could be effectively relieved by IPS and EPS ((I,J)). Besides, the expression of Erk2 was also significantly decreased (p < 0.01, (H)).
4. Discussion
With the acceleration of the pace of modern society and the improvement of living standards, people are not limited to simple dietary intake, and begin to look for safer and more efficient nutritional supplements. Agaricus, Cordyceps, Bacillus tuber, Ganoderma lucidum, Chaga and Shiitake mushrooms, Schizophyllum commune, and other fungi are commonly used in nutritious foods and cosmetics. Also, they are often used as dietary supplements (Hyde et al., Citation2010).
Gutteridge J M et al. defined antioxidants as substances that can effectively inhibit the oxidation of substrates under the condition that the concentration of the oxidizable substrate is lower than that of the substrate. The intake of antioxidants can scavenge the extra ROS in the body, protect the biological macromolecules, such as DNA, RNA, and enzymes. Polysaccharides obtained from mushrooms show excellent free radical scavenging effects (Gutteridge & Halliwell, Citation1992).
Schizophyllum has been found to effectively extend the lifespan of nematodes by co-fermenting with Pueraria (Deng et al., Citation2022). In addition to its anti-aging effect as a fermented strain, Schizophyllum is also an active substance worthy of separate study. Schizophyllan, as one type of polysaccharides, has been found to have antioxidant and mediate effective antisense activity in cancer cells. Besides, it also has potential as a biomaterial (Sutivisedsak et al., Citation2016). While, there are not enough literatures to discuss the sources of polysaccharides from a certain fungus. Research in antioxidant activities between intracellular and extracellular polysaccharides from S. commune was also limited.
In the study, EPS and IPS showed the highest scavenging rate of DPPH• free radicals (66.72% and 30.56%, respectively) at the same concentration of 1.2 mg/mL. EPS and IPS at the concentration of 5.0 mg/mL, both had the highest •OH free radicals scavenging rate. Also, IPS had the highest scavenging rate of •OH and DPPH free radicals (71.85% and 52.31%, respectively). The scavenging abilities of EPS and IPS were found much higher than those reported by Jiang et al. What’s more, IPS and EPS were found that they also had strong chelating ability and reducing activity to Fe2+, indicating that they have anti-oxidative effect and thus have anti-aging effect. Besides, slight damage to the HSFs of IPS and EPS was found, and certain proliferation effects were observed, instead ((A)). Apart from the cell proliferation of polysaccharides from S. commune, Lin et al. showed that Schizophyllan can also improve cellular and humoral immune functions in vivo, thereby enhancing the anti-aging effect (Xia & Lin, Citation1990). Although there is a lack of experimental data in vivo, we further analysed the gene expression of cellular signalling pathways in vitro. The Keap1-Nrf2/ARE signalling pathway is considered to be an important mechanism in response to oxidative stress injury. Some herbal extracts or macromolecules from microorganisms show excellent activation of Nrf2, thus, resulting in antioxidant effects (Chaiprasongsuk & Panich, Citation2022; Rubiolo et al., Citation2008; Yang et al., Citation2014). In the study, IPS and EPS showed a significant increase in the expression of Nrf2, which further inducing the production of antioxidant enzymes. Similar results were obtained when discussing the polysaccharide extract of Lycium barbarum. According to the literature, the crude polysaccharide extract of Lycium barbarum activated Nrf2, further increased the content of glutathione peroxidase and superoxide dismutase, and meanwhile reduced lipid peroxidation, which finally repaired the cell oxidative damage caused by ultraviolet light (Yang et al., Citation2014).
The downstream of Keap1-Nrf2/ARE signalling pathway encodes the antioxidant enzymes, which activate the self-response to oxidative stress. Compounds have been reported to have antioxidant effects by activating Nrf2 and its downstream genes. Resveratrol, a famous antioxidant, can promote the expression of SOD by activating Nrf2, thereby reducing the oxidative stress damage of primary rat liver cells induced by H2O2 stimulation (Rubiolo et al., Citation2008). EPS and IPS showed great total antioxidant activities, and significantly increased the cellular SOD activities. Besides, the antioxidant enzymes GSTs-related gene expressions were also accelerated. That was the reason to explain the protective effects of IPS and EPS, against the oxidative stress damage of HSFs caused by H2O2 stimulation. Presumably, they activate the Nrf2/ARE signalling pathway to regulate downstream oxidative stress positive regulator genes, such as genes for antioxidant and detoxification enzymes (Shih & Yen, Citation2007).
There is a theory on the activation of the Keap1-Nrf2/ARE signalling pathway: MAPK pathway. MAPK pathway is widely studied and verified. Mitogen-Activated Protein Kinases (MAPKs) – a family of serine/threonine protein kinases – mediate various cellular processes in response to external stress signals (Han et al., Citation2002 Kim & Choi, Citation2015;). Jnk and p38 can activate MAPK (Shih et al., Citation2020). In the study, the expression of Erk2, Jnk, and p38 were all inhibited by IPS and EPS. Although the regulation of up-stream pathway was not proved in the study, it was possible to speculate that MAPK plays an important part in the acceleration of Keap1-Nrf2/ARE signalling pathway. However, the body is complex and affected in lots of ways. It is without a doubt that there may be existed other parameters to affect Nrf2.
On this basis, in the future, high-throughput transcriptome technology, such as RNA-Seq, will more meaningfully reveal the internal mechanism of the interaction between the target signalling pathway and its upstream genes. It is helpful to clarify the dynamic regulation map of fungal polysaccharides against oxidative stress. We hope to reveal the scientific connotation of natural source antioxidants more comprehensively. Besides, continued efforts in structural identification are still needed. This research will stimulate the application of EPS and IPS in food and health products.
5. Conclusion
We found that both IPS and EPS achieved good antioxidant effects by scavenging free radicals, chelating, and reducing Fe2+, although different degrees could be observed. Also, the IPS and EPS both showed repairing effects on oxidatively stressed HSFs. Regarding the influence on the Keap1-Nrf2/ARE signalling pathway, we found that these polysaccharides both had a significant promotion effect. However, the induced downstream genes related to antioxidant enzymes differed. This indicated that although different downstream antioxidant enzymes were initiated by IPS and EPS by means of Keap1-Nrf2/ARE signalling pathway regulation, they achieved antioxidant effects. Although the regulation of up-stream pathway was not proved in the study, the inhibited Erk2, p38, and Jnk1 may be related to the increased Nrf2.
Author contributions
JCZ, CTW, and QA provided concepts and designed experiments; WJC, YDY, LYL, and TH conducted experiments; FQD, DZ, XQS, and ML analysed data; WJC wrote the manuscript, JCZ, CTW read and commented on the first draft. All authors have read and approved the final manuscript.
Supplemental Material
Download MS Word (309.6 KB)Acknowledgements
We are very grateful to our friends who helped us complete the manuscript and the associated financial support
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used and/or investigated during the present study are available from the corresponding author upon reasonable request.
References
- Abdullah, N., Gopal, D. M. V., & Abdulla, M. A. (2012). Effect of soya beans and soya beans fermented with Schizophyllum commune Fr. on ethanol-induced gastric ulcer in Sprague-Dawley rats. Acta Alimentaria: An International Journal of Food Science, 41(3). https://doi.org/10.1556/AAlim.41.2012.3.5
- Baird, L., & Dinkova, K. A. (2011). The cytoprotective role of the Keap1–Nrf2 pathway. Archives of Toxicology, 241–272. https://doi.org/10.1007/s00204-011-0674-5
- Bohn, J. A., & Bemiller, J. N. (1995). (1-3)-beta-D-Glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohydrate Polymers, 28(1), 3–14. https://doi.org/10.1016/0144-8617(95)00076-3
- Boo, Y. C. (2020). Natural Nrf2 modulators for skin protection. Antioxidants, 9(9). https://doi.org/10.3390/antiox9090812
- Cai, W. F., Hu, T., Bakry, A. M., Zheng, Z. M., Xiao, Y. D., & Huang, Q. L. (2018). Effect of ultrasound on size, morphology, stability and antioxidant activity of selenium nanoparticles dispersed by a hyperbranched polysaccharide from Lignosus rhinocerotis. Ultrasonics - Sonochemistry, 42, 823–831. https://doi.org/10.1016/j.ultsonch.2017.12.022
- Chaiprasongsuk, A., & Panich, U. (2022). Role of phytochemicals in skin photoprotection via regulation of Nrf2. Frontiers in Pharmacology. https://doi.org/10.3389/fphar.2022.823881
- Chen, T., Duan, J. L., Wang, F., Yuan, Z. Q., Xue, J. Y., Lu, T., Huang, W. T., Liu, Y. F., & Zhang, Y. L. (2022). GSTM3 deficiency impedes DNA mismatch repair to promote gastric tumorigenesis via CAND1/NRF2-KEAP1 signaling. Cancer Letters, 538, 215692. https://doi.org/10.1016/j.canlet.2022.215692
- Chen, Z. L. (2018). The anti-oxidative stress effect of silencing information regulator 2 related enzymes 1/nuclear factor E2 related factor 2 signaling pathway in hypertension. Chinese Journal of Circulation, 33(10), 1035–1037. https://doi.org/10.3969/j.issn.1000-3614.2018.10.022
- Chien, R. C., Yen, M. T., Tseng, Y. H., & Mau, J. L. (2015). Chemical characteristics and anti-proliferation activities of Ganoderma tsugae polysaccharides. Carbohydrate Polymers, 128, 90–98. https://doi.org/10.1016/j.carbpol.2015.03.088
- Choi, H. D., Han, J. J., Yang, J. H., Lee, S. H., Kim, Y. S., Chung, G. H., & Hahm, D. H. (2013). Effect of soy phosphatidylserine supplemented diet on skin wrinkle and moisture in vivo and clinical trial. Journal of the Korean Society for Applied Biological Chemistry, 56(2), 227–223. https://doi.org/10.1007/s13765-013-3012-1
- Deng, Y. F., Liu, H., Huang, Q., & Tu, L. Y. (2022). Mechanism of longevity extension of caenorhabditis elegans induced by Schizophyllum commune fermented supernatant with added radix puerariae. Food Chemistry, 9, 847064. https://doi.org/10.3389/fnut.2022.847064
- Fan, J., Zhuang, Y. L., & Li, B. F. (2013). Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients, 5(1), 223–233. https://doi.org/10.3390/nu5010223
- Fu, H., Zhang, Y., An, Q., Wang, D., You, S., Zhao, D., Zhang, J., Wang, C., & Li, M. (2022). Anti-photoaging effect of rhodiola rosea fermented by lactobacillus plantarum on UVA-damaged fibroblasts. Nutrients, 14(11), 2324. https://doi.org/10.3390/nu14112324
- Gutteridge, J. M., & Halliwell, B. (1992). Comments on review of free radicals in biology and medicine. Free Radical Biology and Medicine, 12(1), 93–95. https://doi.org/10.1016/0891-5849(92)90062-L
- Han, I. O., Kim, K.-W., Ryu, J. H., & Kim, W.-K. (2002). P38 mitogen-activated protein kinase mediates lipopolysaccharide, not interferon-gamma, -induced inducible nitric oxide synthase expression in mouse BV2 microglial cells. Neuroscience Letters, 325(1), 9–12. https://doi.org/10.1016/S0304-3940(02)00218-5
- Hou, H., Li, B., Zhang, Z., Xue, C., Yu, G., Wang, J., Bao, Y., Bu, L., Sun, J., Peng, Z., & Su, S. (2012). Moisture absorption and retention properties, and activity in alleviating skin photodamage of collagen polypeptide from marine fish skin. Food Chemistry, 135(3), 1432–1143. https://doi.org/10.1016/j.foodchem.2012.06.009
- Hseu, Y. C., Korivi, M., & Yang, H. W. (2015). Dermato-protective properties of ergothioneine through induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated human keratinocytes. Free Radical Biology and Medicine, 86, 102–117. https://doi.org/10.1016/j.freeradbiomed.2015.05.026
- Hyde, K. D., Bahkali, A. H., & Moslem, M. A. (2010). Fungi-an unusual source for cosmetics. Fungal Diversity, 43(1), 1–9. https://doi.org/10.1007/s13225-010-0043-3
- Johnson, G. L., & Lapadat, R. (2002). Mitogen-Activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science, 298(5600), 1911–1912. https://doi.org/10.1126/science.1072682
- Kang, Y. P., Mockabee, M. A., Jiang, C., Falzone, A., Prieto, F. N., Stone, E., Harris, I. S., & DeNicola, G. M. (2020). Non-canonical glutamate-cysteine ligase activity protects against ferroptosis. Cell Metabolism, 33(1). https://doi.org/10.1016/j.cmet.2020.12.007
- Kaspar, J. W., & Jaiswal, A. K. (2010). Antioxidant- induced phosphorylation of tyrosine 486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to the antioxidant response element and activate defensive gene expression. Journal of Biology Chemistry, 285(1), 153–162. https://doi.org/10.1074/jbc.M109.040022
- Katiyar, S., Elmets, C. A., & Katiyar, S. K. (2007). Green tea and skin cancer: Photoimmunology, angiogenesis and DNA repair. The Journal of Nutritional Biochemistry, 18(5), 287–296. https://doi.org/10.1016/j.jnutbio.2006.08.004
- Kim, E. K., & Choi, E.-J. (2015). Compromised MAPK signaling in human diseases: An update. Archives of Toxicology, 89(6), 867–882. https://doi.org/10.1007/s00204-015-1472-2
- Klaus, A., Kozarski, M., Niksic, M., Jakovljevic, D., Todorovic, N., & Van Griensven, L. J. L. D. (2011). Antioxidative activities and chemical characterization of polysaccharides extracted from the basidiomycete schizophyllum commune. Lwt-food Science and Technology, 44(10), 2005–2011. https://doi.org/10.1016/j.lwt.2011.05.010
- Kobayashi, M., & Yamamoto, M. (2005). Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxidants and Redox Signaling, 385–394. https://doi.org/10.1089/ars.2005.7.385
- Komatsu, N., Okubo, S., & Kikumoto, S. (1969). Host-mediated antitumor action of schizophyllan, a glucan produced by Schizophyllum commune. Gan, 60(2), 137. https://doi.org/10.1016/0014-4827(69)90222-5
- Kozarski, M., Klaus, A., Niksic, M., Vrvic, M. M., Todorovic, N., Jakovljevic, D., & Van Griensven, L. J. (2012). Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma Applanatum, Ganoderma Lucidum, Lentinus Edodes and Trametes Versicolor. Journal of Food Composition and Analysis, 26(1–2), 144–153. https://doi.org/10.1016/j.jfca.2012.02.004
- Ku, M. J., Lee, M. S., Moon, H. J., & Lee, Y. H. (2008). Antioxidation effects of polysaccharide fucoidan extracted from seaweeds in skin photoaging. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 22(S1). https://doi.org/10.1096/fasebj.22.1_supplement.647.1
- Lephart, E. D. (2016). Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Research Reviews, 31, 36–54. https://doi.org/10.1016/j.arr.2016.08.001
- Li, Q., Bai, D., & Qin, L. (2020). Protective effect of d-tetramannuronic acid tetrasodium salt on UVA-induced photo-aging in HaCaT cells. Biomedicine and Pharmacotherapy, 126, 110094. https://doi.org/10.1016/j.biopha.2020.110094
- Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods: A Companion to Methods in Enzymology, 25(4), 402–408. https://doi.org/10.1006/meth.2001.1262
- Maryam, J. S., Ali, G., & Mandana, B. (2016). Optimization of ultrasound-assisted extraction, preliminary characterization and in vitro antioxidant activity of polysaccharides from green pea pods. Foods (basel, Switzerland), 5(4), 78. https://doi.org/10.3390/foods5040078
- Moon, H. J., Lee, S. H., Ku, M. J., Yu, B. C., Jeon, M. J., Jeong, S. H., Stonik, V. A., Zvyagintseva, T. N., Ermakova, S. P., & Lee, Y. H. (2009). Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. European Journal of Dermatology, 19(2), 129–134. https://doi.org/10.1684/ejd.2008.0611
- Moon, H. J., Lee, S. R., Shim, S. N., Jeong, S. H., Stonik, V. A., Rasskazov, V. A., Zvyagintseva, T., & Lee, Y. H. (2008). Fucoidan inhibits UVB-induced MMP-1 expression in human skin fibroblasts. Biological and Pharmaceutical Bulletin, 31(2), 284–289. https://doi.org/10.1248/bpb.31.284
- Muhammad, A., Yusra, U., Sarmad, A. Q., & Nimrah, K. (2020). Improved exopolysaccharide production from Bacillus licheniformis MS3: Optimization and structural/functional characterization. International Journal of Biological Macromolecules, 151, 984–992. https://doi.org/10.1016/j.ijbiomac.2019.11.094
- Poprac, P., Jomova, K., & Simunkova, M. (2017). Targeting free radicals in oxidative stress-related human diseases. Trends in Pharmacological Sciences, 38(7), 592–607. https://doi.org/10.1016/j.tips.2017.04.005
- Prasad, N. R., Ramachandran, S., Pugalendi, K. V., & Menon, V. P. (2007). Ferulic acid inhibits UV-B-induced oxidative stress in human lymphocytes. Nutrition Research, 27(9), 559–564. https://doi.org/10.1016/j.nutres.2007.06.011
- Rubiolo, J. A., Mithieux, G., & Vega, F. V. (2008). Resveratrol protects primary rat hepatocytes against oxidative stress damage: Activation of the Nrf2 transcription factor and augmented activities of antioxidantenzymes. European Journal of Pharmacology, 591(1-3), 66–72. https://doi.org/10.1016/j.ejphar.2008.06.067
- Saha, S., Buttari, B., Panieri, E., Profumo, E., & Saso, L. (2020). An overview of Nrf2 signaling pathway and its role in inflammation. Molecules (Basel, Switzerland), 25(22). https://doi.org/10.3390/molecules25225474
- Schulz, D., Rau, U., & Wagner, F. (1992). Characteristics of films prepared from native and modified branched β-1, 3- d -glucans. Carbohydrate Polymers, 18(18), 295–299. https://doi.org/10.1016/0144-8617(92)90095-8
- Sevag, M. G., Lackman, D. B., & Smolens, J. (1938). Isolation of components of streptococcal nucleoproteins in serologically active form. Journal of Biology Chemistry, 124(2), 0425–0436. https://doi.org/10.1016/S0021-9258(18)74048-9
- Shao, P., Shao, J. M., Han, L. F., Lv, R. L., & Sun, P. L. (2015). Separation, preliminary characterization, and moisture-preserving activity of polysaccharides from Ulva fasciata. International Journal of Biological Macromolecules, 72, 924–930. https://doi.org/10.1016/j.ijbiomac.2014.09.048
- Shi, X., Cheng, W., Wang, Q., Zhang, J., Wang, C., Li, M., Zhao, D., Wang, D., & An, Q. (2021). Exploring the protective and reparative mechanisms of G. Lucidum polysaccharides against H2O2-induced oxidative stress in human skin fibroblasts. Clinical Cosmetic and Investigational Dermatology, 14, 1481–1496. https://doi.org/10.2147/CCID.S334527
- Shi, Y. Y., Xiong, Q. P., Wang, X. L., Li, X., Yu, C. H., Wu, J., Yi, J., Zhao, X. J., Xu, Y., & Cui, H. (2016). Characterization of a novel purified polysaccharide from the flesh of Cipangopaludina chinensis. Carbohydrate Polymers, 136, 875–883. https://doi.org/10.1016/j.carbpol.2015.09.062
- Shih, J. H., Tsai, Y. F., Li, I. H., Chen, M. H., & Huang, Y. S. (2020). Hp-s1 ganglioside suppresses proinflammatory responses by inhibiting MyD88-dependent NF-κB and JNK/p38 MAPK pathways in lipopolysaccharide-stimulated microglial cells. Marine Drugs, 18(10), 496. https://doi.org/10.3390/md18100496
- Shih, P. H., & Yen, G. C. (2007). Differential expressions of antioxidant status in aging rats: The role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology, 8(2), 71–80. https://doi.org/10.1007/s10522-006-9033-y
- Song, Y. R., Han, A. R., & Park, S. G. (2020). Effect of enzyme-assisted extraction on the physicochemical properties and bioactive potential of lotus leaf polysaccharides. International Journal of Biological Macromolecules, 153, 169–179. https://doi.org/10.1016/j.ijbiomac.2020.02.252
- Su, Y., Zhang, Y., Fu, H., Yao, F., Liu, P., Mo, Q., Wang, D., Zhao, D., Wang, C., & Li, M. (2022). Physicochemical and anti-UVB-induced skin inflammatory properties of Lacticaseibacillus paracasei Subsp. paracasei SS-01 Strain Exopolysaccharide. Fermentation, 8(5), 198. https://doi.org/10.3390/fermentation8050198
- Sun, D., Cui, S. C., Ma, H. J., Zhu, P. F., Li, N., Zhang, X. W., Zhang, L. N., Xuan, L. J., & Li, J. Y. (2022a). Salvianolate ameliorates renal tubular injury through the Keap1/Nrf2/ARE pathway in mouse kidney ischemia-reperfusion injury. Journal of Ethnopharmacology, 293, 115331. https://doi.org/10.1016/j.jep.2022.115331
- Sun, Q., Fang, J., Wang, Z., Song, Z., Geng, J., Wang, D., Wang, C., & Li, M. (2022b). Two laminaria japonica fermentation broths alleviate oxidative stress and inflammatory response caused by UVB damage: Photoprotective and reparative effects. Marine Drugs, 20(10), 650. https://doi.org/10.3390/md20100650
- Sutivisedsak, N., Leathers, T. D., Biresaw, G., Nunnally Melinda, S., & Bischoff Kenneth, M. (2016). Simplified process for preparation of schizophyllan solutions for biomaterial applications. Preparative Biochemistry and Biotechnology, 46(3). https://doi.org/10.1080/10826068.2015.1031392
- Wang, J. Y., Liu, Y. C., & Han, W. (2018). Analysis on enzyme production of Schizophyllum commune fermented with corn bran fiber and cloning, expression and characterization of xylanase gene. Food and Fermentation Industries, 44(5), 6.
- Wu, D., Liu, P. P., & Li, M. (2019). Evaluation of in vitro anti-oxidation and anti-aging effects of water extract and fermentation broth of Pueraria lobata root. Science and Technology of Food Industry, 40(12), 285–290 + 294.
- Xia, D., & Lin, Z. B. (1990). Effects of Schizophyllum intracellular polysaccharide and extracellular polysaccharide on the immune function of mice. Acta Pharmaceutica Sinica, 25(3), 161–166. CNKI:SUN:YXXB.0.1990-03-000
- Xiang, G., Zheng, C., & Zheng, Q. (2017). Salvianolic acid A attenuates early brain injury after subarachnoid hemorrhage in rats by regulating ERK/P38/Nrf2 signaling. American Journal of Translational Research, 9(12), 5643–5652.
- Yang, S., Park, S. H., Sae, O. W., Kwon, K., Yu, E., Lee, C. W., Son, Y. K., Kim, C., Lee, B. H., Cho, J. Y., Kim, Y. J., & Lee, J. (2022). Antioxidant activities and mechanisms of tomentosin in human keratinocytes. Antioxidants, 11(5). https://doi.org/10.3390/antiox11050990
- Yang, Y., Li, W., & Li, Y. (2014). Dietary lycium barbarum polysaccharide induces Nrf2/ARE pathway and ameliorates insulin resistance induced by high-fat via activation of PI3K/AKT signaling. Oxidative Medicine and Cellular Longevity, 2014(1), 145641. https://doi.org/10.1155/2014/145641
- Zhang, L., Zhang, Q., & Zheng, Y. (2018). Study of Schiff base formation between dialdehyde cellulose and proteins, and its application for the deproteinization of crude polysaccharide extracts. Industrial Crops and Products, 112, 532–540. https://doi.org/10.1016/j.indcrop.2017.12.056
- Zhang, Y., Liu, P., Fu, H., Wang, D., Zhao, D., Zhang, J., Wang, C., & Li, M. (2022). Effects of Lactobacillus Kefiri fermentation supernatant on skin aging caused by oxidative stress. Journal of Functional Foods, 96, 105222. https://doi.org/10.1016/j.jff.2022.105222
- Zhao, D., Xu, D. N., & Wang, D. D. (2016). Component detection of Ganoderma lucidum fermentation broth and evaluation of whitening and anti-aging effects. Daily Chemical Industry, 46((04|4)), 226–230 + 242. https://doi.org/10.13218/j.cnki.csdc.2016.04.010
- Zhe, Y., & Chen, C. M. (2019). Characterization of physicochemical and biological properties of Schizophyllum commune polysaccharide extracted with different methods. International Journal of Biological Macromolecules, 10(156), 1425–1434. https://doi.org/10.1016/j.ijbiomac.2019.11.183
- Zhuang, Y. L., Hou, H., Zhao, X., Zhang, Z. H., & Li, B. F. (2009). Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV irradiation. Journal of Food Science, 74(6), H183–H188. https://doi.org/10.1111/j.1750-3841.2009.01236.x