ABSTRACT
Kaempferol is a flavonoid present in many eatable plants. Researchers discovered a link between consuming foods high in kaempferol and lowering the threat for acquiring many diseases, including cardiovascular disease, diabetes, obesity and cancer. Kaempferol can inhibit AHR transcription, modulate ERK signalling pathway and NF-κB pathways, block MAPK and AP1 signalling pathways and perform more anticancer roles. Kaempferol also has the ability to act against diabetes via suppressed phosphorylation of (IKK) IkB kinase, (IRS-1) insulin receptor substrate-1 and (IKK) IkB kinase through the hepatic IKK/NF-B signalling pathways and significantly enhanced insulin secretion and synthesis. Kaempferol protects cardiomyocytes from anoxia/reoxygenation (A/R)-induced damage by lowering LDH release, improving cell survival, reducing A/R-induced ROS formation, and release of cytochrome c. This knowledge may aid in understanding health advantages of medicinal plants that contain kaempferol and may lead to the development of the flavonoid as a potential agents for disease aversion and therapy.
Introduction
Flavonoids are a kind of secondary metabolite of plants with diphenylpropane structure. These are present throughout plant kingdom and are ubiquitous components of fruits, several drinks and vegetables. Flavonoids have a role in lower risk of chronic illnesses linked with a plant-based diet. Some epidemiological researchers have discovered a link between intake of foods high in flavonoids and lower threat of emerging cardiovascular disease and cancer. In vitro and in vivo studies have displayed probable pathways through which flavonols may protect against cardiovascular diseases and cancer. Studies also showed that some flavonols might be beneficial in the therapeutic management of a variety of disorders. Being a natural flavonoid, kaempferol can be administered alone or with other drugs (Wang et al., Citation2019). But limited data is available on comparative studies of drugs and kaempferol being used as treatment in pre-clinical or clinical trials. Some of the data comes from research on herbs utilized in traditional medication to cure a variety of diseases, which indicated that flavonols are frequent bioactive metabolites of plants (Jan et al., Citation2022).
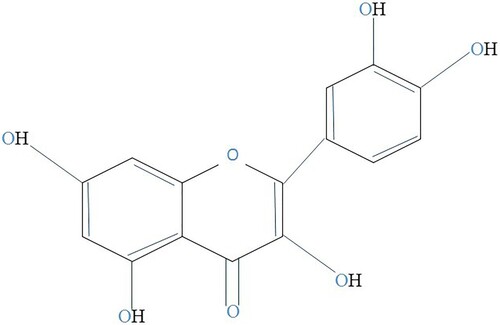
The flavonol kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) is yellow molecule with lesser molecular weight (MW: 286.2 g/mol) discovered in foods resulting from plants and herbal remedies. The goal of this review is to understanding the preventative and beneficial capabilities of kaempferol-comprising herbs, as well as the development of this flavonoid as a potential agent for both the avoidance and management of certain illnesses (Kashyap et al., Citation2017). Kaempferol has diphenylpropane structure (C6–C3–C6) and it is synthesised by combining four molecules of 4-coumaroyl-CoA (C6-C3) with 3 molecules of the malonylCoA (C6). The flavonol (C6–C3–C6) naringenin chalcone is formed as a result of this response, which is catalysed by the enzyme chalcone synthase. The enzyme chalcone isomerase (EC 5.5.1.6), which catalyses the closing of the C3 ring, converts this chalcone into the flavanone naringenin. To create dihydrokaempferol, enzyme flavanone 3-dioxygenase (EC 1.14.11.9) inserts hydroxyl group at C3 in a naringenin. Lastly, the enzyme flavonol synthase (EC 1.14.11.23) creates kaempferol by introducing double bond into dihydrokaempferol at C2–C3. Kaempferol-3-(p-coumaroyltriglucoside) is not frequently found in herbs because its bioavailability necessitates the existence of three extra enzymes which are not widely disseminated: flavonol-3-O-diglycoside glucosyltransferase (EC 2.4.1.240), flavonol-3-O-glucoside glucosyltransferase (EC 2.4.1.239), and flavonol-3-O-triglucoside p-coumaroyltransferase (Haeri et al., Citation2023). The chemical structure of kaempferol was shown in .
Pharmacokinetics of kaempferol
Various in vitro research had shown that kaempferol has a diverse set of natural processes. Some of these accomplishments, though, might be irrelevant in vivo analysis if flavonoid does not approach targeted tissues and organs at precise levels for a particular period of time. In other research, kaempferol's rapid metabolism and limited levels of bioavailability can make it difficult for it to produce various bioactivities in vivo analysis. Because kaempferol is a common dietary component and the oral way of administration is the favoured way of management for the majority of medicines, it is critical to comprehend the outcome of the kaempferol in the body when consumed through oral route. This flavonoid pharmacokinetics has been examined in vivo and in vitro clinical trials. Flavanols like kaempferol are widely consumed as glycosides. High polarity of glycosides is known to inhibit concentration, while the transitional polarity of the aglycones helps it. Though this implies that the absorption of glycoside requires prior hydrolysis to most absorbable aglycones, research have demonstrated that glycosides can be consumed without hydrolysis (Zabela et al., Citation2016).
Kaempferol is primarily absorbed in the small intestine, similar to other flavonoids. Kaempferol’s lipophilicity makes passive diffusion easier, but there is evidence that it can also be absorbed through assisted diffusion or active transport. Intestinal conjugation enzymes can metabolize kaempferol in the small intestine (to glucuronides and sulfoconjugates). Like other flavonoids, kaempferol glycosides and kaempferol are extensively metabolized in the colon bacteria. Colonic bacteria have the ability to hydrolyse glycosides into aglycones and break the C3 ring of aglycones to produce simple phenolic compounds that can be absorbed or ejected in faeces, such as 4-hydroxyphenylacetic acid, 4-methylphenol, and phloroglucinol (Zhang et al., Citation2015a). After absorption, kaempferol undergoes significant liver metabolism to produce glucurono- and sulfo-conjugated derivatives. These conjugated types of kaempferol, as well as other phenolic substances generated by the colon probiotics, some kaempferol glycosides and originally kaempferol, can enter the tissues and systemic circulation, where they are eliminated in urine. The % of kaempferol defecated in urine was determined to be between 1.9% and 2.5% of the total quantity consumed. Kaempferol's limited oral bioavailability can be addressed by employing alternate modes of administration, such as intravenous (Zhang et al., Citation2015b). Half-life of kaempferol ranges from 2.9 to 3.8 min when administered intravenously. It takes about 3.8 min for a specific concentration of kaempferol to be reduced to half in blood. Half-life of kaempferol depends upon number of factors, i.e. dose, administration route and the metabolism of the individual (Zabela et al., Citation2016).
Pharmacodynamics of kaempferol is complex and it is yet to be studied further, to completely understand the underlying processed. It also depends heavily on the condition and/or diseases that are being treated. In some cases, kaempferol has been shown to have antioxidant, anti-inflammatory, and anti-cancer properties. In other cases, kaempferol has been shown to have estrogenic, antidiabetic, and neuroprotective properties. Kaempferol is distributed throughout the body. Highest concentrations are found in the liver, kidneys and colon. Lower concentrations were found in the brain, heart, and lungs, according to a study done on rats. It was also speculated that tissue distribution of kaempferol varies depending on the dose and the route of administration. During the study, it was also found out that the distribution of kaempferol was different after oral and intravenous administration. After oral administration, kaempferol was more concentrated in the liver and kidneys, while after intravenous administration, kaempferol was more concentrated in the brain and heart (Barve et al., Citation2009).
When kaempferol is consumed, the liver and intestines break it down into a variety of metabolites. 4-hydroxyphenylacetic acid (4-HPAA) is the main metabolite of kaempferol. It is produced by the liver and is excreted in the urine. 3-methylkaempferol is another major metabolite of kaempferol. It is produced in the gut and is excreted in the urine. Isorhamnetin is a minor metabolite of kaempferol. It is produced by the liver and is excreted in the urine (Kim et al., Citation2008).
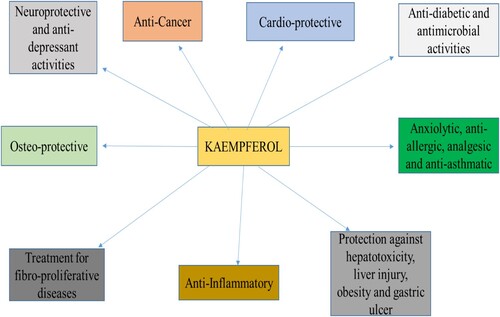
Health benefits of kaempferol are presented in .
Health perspectives
Anti-cancerous activity of kaempferol
Epidemiological research (cohort studies and case control) shows that consuming foods high in kaempferol may lower the incidence of cancer, such as pancreatic cancer, lung cancer, gastric cancer, and ovarian cancer. While few studies have looked at kaempferol's anti-carcinogenic impact on animal models, many preclinical studies show that this kaempferol possesses cancer-preventive and cancer-treatment characteristics (Shahbaz et al., Citation2023).
The most widely accepted theory of carcinogenesis (the somatic mutation theory of cancer) states that DNA mutations cause cancer, and numerous studies have shown that kaempferol may shield DNA from damage caused by various carcinogens. It is commonly believed that the development of malignant tumour necessitates the acquisition of various capacities (the supposed biomarkers of tumour), such as apoptotic sensitivity, enhanced angiogenesis, or the ability of metastasis and invasion. Cancerous cells must gain apoptosis resistance to form a tumour, and this can be averted by apoptosis triggered by kaempferol. Solid tumour development can be done by the creation of newly originated blood vessels called angiogenesis without expansion of vessels, tissue tumour volume is limited to diffusion distance of approximately 0.2 mm. Angiogenesis is called to be activated in malignant tumours and kaempferol has been discovered to block the new blood vessels in vitro analysis. It is widely acknowledged that the metastatic dissemination of original tumours comprising of roughly 90% of all tumour fatalities, the procedure according to which cells from localized tumour penetrate adjoined tissues and develop metastasis to the distant organs might lead to the most practical procedure linked to carcinogenesis. Kaempferol has been shown in studies to suppress this mechanism in vivo and in vitro (Liu et al., Citation2019).
ROS has central role in cancer development which has been displayed by scientific research demonstrating that the cancer cells commonly contain excessive amounts of ROS which can cause cell malignant changes, thus decreasing cellular ROS levels can reverse the malignant phenotype of tumour cells. Antioxidant agents may have a protective function against cancer development because they can avert the build-up and/or reduction of extreme cellular levels of reactive oxygen species. For example, cellular levels of ROS are reduced by the production of antioxidant enzymes into cancer cells; these cancer cells restored to original look, their development rate normalized, and they were further no longer able to generate tumours in athymic mice. These research showed that kaempferol has suppressive role against different cancer cells lines such as gastric, prostate, cervical, bladder, oesophageal, intestinal, ovarian, and thyroid cancers through suppressing the invasion and migration stages of cancers (Sheik et al., Citation2021). Inflammatory cells, cytokines, and chemokines are also present in the early stages of all malignancies in people and research animal models. Tumour growth is aided by the overexpression of inflammatory cytokines and transfer of inflammatory cells. Additionally, targeting of the inflammatory mediators (cytokines and chemokines, such as IL-1and TNF), transcription components convoluted in infection (including STAT3 and NF-B) or inflammation causing cells reduces the spread and incidence of tumour. Non-steroidal anti-infectious medicines have also been shown to lessen the likelihood of some malignancies forming (for instance breast and colon tumour) and decrease the lethality resulted by these tumours. These findings show that kaempferol's anti-infectious characteristics may lead a key function in the flavonoid's cancer-reducing effect. To become genotoxic, numerous chemical carcinomas must be enzymatically stimulated, and cytochrome P450 enzymes are most notable enzymes implicated in this stimulation. Furthermore, kaempferol may suppress P450 and hence limit the stimulation of carcinogenic substances (Nandi et al., Citation2023).
Kaempferol acts an effective antagonist of (AhR) aryl hydrocarbon receptor, which is triggered by the human carcinogens found in cigarette smoke and air pollution. This flavonoid also decreased the potential of cigarette smoke vaporize to trigger the formation of immortalized epithelial cells present in lungs, according to the researchers. In the liver of mice, oral treatment of the kaempferol and Ginkgo biloba extract (EGb) that contain 24% flavonol at 100 mg/kg body weight reduced AhR conversion caused by 3-methylcholanthrene. Kaempferol is also a powerful antagonist of sulfotransferase 1A1 having no competition with other flavonol, an enzyme which is able to develop reactive electrophiles from bioactivated procarcinogens. Kaempferol might promote phase 2 purifying enzymes as like quinone reductase in addition to blocking many phase I enzymes. Kaempferol has been shown to lower cellular levels of carcinogen 7,12-dimethylbenz(a)-anthracene by increasing level of P-glycoprotein-mediated efflux of carcinogen (Suguna & Umesha, Citation2023).
Hypoxia-inducible factor 1 (HIF-1) is a major regulator of homeostasis of oxygen. HIF-1 activation is recognized to have critical role in most important features of carcinogenesis, such as angiogenesis, cell survival, invasion, metastasis, metabolic reprogramming and cellular immortalization. HIF-1 activity is seen in the majority of human malignancies and has been linked to higher mortality among patients. HIF-1 expression was shown to be higher in 13 tumour kinds, involving colon, lung, breast, and prostate cancers, which are most frequent malignancies in industrialized nations. These findings show that HIF-1 stimulation is critical function in cancer causing factors and, as such, a significant goal for tumour prevention through chemicals. The impact of kaempferol on HIF-1 activity, impression levels, and localization in hepatoma cancer cells was studied, and it was shown that this dietary flavonol suppressed transcription component in the lower micro-molar series. Kaempferol also inhibited HIF-1 expression in ovarian carcinomas (Simington, Citation2022).
The ribosomal S6 kinase 2(RSK2) is a member of the (RSK) p90 protein family and it is a broadly articulated threonine/serine kinase, is triggered in response to numerous peptide hormones and growth factors via phosphoinositide-dependent kinase 1 and extracellular signal regulated kinase 1/2. Its activation is implicated in carcinogen-induced transformation of cell and proliferation. Kaempferol in MCF-7 and LNCaP cancer cells lines suppresses the cell growth, ribosomal S6 kinase 2(RSK2) proliferation, development of hormone-depending malignancies and induces induce differentiation (Murden et al., Citation2016).
Aside from its cancer-preventive characteristics, kaempferol has demonstrated effects that might be important to tumour prevention. Various reports had determined that the kaempferol and some glycosides of kaempferol activate cell mortality in a diversity of carcinomas from various tissues, comprising oesophagus, liver, ovary, lung, colon, breast, prostate, pancreas, lymph/blood, brain, uterus, thyroid and bone. Numerous studies have found that kaempferol causes cell mortality via apoptosis, and the potential pathways, i.e. caspase-7, caspase-3, and caspase-9. Caspases are kind of cysteine protease that is included in the start and implementation of apoptotic cell death. Kaempferol has been shown to activate caspase-7, caspase-3, and caspase-9. This flavonoid may stimulate apoptosis-inducing factor (AIF) that is implicated in start of caspase-independent apoptosis trail. Various investigations have found that kaempferol lowers the anti-apoptotic proteins such as Bcl-Xl and Bcl-2 while increasing pro-apoptotic proteins Bad and Bax. This dietary supplement can also reduce the expression of the anti-poptotic protein (XIAP) Xlinked regulator of protein of apoptosis. The loss of mitochondrial membrane potential, (PARP) PoliADP-ribose polymerase activation and cytochrome c release are significant proceedings in initiation of cell death, and kaempferol has been discovered to induce PARP and damage of mitochondrial membrane potential and stimulate release of cytochrome c (He et al., Citation2019). showed the anticancer mechanisms associated with kaempferol.
Figure 3. Anticancer mechanisms associated with kaempferol. Figure shows kaempferol induces apoptosis in tumour cells through a variety of signalling pathways and mitochondrial mechanisms. Kaempferol inhibits the PI3K/AKT/mTOR pathway, inhibition leads to the activation of pro-apoptotic proteins, p53 and caspases (CASP). Kaempferol suppresses ERK pathway, leads to the activation of MAPK. The inhibition of NF-κB pathway activates pro-apoptotic protein CASP8. Mitochondrial targeting causes cytochrome-c release, which activates caspases.
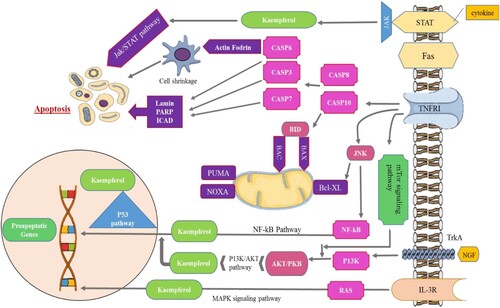
Kaempferol causes energy failure by inhibiting glucose intake and complex I of mitochondrial respiratory series; this leads in the autophagy induction, which can mitigate kaempferol's ability to trigger apoptosis to some extent. Kaempferol has capacity to limit the activation of (FAS) fatty acid synthase that is lipogenic enzyme which is activated in carcinomas has been linked to its potential to trigger apoptosis in cancer cells. Several anticancer medications, including doxorubicin, etoposide, irinotecan, and topotecan, target the enzymes DNA topoisomerases 1 and 2, while kaempferol has discovered to lower topoisomerase 1and topoisomerase 2. Kaempferol results in cell cycle arrest in the G2/M, which could be achieved by inhibiting the activation of cyclin-dependent kinase (CDK1). The proteasomes are enlarged complexes of protein that use proteolysis to breakdown damaged or unwanted proteins; these complexes have to play a crucial role in controlling levels of protein and are required for cell existence. Proteosoma suppressors (e.g. bortezomib) are a novel family of anti-tumour drugs, while kaempferol has been reported to behave as proteasome suppressor in the human leukaemia cells. Kaempferol's anti-proliferative actions in carcinomas may possibly be controlled via the suppression of the ERK/ MAPK pathway. Similarly, low doses of kaempferol have been shown to lower cellular ROS levels and elicit antioxidant effects. Evidence shows that kaempferol might be used with a number of anticancer medicines to enhance their therapeutic effectiveness. According to studies, kaempferol may make carcinomas more sensitive to the cytotoxic impacts of cisplatin, cytarabine, 5-fluorouracil, mitoxantrone, doxorubicin, and stimulated metabolite of (SN-38) irinotecan. Additionally, it also boosts the cytotoxic effects of (TRAIL) tumour necrosis factor related to apoptosis inducing ligand. These findings indicate that kaempferol may have practical uses as an adjuvant treatment in the therapy of some malignancies (Sharma et al., Citation2022).
Anti-oxidant and anti-inflammatory effects of kaempferol
Inflammation is non-specific immune response that happens due to an injury or illness. Under normal settings, the inflammatory response is self-limiting; nevertheless, in some illnesses, the inflammatory response can become uncontrolled, resulting in persistent or chronic inflammatory disorders. Many studies have demonstrated that persistent inflammation caused by oxidative stress may end up in illnesses such as cancer, cardiovascular disease, and neurological issues. The chronic inflammatory diseases, like Hepatitis B virus infection and Helicobacter pylori infection, have been related to hepatic and gastric carcinomas, respectively. Due to its anti-inflammatory and anti-oxidant properties, kaempferol has inspired numerous experts to research its molecular processes and possible application in treatment of inflammatory illnesses (Alam et al., Citation2020).
Kaempferol reduces brain damage caused due to reperfusion/ischemia. In a subsequent investigation, kaempferol inhibited enhancement in the ROS levels generated by lipopolysaccharide in the rat macrophages and alleviated bladder hyperactivity triggered due to potassium chloride following bladder damage induced by potamine sulphate. Kaempferol's inhibitory impact on generation of ROS in bladder tissue was also established. Alternative experiment revealed that aforementioned inhibitory consequences were achieved via inhibiting the stimulation of the enzymes (COX-2) cyclooxygenase-2 and inducible (iNOS) nitric oxide synthase. Cyclophosphamide induced cystitis in the rats that is well-known cystitis model, has also shown to be facilitated by COX-2 and iNOS activation. Additionally, it has been demonstrated that a COX-2 inhibitor or an iNOS inhibitor will lessen bladder hyperactivity brought on by inflammation (Wang et al., Citation2020).
Kaempferol as a modulator of anti-oxidant enzymes
Kaempferol is an effective free radical and superoxide radical scavenger that reduces microsomal lipid peroxidation. Antioxidant enzymes are essential for neutralising superoxide radicals and free radicals produced by a variety of sources. These enzymes are thought to play an essential part in kaempferol's impact. According to a study, kaempferol may help the alcohol- and PUFA-induced oxidative stress rat liver tissue express more glutathione-S-transferase, catalase, glutathione peroxidase, and reduced glutathione levels. Furthermore, kaempferol can counteract the diethylnitrosamine-induced reduction in mRNA expression and activity of the anti-oxidant enzymes including glutathione-S-transferase, catalase, and glutathione peroxidase. Kaempferol may thus be useful in reducing the negative effects of high amounts of the free radicals during inflammatory illnesses. Kaempferol is being studied for its effects on the glutathione that is tripeptide that is important in detoxification of drugs and can reduce ROS damage. Kaempferol has been shown in-vitro to ameliorate experimental anti-encephalitis. Furthermore, kaempferol can counteract the decline in glutathione, catalase and glutathione peroxidase levels reported in gentamicin and cisplatin treated rats’ kidney tissue. The effect of kaempferol on glutathione levels into body has to be studied further since these findings may clarify its involvement in suppression of inflammation or possibly carcinogenesis (Devi et al., Citation2015).
NF-kB is a transcription factor that may be activated by the range of factors such as cytokines, bacteria, stress, viruses and free radicals. Many genes such as angiogenic factors, regulatory molecules of cell-cycle, enzymes, and cytokines are regulated by this transcription factor. Kaempferol has been shown to block the overproduction of pro-inflammatory cytokines in BALF, such as IL-6, IL-1, and V as well such as significantly lower activation of NF-B and MAPKs signalling pathways activated by LPS in mice with LPS-induced acute lung damage. Another study discovered that kaempferol in LPS-stimulated macrophages prevented from the translocation of NF-kB into the nucleus. It also blocks the LPS-induced NF-B signalling cascade in RAW 264.7 cells by targeting IKK. The positive effects of kaempferol regulation of NF-kB have been explored in numerous inflammatory disorders models. It had been demonstrated that kaempferol might inhibit or improve Japanese encephalomyelitis, presumably by preventing NF-kB (Qattan et al., Citation2022). A study found that kaempferol protects against rheumatoid arthritis, perhaps via inhibiting ERK1/2 phosphorylation, NF-kB-p65 and p38. Kaempferol's reduction of NF-kB is expected to play a major role in anti-inflammatory effect. STAT3 controls the expression of genes used in angiogenesis, cell differentiation, cell death, proliferation, and immunological responses. STAT3 is frequently active in many carcinomas and interferes with carcinogenesis at various stages. A drop in survivin protein levels caused by kaempferol contributes to apoptosis that is TRAIL-induced. In kaempferol-treated cells, inhibiting proteasomal degradation with MG132 restores levels of survivin protein in two glial cell lines. As a result, survivin overexpression reduces TRAI-induced apoptosis with kaempfrol. Furthermore, revealed that the kaempferol causes the inhibition of phosphorylated Akt, lowering survivin protein level even further. Furthermore, kaempferol inhibits survivin via inhibiting the activity of the serine/threonine kinase Akt, because the active phosphorylated Akt increases stability of survivin. However, the combination of TRAIL and kaempferol therapy causes caspase-8 cleavage (activity), resulting in surviving independent proapoptotic action that does not block the activation of caspase-8. Other effects of kaempferol include concentration-dependent inhibition of apoptosis proteins such for instance Bcl-2, Bcl-xL, and Mcl-1, all of which belong to antiapoptotic Bcl-2 family. Kaempferol has been shown to drastically inhibit the production of IL-8 in the human lung epithelial cells that are LPS-stimulated. In addition to the previously documented antioxidant properties, this study found that kaempferol had PARP-1-inhibiting action. The suppression of PARP-1 and the maintenance of energy generation and cellular NAD1 may play a role in kaempferol's anti-inflammatory action (Estakhri et al., Citation2020).
Inflammatory mediators and cytokines play critical roles in the inflammatory response. Inhibiting these targets is thus used to avoid and lessen the harm caused by inflammation. Kaempferol significantly lessens LPS-induced proinflammatory cytokines such as IL-8 and IL-6 and prostaglandin (PGF2 α and PGE2) secretion in foetal membranes; prostaglandin production and gene expression of IL-1 induced COX-2 in the myometrium or IL-1 induced MMP-9 action in myometrial and amnion cells. It also lowers nuclear c-Jun expression and binding activity of IL-1β-induced NF-κB p65 DNA. Additionally, Tyk-STAT signalling was found to be inhibited by kaempferol in both OVA-challenged mice and BEAS2B cells that had been activated by LPS. Tyk2, which is in charge of producing eotaxin-1, was activated by LPS, but kaempferol stopped this from happening. Kaempferol similarly prevented Tyk2 activation in mice given OVA. In Prevotella intermedia LPS-stimulated RAW264.7 cells, another study discovered that kaempferol decreases NO production and iNOS protein expression at the translational level through HO-1-mediated ROS reduction and might be an effective regulator of the immune system's reaction in the therapeutic management of periodontal disease. Kaempferol reduces LPS-mediated HMGB1 release as well as LPS- or HMGB1-mediated cell-adhesion molecule expression and barrier permeability. Further research indicated that kaempferol suppresses toll-like receptor (TLR) 2/4 or HMGB1 receptor of cell-surface, but not the RAGE receptor. These findings imply that kaempferol inhibits HMGB1-mediated pro-inflammatory responses, making it effective as a treatment for vascular inflammatory disorders (Lee et al., Citation2015).
Anti-diabetic activity of kaempferol
Obesity and type 2-diabetes have become more prevalent in both developed and developing nations during the last century. Diabetes had a global prevalence of 280 million individuals (6.2% of the entire population) in 2010, and it is estimated that by 2030, the figure will have risen to more than 7.5% of the whole population. The rise in type 2 diabetes is directly related to the rise in obesity. Excess weight is responsible for around 90% of type 2 diabetes. Furthermore, due to obesity and the accompanying metabolic syndrome, roughly 197 million people globally have reduced glucose tolerance (Yang et al., Citation2021a; Zang et al., Citation2015).
One of the most common diabetic microvascular problems is diabetic retinopathy. Glucose at the concentration of about 25 mM increases expression levels of mRNA of the placenta growth factor (PGF) and VEGF, in addition the quantities of the secreted PGF and VEGF in (HRECs) human retinal endothelial cells. Under high-glucose circumstances, the injection of kaempferol at a range of 5–25 M significantly reduced migratory distance, cell proliferation, and HRECs growth. Furthermore, kaempferol inhibited Erk1/2, Src, and Akt1 activation and reduced PI3K impression. Researchers have investigated kaempferol's cytoprotective impact against palmitic acid-prompted pancreatic-cell mortality via autophagy regulation through the mTOR/AMPK signalling trail. Kaempferol elevates apoptotic cellular lethal action and increases cell existence while also improving the production of LC3 puncta and LC3-2 protein, according to scientists. These findings imply that kaempferol protects from lipotoxicity by activating autophagy through AMPK/mTOR pathway. Similarly, an early meta-analysis revealed the positive effect of kaempferol intake in the diet. A meta-analysis was done to explore the connection among diabetes onset and flavonoids intake. Researchers calculated the summary of risk using a fixed effect model and included cohort studies from 2013. The findings revealed a substantial negative relationship between flavonoids intake and diabetes development (Kim & Park, Citation2020).
The phosphorylation of (IKK) IkB kinase, (IRS-1) insulin receptor substrate-1 and (IKK) IkB kinase via the hepatic IKK/NF-B signalling pathway was suppressed while blood lipids and insulin levels were improved in diabetic rats treated with kaempferol at doses of 50 and 150 mg/kg. Furthermore, previous research has shown that kaempferol can enhance glucose balance, hyperglycaemia, and increased levels of blood insulin in obese mice that are fed on high fat diet. These research imply that kaempferol may operate as a drug can be used as anti-diabetic by increasing peripheral blood insulin sensitivity and guarding in contrary to pancreatic-cell failure. Other research has found that feeding a diet rich in fat to the mice C57BL/6J reverses the result on parameters including triglyceride concentration, body weight, adipose tissue, (PPAR-γ) activated receptor of peroxisome proliferator, blood glucose level, (haemoglobin A[1c]) serum HbA1c levels, and expression of sterol regulatory element binding protein (SREBP-1c) expression. Scientists discovered that kaempferol increases cell survival, lowers caspase-3 activity and inhibits apoptosis in human islets and INS-1E cells. Additionally, it prevents lipotoxicity from causing the antiapoptotic proteins Akt and Bcl-2 to be down regulated. Along with increasing duodenal homeobox-1 (PDX-1) and pancreatic expression, kaempferol significantly enhanced insulin secretion and synthesis. It also improved CREB phosphorylation, protein kinase A activation, and cyclic adenosine monophosphate (cAMP) generation. Additionally, it controlled transcriptional activity in cells by activating the signalling pathway of CREB/PDX-1/cAMP/PKA (Alkhalidy et al., Citation2018).
Cardio-protective effect of kaempferol
In male albino rats with diabetes, kaempferol protects against myocardial (IR) ischemia-reperfusion injury, according to a recent study. It dramatically lowers hyperglycaemia, suppresses the receptor for advanced glycation end products (RAGE) axis, normalizes oxidation and retains morphological alterations, maintains hemodynamic function. Additionally, it reduces levels of TNF-, IL-6, and NF-B, suppresses c-JNK, and increases the ERK1/2 and p38 proteins. Similar to this, by lowering the expression of protein kaempferol prevented apoptosis that promote apoptosis, expression of protein also increased by caspase-3 and Bax that prevent apoptosis, such as Bcl-2. Additionally, it improved the level of (Bcl-2) ant apoptotic protein. In an previous research, it was shown that kaempferol (a) upregulates ERK1/ERK2; (b) reduces expressions of IL-6, TNF-α, and NFκB; (c) downregulates JNK and p38 proteins; (d) enhances the level of ant apoptotic proteins (Bcl-2) in the IR model of myocardial injury; (e) decreases the level of pro-apoptotic proteins such as caspase-3 and Bax, TUNEL positive cells, and (f) induces apoptosis. The function of flavonoids in cardiovascular protection was evaluated using a meta-analysis by researchers. Information from the Cochrane, MEDLINE, and EMBASE databases were used, which was then subjected to inclusion and exclusion standards prior to being included in the meta-analysis. Numerous flavonoids demonstrated dose-dependent cardioprotective benefits, according to an analysis of 133 clinical studies (Chen et al., Citation2022).
It is related to the control of (OPN)-osteopontin linked signalling pathways that the nuclear factor-b (NF-B) is activated in primary human umbilical vein endothelial cells (HUVECs). On the other hand, kaempferol was discovered to diminish the generation of TNF-, ROS, NF-B, OPN, (P-IB) inhibitor of NF-B alpha phosphorylation, IL-6, and lowered (Nox4) nicotinamide adenine dinucleotide phosphate-oxidase 4, P-IB, and v3 integrin expression. In a similar vein, it was shown that kaempferol protects rats against myocardial I/R damage by lowering TUNEL-positive cell rates and decreasing infarct size. It lowers cytochrome C in cytoplasm, lactate dehydrogenase, cleaved caspase 3, TNF-, malondialdehyde levels and creatine kinase, while increasing left ventricular generated stress and its max down/up rate (dt/dp maximum). Additionally, kaempferol also has effect on superoxide dismutase, glutathione disulphide/glutathione, (P-GSK-3beta) phospho-GSK-3 beta, and kinase-3 beta of total glycogen synthase (Shrivastava et al., Citation2016).
According to the findings, kaempferol protects cardiomyocytes from anoxia/reoxygenation (A/R)-induced damage by lowering LDH release, improving cell survival, reducing A/R-induced ROS formation, cytochrome c release, and loss of Δψm from mitochondria in cytosol. Moreover, it upregulated the impression of human silent info regulator Type 1, activated caspase-3, inhibited the opening of A/R-stimulated mPTP, and improved the impression of Bcl 2. Likewise, it suppressed the (VSMC) vascular smooth muscle cell migration and proliferation, BMP4 signalling pathway and moderated the expression levels of microRNA. It additionally activated the BMP signalling pathway, induced apoptotic cell death, downregulated DOCK4, 7, and 5, antagonized PDGF-mediated pro-migratory effect and induced miR-21 expression. Furthermore, intraperitoneal treatment of (STZ; 40 mg/kg BW) streptozotocin produced diabetes in mature male rats, but kaempferol (100 mg/kg BW) reduced total ATPase, Ca(2+)-ATPase, Mg(2+) K(+)/Na(+)-ATPase-ATPase actions in tissues and erythrocytes (Duan et al., Citation2017).
In male Wistar rats with (MI) myocardial infarction, oral administration of kaempferol 5, 10, and 20 mg/kg/day, i.p. significantly increased arterial pressure, decreased left ventricular pressure, decreased left ventricular end-diastolic pressure, and increased catalase, glutathione, and superoxide dismutase concentrations. Malondialdehyde, TNF-, serum IL-6, heart damage indicators such as creatine kinase-MB and lactate dehydrogenase and the Bax/Bcl-2 ratio all decreased after kaempferol administration. Similar to this, a team of researchers investigated how well kaempferol relaxed the smooth muscle cells in pig coronary arteries. These scientists found that kaempferol enhances modification brought on either endogenous NO, exogenous NO, or hyperpolarization that is endothelial-dependent, possibly through activating KCa 1.1 channels. However, when lipopolysaccharide (LPS) was added to ATP-induced cardiac fibroblasts, the results showed that kaempferol (12.5 and 25 g/mL) dramatically lowered the production of TNF-, IL-1, IL-6, and IL-18 inhibited the starting of NF-B and Akt (Hosseini et al., Citation2023).
Anti-obesity effect of kaempferol
Obesity and associated metabolic problems have become significant health concerns in recent years. Type II diabetes mellitus (c), coronary heart disease (CHD), stroke, hypertension, hyperlipidaemia, inflammatory bowel disease (IBD), non-alcoholic fatty liver disease (NAFLD), and cancer are all elevated by obesity. Obesity is linked to persistent, low-grade generalised inflammation. Chronic inflammation, often known as “metabolic inflammation,” is characterised by systemic inflammatory responses or low-level local in metabolic diseases. Metabolic inflammation is thought to be a sterile mechanism caused by nutrition, particularly excess dietary fat. In obesity and associated metabolic disorders, adipose tissue serves as a key cytokine sink. There is a lot of proof that inflammation can be brought on by hypoxia in adipose tissue that is developing. (NAFLD) Inflammation and steatosis are hallmarks of non-alcoholic fatty liver disease, which is also connected to obesity and is the hepatic manifestation of a metabolic condition (Torres-Villarreal et al., Citation2019).
Previous research has shown that natural chemicals taken from traditional medicine or food can modulate the metabolic syndrome and obesity. Because of their particular structural properties, several types of flavonoids have varied biological actions including anti-obesity antioxidant, anti-diabetic, anti-inflammatory and anti-carcinogenic effects. Notably, kaempferol (3,5,7-trihydroxy-2-[4-hydroxyphenyl]-4H-1-benzopyran-4-one), a prominent flavonol available in a wide range of fruits and vegetables, has received increasing interest for its biological features. Kaempferol has previously been shown to preserve the intestinal epithelial barrier and prevent inflammation in the intestines. Kaempferol has a significant therapeutic potential for treating obesity, according to recent studies. It may be able to decrease the development of adipose tissue in obese mice, enhance insulin sensitivity in diabetic rats, and block adipogenesis in adipose cell line 3T3-L1. Recent research suggests that kaempferol may have prebiotic potential because of its capacity to alter the metabolism and composition of gut bacteria (Zang et al., Citation2015).
According to studies, kaepferol reduces the amounts of lipid formation in adipocytes and zebrafish. SREBP-1C fatty acid synthetic proteins, lipin1 LPAAT (lysophosphatidic acid acyltransferase), DGAT1 (triglyceride artificial enzymes), and FASN impression levels are decreased. Additionally, kaempferol suppresses (mTOR) rapamycin and phosphorylation of (protein kinase B) AKT, slows cell cycle progression from the synthesis to G2/M stages, and dose-dependently modifies cyclins. Additionally, kaempferol upregulates the anti-early adipose components (pref-1 (preadipocyte factor-1), and KLF2) and downregulates the pro-early adipogenic components (KLFs, CCAAT-enhancer binding proteins, and Krüppel-like factors 4 and 5). Late adipogenic factors such as C/EBP and (PPAR) peroxisome proliferator-activated receptor have been proved to be suppressed by kaempferol. In addition, a group of researchers find that kaempferol downregulates 3T3-L1 adipocytes through various mechanisms including the reduction of levels of adipogenic transcription factors such as Cebpβ, Rxrβ, Pparγ, Srebp1, Rorα, Lxrβ and genes involved in the biosynthesis of triglyceride such as Agpat2, Gpd1, Dgat2 and improvement of lipolysis-related genes like Lsr, Tnfα, and Cel. In addition, kaempferol suppresses rosiglitazone instigate PPARγ transcriptional activity (Bian et al., Citation2022).
Results of a different study point to integrative responses involving kaempferol's anti-inflammatory and prebiotic properties as the mechanism by which it protects against HFD-induced obesity. Furthermore, the TLR4/NF-B pathway may operate as a mediator for kaempferol's modulatory effect. The present investigation was restricted to correlation analyses that cannot demonstrate causality, despite the identification of particular intestinal inflammation and microbiota pathways related to kaempferol-mediated reduction of obesity. Therefore, additional research is required to determine the particular molecular pathways involved. However, research supports the idea that kaempferol-rich meals or supplements could be protective and lower the incidence of obesity. It is also a promising field for the development of medicines based on flavonoids for the management of metabolic syndrome linked to obesity (Lee et al., Citation2015).
Anti-asthmatic role of kaempferol
Asthma is a broad category of non-communicable respiratory disease characterized by varying degrees of airflow limitation, bronchoconstriction, mucus hypersecretion, and oedema brought on by persistent (AHR) airway hyperresponsiveness to environmental stimuli, airway remodelling, and airway inflammation. The most prevalent type of asthma in the general population is allergic asthma, which typically develops in childhood and is triggered by allergens. This type of asthma causes a significant immune response involving both immune system cells such as dendritic cells, mast cells, Th2-lymphocytes, macrophages, and eosinophils and structural cells (such as smooth muscle cells and epithelial cells). There is an urgent need for innovative treatments with complex anti-asthmatic profiles, ideally with few side effects, given the rising prevalence of allergic asthma worldwide and the increasing resistance to the traditional therapy such as controllers, relievers, and a small number of biological medicines. Secondary plant metabolites known as flavonoids have a characteristic C6–C3–C6 skeleton structure, which consists of two benzene rings connected by pyran rings (Molitorisova et al., Citation2021).
Flavonoids have been found to reduce asthma symptoms and control immune reactions in both of the experimental and clinical studies. Kaempferol plays important role as a modulator of the inflammatory genes expression that are activated by certain cellular mechanisms of (PKC) protein kinase C, (MAPK) mitogen activated protein kinase, (PI3K) phosphatidylinositol 3-kinases, (JAK/STAT) Janus Kinase-Signal Transducer and Activator of Transcription pathways that control the purpose of transcription factors such as nuclear factor κB (NF-κB), activator protein-1 (AP-1) (Chung et al., Citation2015).
The substance reduces the induction of pro-inflammatory enzymes, including (COX2) cyclooxygenase 2, (PLA2) phospholipase A2, and (iNOS) inducible nitric oxide-synthase, which prevent the production of inflammatory mediators of arachidonic acid (for example, prostaglandins). Kaempferol’s antioxidant properties enable it to preserve the cellular oxidant–antioxidant equilibrium by interacting with reactive oxygen/nitrogen species and scavenging free and superoxide radicals. By preventing the release of the interleukins IL-1ß, IL-6, IL-18 (TNF-), tumour necrosis factor-, and (TSLP) thymic synaptophysin, kaempferol interferes with immunological responses (Khazdair et al., Citation2021).
Based on the dose–response relationship, the highest dose of the kaempferol (20 mg/kg b.w. p.o.) was used for a 21-day long-term peroral dosing. By closely emulating the bronchodilatory effects of reference long acting 2 agonist salmeterol, sRaw was found to significantly reduce histamine-induced airway hyperreactivity in rats given kaempferol treatment. The effects of short-term (20 mg/kg b.w. p.o., 21 days) and high-dose (20 mg/kg b.w. p.o., 72 h) kaempferol treatment on (TSM) tracheal smooth muscle responsiveness were examined in vitro. Following the infusion of 100 l of the constrictor mediator histamine into cumulative dosages that ranges 10 nM–1 mM, the contractile amplitude of isolated TSM strips was evaluated. Comparatively to healthy control group of the unsensitized animals (CON), animal sensitization with OVA significantly increased TSM contractility. All three single dosages of kaempferol decreased the peak intensity of smooth muscle contractions brought on by histamine (Rakha et al., Citation2022).
According to the traditional asthma model, immune cells that generate the cytokines IL-13, IL-5, IL-4, and IL-9, which are linked to a number of inflammatory changes, are primarily responsible for orchestrating Th2-allergic inflammation. The genesis of Th2-mediated allergic inflammation was demonstrated in an experimental model by increased levels of the cytokines IL-5, IL-4, IL-13, and GM-CSF as well as increased amounts of eosinophils in BAL fluid of sensitised mice. When a Th2-allergic response first begins, Th2-cell survival and the cytokine IL-4 regulate proliferation and help B-lymphocytes produce IgE, but pleiotropic IL-13 seems to play a bigger role during an allergic reaction. The primary effector cells of late asthmatic phase, in which inflammation, airflow restriction, and remodelling of the airway wall continue to occur, are eosinophils. In allergic asthma, a complicated interplay between 355 IL-13, IL-3, IL-5, CC-chemokine signalling, and GM-CSF regulates the lifespan of eosinophils. When compared to the standard anti-inflammatory drug budesonide, the 21-day dose of kaempferol dramatically decreased the 357 levels of the pro-inflammatory Th2-cytokines such as IL-5, IL-13, and GM-CSF as well as the number of eosinophils in BAL fluid (Barliana et al., Citation2022).
Effect of kaempferol on reproduction associated disorders
Progesterone is a steroid hormone that has a variety of functions in ordinary human physiology, most notably in the female reproductive system. The majority of these tasks are carried out by progesterone mandatory to nuclear progesterone receptors such as PRB and PRA and functioning as transcription component to control downregulated targeted genes. The functions of progesterone hormone in the female reproductive organs such as uterus, breasts and ovaries have received the greatest attention. Progesterone, specifically, is renowned for its capacity to inhibit oestrogen-activated endometrial epithelial growth. As a result, progesterone is used to treat undesired symptoms like premenopausal irregular blood loss or increased uterine growth in disorders like endometriosis. Because progesterone is not orally accessible until manufactured as micronized progesterone, these remedies are often for artificial progestins that link the progesterone receptor. Recent studies, however, indicate that women are progressively turning to botanical enhancements to relieve these and other ailments, such as premenstrual syndrome, infertility, and menopause. Customer spending on herbal nutrients climbed by more than 17% in 2020, reaching more than USD 11 billion nationally (Duračka et al., Citation2019).
Unluckily, though these extra nutrients may offer women alternative preventive options, they are frequently not controlled by Food and Drug Administration (FDA) of the United States and thus are not subject to severe analysis to recognize active substances, inherent toxicity, operational doses, or drug-supplement connections. As a result, quantifying and characterizing active chemicals in botanical supplements, as well as their biological goals within animal and cellular structures, are critical. Numerous readings have found chemicals in botanical supplements that can bind to human steroid receptors then activate their downregulation consequences. Usually, these substances comprised phytoestrogens that can fix oestrogen receptor, nevertheless lately, phytoprogestins, which can connect the progesterone receptor, have been discovered. Artificial progestins are frequently discovered as therapies for a variety of undesirable symptoms associated with the feminine reproductive tract (Wu et al., Citation2017).
Though these substances have contemporary theoretical implications in women's well-being, the original chemicals in botanical dietary supplements that operate to modulate PR signalling are less thoroughly explored. As a result, there is an increasing requirement to comprehend the components of these supplements as well as their varied consequences. As a result, an in vivo investigation was conducted to compare the impacts of two formerly found phytoprogestins, kaempferol and apigenin, to progesterone therapy (Jamalan et al., Citation2016).
According to immune-histochemical data, kaempferol has some progestogenic action, although not in the same way as oestrogen. For example, although progesterone therapy increased the expression of the recognized target gene HAND2 of progesterone, kaempferol increased HAND2 impression to same level. Therefore, kaempferol, like progesterone, boosted the expression of FKBP5 and ZBTB16, two additional progesterone-inducible genes. Interestingly, in RNAseq data, FKBP5 is among one of the key drivers of androgen response pathway in the GSEA, which was increased due to progesterone. While RNAseq showed that kaempferol was not knowingly increase FKBP5 impression, this might be because of single duplicate of RNA, such as IHC shows that at the protein level, kaempferol increases FKBP5 impression. Kaempferol-managed tissues marked for the PCNA showed greater impression of PCNA in luminal epithelium than progesterone-treated organs. This is consistent with prior findings showing, while kaempferol does not limit PCNA production on its own, it can reduce proliferation when combined with an estrogenic drug like genistein. According to the findings, kaempferol has some progestogenic action. However, additional research is required to determine amount of their activities in vivo, chiefly when comparing rat and mouse uteri (Santos et al., Citation2019).
Progesterone therapy affected far more transcripts than kaempferol treatment, according to RNAseq data. While kaempferol therapy did modify several transcriptions in a comparable way to progesterone prevention, it also exhibited some distinct impacts. MMP3 and LEFTY1, two of the transcripts elevated solely by kaempferol therapy, are recognized to be changed during menstrual blood flow. MMP3 has also been observed to rise in cells of ovarian granulosa visible to P4, implying its overexpression in the findings might be indicative of the kaempferol’s progestogenic effect. Research also found that kaempferol therapy increased the expression of LEFTY1 transcripts. Interestingly, LEFTY1 is produced through the oestrus sequence but has been found to trigger MMP3 overexpression, implying a link between these transcripts and progesterone modulation (Mikani, Citation2019).
Several transcripts known to be elevated by both kaempferol and progesterone therapies play important role in effective conceptus implantation in suitably decidualized endometrium and this process is facilitated by PR signalling. ATF3 has been demonstrated to increase spheroidal adherence to endometrial cells in a human implantation model and it diminished in endometria of individuals undergoing periodic implantation failure. Furthermore, knocking out ATF3 in human endometrial model hampered decidualization, indicating possible explanation for periodic implantation failure found in individuals through inadequate ATF3. Another key gene is ATF3 in UPR pathway, with multiple transcripts considerably elevated by both progesterone and kaempferol therapies. SFRP4 has also been found to be substantially expressed in the decidualizing endometrium. SFRP4 has also been found to be considerably diminished in infertile women’s uterine lavage samples, which may compromise the normal endometrial growth required for implantation to be successful. Lrp2 expression that was increased 2.61 fold by kaempferol over progesterone in RNAseq data is increased by the progesterone therapy and peaks during implantation window into mice endometria. These findings indicate that the kaempferol therapy may possess progestogenic characteristics, since it upregulates numerous transcripts required in optimal endometrial procedures for effective conception. While MRAP2, which is elevated by progesterone and kaempferol, has been found to be suppressed during human pre-receptive and the receptive endometria and raised in infertile individuals’ endometria. These findings imply that kaempferol and progesterone may not have fully distinct pro-implantation effects. Future research might concentrate on kaempferol medication and how it affects implantation and decidualization (Zeng et al., Citation2022).
Surprisingly, kaempferol therapy downregulated many more transcripts than it elevated. This is consistent with research, which shows that when steroid hormones like oestrogen bind to nuclear receptors, the majority of changed transcripts are suppressed rather than triggered. Many of the transcripts that kaempferol suppresses have an impact in fertility or the gestation phase of a foetus. In female mice, for example, knocking out Six6ox1 or 4930447C04Rik, which are solely decreased by kaempferol, leads in oocyte insufficiency and infertility. Many placental development difficulties resulted in lower fertility in mice with Asb4/deletion, which was downregulated by both kaempferol and progesterone therapies. Similarly, Nr5a2, which is solely decreased by kaempferol, is necessary for proper placental development and decidualization in both human and animal models. While findings indicate that kaempferol medication may suppress the expression of genes essential for successful pregnancies, it was also discovered that kaempferol treatment increases the development of transcripts required for healthy pregnancies. As a result, further study is required to completely comprehend the association between kaempferol medication and implantation occurrences (Yao et al., Citation2019).
These genes are of importance because there is a link in the literature between using progesterone-only contraception and a lower risk of endometrial and ovarian cancer. CEMIP, which is downregulated by both kaempferol and progesterone therapies, is increased in epithelial ovarian cancer tissues. CEMIP knockdown has also been demonstrated to reduce oncogenic features in a human ovarian cancer cell line, including invasion, proliferation, and migration. Furthermore, as compared to controls, OGDHL is elevated in epithelial ovarian cancer samples, but it is inhibited by both progesterone and kaempferol therapies. Similarly, both kaempferol and progesterone therapy decreased RSAD2, which is both elevated and related with worse progression in endometrial cancer patients. RSAD2 is likewise a key gene in the IAR pathway, with transcripts considerably reduced by both kaempferol and progesterone therapy. Several findings imply that kaempferol medication may reduce the expression of transcripts associated in several forms of cancer. However, OSR2 expression is often decreased in endometrial cancer, indicating that certain transcripts linked with risk have different expression patterns as a result of kaempferol therapy (Wang et al., Citation2020).
According to the literature, another pattern discovered by RNAseq data was that numerous genes downregulated by kaempferol medication are associated with (PCOS) polycystic ovarian syndrome. PCOS is distinguished by the production of many cysts on the ovaries, as well as an abnormally elevated level of androgens and low systemic levels of progesterone leading to decreased ovulation. SLC5A3 expression was observed to be reduced in endometrial tissue from women with PCOS when treated with kaempferol. Furthermore, this reduced expression contributed to the emergence of insulin intolerance, which is prevalent in PCOS patients. Similarly, reduced Procr expression, which was likewise reduced by progesterone and kaempferol therapy, resulted in impaired ovarian follicle formation and a PCOS phenotype in mice. CXCL14 transcripts have been reported to be lowered in human luteinized granulosa cells from PCOS women by both kaempferol and progesterone therapy. Furthermore, a paucity of CXCL14 in these cells is likely related to their reduced ability to make progesterone, since treatment with higher amounts of CXCL14 consistently boosted progesterone synthesis. Kaempferol therapy also reduced Pdgfd activation. When compared to samples from control individuals, both serum and follicular fluid from women with PCOS contained lower levels of PDGFD protein. PDGFD levels were also observed to be lower in a PCOS rat model. Data imply that kaempferol therapy may induce gene expression comparable to that reported in PCOS models, which is corroborated by one of the important GSEA pathways associated to androgen signalling. However, both kaempferol and progesterone therapy reduced C3 transcript expression, which has been demonstrated to be elevated in patients with PCOS along with other related pathway components and is considered to contribute to the inflammatory element of the condition. This shows that the effects of kaempferol medication on PCOS pathways and regulated transcripts may not be completely consistent and that further research is needed (Zhou et al., Citation2015).
Kaempferol and vascular endothelium
The vascular endothelium might be thought of as an active obstacle that prevents cells and plasma from moving from blood into surrounding tissues. Inhibiting vascular endothelial swelling is regarded as a critical step in the management of many vascular conditions, as exceptionally generated inflammatory intermediaries can lead to irreversible vascular damage and unnecessary fluid removal from the distribution, causing organ dysfunction, inadequate tissue perfusion, and mortality (Hu et al., Citation2020).
Kaempferol was shown to reduce the pro-inflammatory reaction caused by LPS or (HMGB1) high mobility group box 1 by enhancing obstacle reliability and reducing cell adhesion molecule production. As a result, the anti-inflammatory activities of kaempferol are thought to be based on the downstream of the HMGB1 receptors TLR4 and TLR2, which may be advantageous in the prevention of vascular disorders (Hu et al., Citation2021).
Role of kaempferol in vascular smooth muscle cell migration
In reaction to environmental factors such as growth factor induction or vascular damage, VSMCs keep the potential to transition between dedifferentiated and differentiated phenotypes. TGF- and (BMP) bone morphogenetic protein are key components that suppress VSMC migration and proliferation, boost contractile VSMC gene expression, and promote the contractile phenotype. The (PDGF) platelet-derived growth factor signalling trails can transform this characteristic to another. PDGF may enhance the synthetic phenotype by causing resistance to BMP and TGF signalling (Zhu et al., Citation2022).
Kaempferol may induce the BMP signalling path, promote (miR)-21 microRNA production, and downstream the dedicator of cytokinesis 4 DOCK5, (DOCK4), and DOCK7, hence reducing cell immigration and provoking PDGF-mediated movement, according to research. Kaempferol has the potential to be a treatment for cardiovascular illnesses since it suppresses VSMC migration via altering BMP-controlled miR-21 production and miR control.
Kaempferol against atherosclerosis
Cholesterol-related cardiovascular ailment is among the world's major reasons of death. The main cell types implicated in atherosclerosis include endothelial cells, smooth muscle cells, and macrophages (Ren et al., Citation2019).
Osteopontin (OPN), along with its receptor CD44, is widely regarded as a crucial molecule in atherosclerosis. The plasma and aorta of 6J/C57BL control and ApoE-deficient mice were examined with and without kaempferol. Prior to investigate, the impression of CD44 and OPN, the generation of aortic reactive oxygen species, and the area of atherosclerotic wounds were greater in ApoE-deficient animals with kaempferol compared to the control group. Kaempferol dramatically reduced atherosclerotic lesion development in mice fed with vehicle for 4 weeks, restored vasodilation in reaction when acetylcholine, increased the Emax value, then decreased the EC50 value. OPN plasma concentration was reduced, as was the expression of aortic CD44 and OPN. As a result, KAEMPFEROL inhibited atherosclerosis in the ApoE-deficient animal aorta through regulating the OPN-CD44 pathway. Furthermore, higher plasma (LDL) low-density lipoprotein cholesterol levels have been linked to coronary heart disease and atherosclerosis. Flavonoids boost Sp1 activity due to phosphorylation Thr-739 and Thr-453 on ERK1/2, hence enhancing Sp1 Promoter–DNA interaction area of the gene for the low-density lipoprotein receptor (Xu et al., Citation2017).
Kaempferol was also shown to reduce oxidized (ox)-LDL-induced apoptosis. In ox-LDL-induced human umbilical vein endothelial cells, kaempferol enhanced the ratio of microtubule-associated beclin-1 levels to protein 1 light chain 3. Furthermore, Akt and p-mammalian target of rapamycin (p-mTOR) are phosphorylated expression were reduced. In ox-LDL-induced HUVECs, insulin inhibited the response of kaempferol on apoptosis and cell survival. These findings suggest that KAEMPFEROL reduces ox-LDL-induced mortality in human endothelial cells by increasing autophagy via the Akt/PI3K/mTOR signalling path (Yao et al., Citation2020).
Effects of kaempferol against thrombosis
Risky cerebral vascular illness is mostly resulted by aberrant vessel for blood coagulation following haemostasis that may involve the malfunction of the fibrinolytic system lead via misaligned fibrinolytic risk factors, such as plasminogen activator and plasmin, and thrombosis caused by a coagulation factor imbalance. By initiating thrombin, which adapts fibrinogen to fibrin and factor XIIIa, a thrombus constituted a fibrin polymer that is insoluble is produced (Choi, Park, et al., Citation2015).
Kaempferol has been shown to decrease pro-coagulant activation and the collaboration of thrombin and fibrinogen by blocking the activation of serine protease, ERK, JNK, p38, and Akt. Furthermore, platelet emergence is a key acute vascular thrombosis event. As a result, another therapy option is to treat excessive platelet aggregation. Reactive oxygen species production in response to collagen stimulation is linked to a variety of platelet activation events, including cytosolic calcium increase, phospholipase C2 activation, granule release, and integrin activity. According to research, kaempferol inhibits the triphosphopyridine nucleotide oxidase produced by collagen activity and effectively inhibits the tyrosine phosphorylation-based glycoprotein VI signalling pathway by treating SH2 oxidative inactivation-comprising phosphatase 2, and thus reduces collagen-triggered platelet activation and thrombosis that depends on platelets. Kaempferol hinders angiogenesis of human retinal endothelial cells under conditions of high glucose (Quintal Martínez & Segura Campos, Citation2023).
Diabetic retinopathy is a frequent microvascular consequence of diabetes and the leading reason of blindness in developed nations. Retinal neovascularization is thought to be an important element in the aetiology of diabetic retinopathy. VEGF has the potential to enhance angiogenesis and vasculogenesis. VEGF levels were found to be elevated in the aqueous humour of DR sufferers and endothelial cells subjected to increased glucose. Endogenous inhibitors of angiogenesis, such as thrombospondin 1 and metallopeptidases with TSP type 1 motif, have been shown to play a significant role in diabetes-associated retinal vascular dysregulation and vascular homeostasis (Tamer et al., Citation2022).
High glucose substantially increased the migration, proliferation, and tube induction of HRECs, which was dose-dependently inhibited by 10, 30 mM kaempferol. Researchers discovered that after 30 mM kaempferol treatment, the impression levels of ADAM mRNA and TSP-1 increased, although ADAM and TSP-1 quantities did not change among increased-normal and glucose (5 mM) glucose settings. High glucose levels raised the mRNA impression levels of VEGF and placental growth factor, as well as the absorption of released PGF and VEGF from HRECs. Kaempferol was shown to dramatically inhibit VEGF stimulates the growth of HUVEC. Consequences show that kaempferol may suppress angiogenic activity in VEGF-induced ECs via regulating the VEGF/VEGF receptor 2 and its downregulated signalling pathways (MEK, AKT/PI3K, and ERK) (Imran et al., Citation2019).
Kaempferol aiding in neurological disorders
The neurodegenerative disease pathogenesis illnesses is complicated, and it may be defined in part using oxidative pressure, which is widely recognized to have an important role in nerve injury. Previous research has shown that reactive oxygen species are implicated in neurodegenerative illnesses and that they are created in elevated quantities in the brain owing to oxidative metabolism. The creation and removal of ROS are in a dynamic equilibrium under normal circumstances. If the equilibrium is disrupted, the quantity of ROS rises owing to reduced activation of anti-oxidant enzymes, impairing brain functioning, notably in the hippocampus part (Siddique, Citation2021).
Kaempferol appears to alleviate cognitive impairment by increasing the production protein content of the (CREB) 2-cAMP/ERK1- protein that binds to sensitive elements signalling pathway. Kaempferol may also increase K+/Na+-ATPase movement and decrease hippocampus oxidative pressure, according to research. Furthermore, an elevation in the amount the flavonol B ring contains hydroxyl group’s leads to the elevation of 2-CREB/ERK1 signalling pathway protein expression in the D-gal-induced mice’s hippocampus perceptive decline (Tahir et al., Citation2021).
Chronic pressure is known to increase the danger of mental and psychosomatic diseases such as depressive disorders and anxiety. Dejection is the another frequent chronic disorder in clinical medication, and by 2020, it is expected to be the second biggest reason of premature mortality or disintegration globally (Beg et al., Citation2018).
The anti-depressant properties of kaempferol were measured using that of the tail analysis, the forced swimming experiment, and the rota-rod analysis in mice that were pressurized owing to the continuous usage of limitations or restriction. The mice in the experiment were given kaempferol orally at a dosage of 30 mg/kg/day for 14 days though being examined. The concluding study findings showed that quercetin or kaempferol significantly diminished restricted time in the FST and TST, demonstrating that flavonols have substantial anti-depressant properties (Ma et al., Citation2017).
Parkinson's disease and role of kaempferol
Parkinson's disease is the second-most common prevalent central nervous system condition. The harm of dopaminergic neurons and the creation of cytoplasmic insertions in the (SN) substantia nigra are pathogenic hallmarks. When Parkinson's disease is current, 80% of striatal dopamine is destroyed, may harm to the deadly area, may foreshadow the removal of cell bodies in the substantia nigra (Naz et al., Citation2020).
It was proposed that inflammasomes are involved in immunological homeostasis and that their dysfunctioning contributes to neurodegradable diseases. The pyrin domain of the NLR family comprising 3 (NLRP3) inflammasome has a role in Parkinson's disease. Kaempferol suppresses NLRP3 inflammasome activity, resulting in NLRP3 inflammasome deactivation and decreased NLRP3 protein expression in command to induce autophagy/macroautophagy in microglia (Han et al., Citation2021).
Pre-ingestion of KAEMPFEROL significantly enhanced 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopamine and (DOPAC) dihydroxy-phenyl acetic acid removal in the striatum, as well as the dopamine/DOPAC proportion and the MPTP-triggered loss of mouse neurons that are positive for tyrosine hydroxylase substantia nigra. In mice with MPTP-induced Parkinson's disease, KAEMPFEROL has a neuroprotective effect, which may be due to its anti-oxidant potential that eliminates free radicals and resulted to the retention of more dopamine neurons (Pan et al., Citation2020).
Earlier research has shown that anhydrosafflor yellow B and KAEMPFEROL 3-O-rutinoside, chemicals extracted from SSFE, a consistent safflower flavonoid extraction, may decrease ROS levels generated by hydrogen peroxide and regenerate tyrosine hydroxylase activation in PC12 cells. Dehydrin flavonoid B and KAEMPFEROL 3-O-rutinoside prevented microtubule variability and decreased cell volume, according to the findings. Furthermore, SSFE in tablet form may decrease astrocyte growth and enhance neurological function in a 6-hydroxydopamine (6-OHDA)-induced Parkinson's disease rat model. 6-OHDA was shown to affect diffusion properties of endothelial cells in a research utilizing a magnetic resonance imaging (MRI) -based, tracer-based method, including lower bending and removal rate constants and prolonged removal half-life of the tracer in the substantia nigra with 6-OHDA-induced wounds. These findings suggested that flavonols may be used to treat Parkinson’s disease (Siddique, Citation2021).
Alzheimer’s disease
According to research, aberrant protein synthesis and high glutamate concentrations may cause excessive reactive oxygen species formation in cells, leading to neuronal death and neurotoxicity. The neuroprotective properties of KAEMPFEROL in HT22 hippocampal neuronal cells treated with glutamate were investigated. Following that, dehydrogenase of lactate tests and Connexin V/fluorescein-5-isothiocyanate-connexin V double labelling techniques remained utilized to validate KAEMPFEROL’s defensive impact on cells HT22. KAEMPFEROL likewise protects neurons by modulating the apoptosis-associated proteins’ expression levels such as Bid, Bcl-2, MAPK, and apoptosis-instigating element (Nejabati & Roshangar, Citation2022).
The build-up and deposition of -amyloid peptides in AD cause progressive impairment of neurons and cell death. One of the possibilities is that oxidative stress is a process that leads to neurodegeneration. Currently, there is no cure for Alzheimer’s illness, although the usage of original anti-oxidants may postpone the infection’s aetiology. These findings imply that KAEMPFEROL might be a beneficial medicine to treat and prevention of neurodegenerative illnesses including Alzheimer’s (Beg et al., Citation2018).
Ischemic stroke and role of kaempferol
Clinical term for ischemic stroke illness is defined by the sudden development of neurological impairments, which is usually caused by an obstruction of an artery. Because of its high metabolic demand, the brain is especially vulnerable to ischemia damage (Wu et al., Citation2017).
According to reports, the development of brain ischemia damage might entail LPS mandatory to TLR4, activating microglia via TLR4 and TLR2, shadowed by the issue of pro-inflammatory molecules via the MyD88/TLR4 signalling pathway, causing additional injury. The blood–brain barrier’s job remains to prevent hazardous compounds within the blood of entering the mind. Neuroinflammation and BBB breakdown are two of the most common causes of brain injury, therefore maintaining anti-neuritis activities and BBB integrity is critical to safeguarding the nerve (Zhang et al., Citation2022).
Anti-bacterial effects of kaempferol
Coccobacillus was long thought to be of minor medical concern, but it has since appeared as a common healthcare community-developed and unit-acquired illness. It mostly results in septicaemia and lung diseases in immunocompromised persons. Its tolerance to antibiotics and enhanced survivability in hostile conditions contribute to its disease causing ability. At the present, the findings of kaempferol-comprising drugs used for this disease are highly potential, which might be significant in light of rising antibiotic resistance (Yeon et al., Citation2019).
A. baumannii was shown to be considerably inhibited by the chemical kaempferol-3,7-O-l-dirhamnoside. A unique nanotechnology application utilizing a mix filled with kaempferol nanocrystals shown extremely promising anti-A. baumannii outcomes; the study concentrated on handling infested lesions. In vitro, propolis extractions containing kaempferol were similarly efficient against A. baumannii. Another kaempferol-containing substance, Jubatum subspecies of geranium ibericum extract, remained shown to stay virtually as efficient as certain industrial antibiotic drugs in contrast with this infectious agent in vitro. Previous studies suggested that a K. fedtschenkoi extraction containing kaempferol was beneficial against this infection (Lee et al., Citation2017).
E.coli are gastrointestinal physiological colonists; colonization normally occurs shortly after birth. In immunocompetent people, they usually do not cause illness, but they can become pathogenic if they move to new places or if their recipient develops immunocompromised. The greatest well-known E. coli disease causing types include the pathogenic strains of E. coli known as enteropathogenic E. coli (EPEC), enterohaemorrhagic E. coli (EHEC), enterotoxigenic E. coli (ETEC), enteroaggregative E. coli (EAEC), enteroinvasive E. coli (EIEC), and diffusely adherent E. coli (DAEC). E. coli infections are most commonly seen in the urogenital and gastrointestinal systems. Although the majority of these infections are curable, the appearance of multi-drug resistance E. coli provides a new therapeutic trial (Jia et al., Citation2019).
The antimicrobial activity contains kaempferol-3,7-O-l-dirhamnoside against E. coli stood modest. Success in this regard was also noted by those who employed S. hymettia extract. B. chinense extract containing Kaempferol and kaempferol-3-O-D-rutinoside was shown to be efficient against this bacteria. Propolis extracts were also proven to be efficient against this infection in studies. Kaempferol in conjunction with silver nanocomponents has also been shown to be efficient against E. coli. Extracts of phytochemicals were similarly effective against E. coli (Li et al., Citation2017).
Anti-fungal effects of kaempferol
Only a few fungus species are dangerous to humans; some produce minor ailments, though others, such as Aspergillus spp. and Candida spp., can lead to lethal infectious diseases that are systemic. According to current studies, Candida species infections in hospital settings are becoming a growing health concern. Though fungi of this species are normally harmless, they could be the reason of oral candidiasis; in female, a considerable proportion will go through from vaginal candidiasis at some point in their life. Candida species infections are primarily identified by risk components (Jan et al., Citation2022).
Similarly, Aspergillus fumigatus, however safe to immunocompetent hosts, causes aspergillosis in immunocompromised people; this is a growing worry as the number of such patients increases. It is vital to highlight that avoiding transmission to this disease is very impractical, as people consume thousands of its conidia on routine basis. One more key element of A. fumigatus infestations is that they can form in the holes remained in tuberculosis sufferers; this is intriguing, given that kaempferol is renowned to be efficient counter to M. tuberculosis, as previously noted. As a result, in this scenario, a kaempferol-comprising drug might together decrease the preliminary illness and function as a prophylactic measure against probable aspergillosis (Ekalu & Habila, Citation2020).
Lastly, Cryptococcus neoformans is one of the toughest fungal infections, killing thousands of afflicted people each year, according to a recent survey. Its significance as an infection of worldwide concern was recognized after the 1970s. Various risk components have been linked to an elevated chance of cryptococcosis infectious disease; for instance, like with further infections, cryptococcosis is especially hazardous in AIDS/HIV patients. Regardless of the accessibility of antifungal medicines, there is an increasing conflict as microorganisms adjust; this, combined with the renowned results of many antifungal medicines, highlights the significance of developing new therapies based on organic substances (Noor et al., Citation2023).
The extract of Mitracarpus scaber, a herb utilized in outdated West African drug, was perhaps the first to demonstrate antifungal activity of a kaempferol-containing molecule. Both isolated kaempferol-3-O-[3-O-acetyl-6-O-(E)-p-coumaroyl] and kaempferol-3-O-[3-O-acetyl-6-O-(E)-b-d-glucopyranoside] and kaempferol 3-O-b-D-glucopyranoside from S. hymettia were reported to be efficacious in vitro contrary to Candida glabrata, Candida albicans, and Candida tropicalis. As previously stated, the extract’s kaempferol component was not as efficient when evaluated in isolated form. Some kaempferol-comprising extractions from Allium ursinum demonstrated considerable antifungal efficacy against Candida albicans. The extractions from Labisa pumila Benth, which was previously described, also exhibit a significant antifungal activity. Bryophyllum pinnatum (Lank.) Oken extract showed promising antifungal action against Candida parapsilosis, Candida albicans, and Cryptococcus neoforman (Saeed et al., Citation2019).
Conclusion
Kaempferol has been shown to have anti-cancer and anti-inflammatory properties via many mechanisms of action. This chemical was discovered to be a powerful free radical and superoxide radical scavenger, while also sustaining the activity of several antioxidant enzymes such as catalase, glutathione-S-transferase and glutathione peroxidase. These effects have been demonstrated to be helpful in animal illness models such as experimental allergic encephalomyelitis, asthma, diabetes, and carcinogenesis. Numerous lines of evidence have pointed to several ways of anticancer activity, including as cell-cycle arrest, anti-proliferation, apoptosis induction, ROS formation, synergism with conventional treatments, and inhibition of angiogenesis and cancer spread. Furthermore, kaempferol can reduce the toxicity associated with traditional drugs while maintaining their therapeutic efficiency. The study of cancer cell lines has revealed several molecular targets of kaempferol; nevertheless, further research utilizing animal models of illness is needed to acquire more definitive information for the molecular basis of kaempferol activity. Furthermore, unique kaempferol nanoparticles/analogs have been produced and shown to have higher anticancer and antioxidant effects than kaempferol itself. Despite its established therapeutic effectiveness in tumour cell lines and animal models, kaempferol has received little attention in clinical trials. More research is needed before kaempferol may be developed into a medication for the treatment of different carcinomas and inflammatory illnesses ().
Table 1. Anticancer potential of kaempferol in the light of published studies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abdullah, A., Talwar, P., d’Hellencourt, C. L., & Ravanan, P. (2019). IRE 1α is critical for kaempferol-induced neuroblastoma differentiation. The FEBS Journal, 286(7), 1375–1392. https://doi.org/10.1111/febs.14776
- Alam, W., Khan, H., Shah, M. A., Cauli, O., & Saso, L. (2020). Kaempferol as a dietary anti-inflammatory agent: Current therapeutic standing. Molecules, 25(18), 4073. https://doi.org/10.3390/molecules25184073
- Alkhalidy, H., Moore, W., Wang, Y., Luo, J., McMillan, R. P., Zhen, W., & Liu, D. (2018). The flavonoid kaempferol ameliorates streptozotocin-induced diabetes by suppressing hepatic glucose production. Molecules, 23(9), 2338. https://doi.org/10.3390/molecules23092338
- Azevedo, C., Correia-Branco, A., Araújo, J. R., Guimaraes, J. T., Keating, E., & Martel, F. (2015). The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutrition and Cancer, 67(3), 504–513. https://doi.org/10.1080/01635581.2015.1002625
- Barliana, M. I., Diantini, A., Subarnas, A., & Abdulah, R. (2022). Kaempferol-3-O-Rhamnoside inhibits the proliferation of Jurkat cells through Jun amino-terminal kinase signaling. The Natural Products Journal, 12(4), 57–63. https://doi.org/10.2174/2210315511666210826102427
- Barve, A., Chen, C., Hebbar, V., Desiderio, J., Saw, C. L. L., & Kong, A. N. (2009). Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharmaceutics & Drug Disposition, 30(7), 356–365. https://doi.org/10.1002/bdd.677
- Beg, T., Jyoti, S., Naz, F., Ali, F., Ali, S. K., Reyad, A. M., & Siddique, Y. H. (2018). Protective effect of kaempferol on the transgenic Drosophila model of Alzheimer's disease. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 17(6), 421–429.
- Bian, Y., Lei, J., Zhong, J., Wang, B., Wan, Y., Li, J., Liao, C., He, Y., Liu, Z., Ito, K., & Zhang, B. (2022). Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. The Journal of Nutritional Biochemistry, 99, 108840. https://doi.org/10.1016/j.jnutbio.2021.108840
- Budisan, L., Gulei, D., Jurj, A., Braicu, C., Zanoaga, O., Cojocneanu, R., Pop, L., Raduly, L., Barbat, A., Moldovan, A., Moldovan, C., Tigu, A., Ionescu, C., Atanasov, A., Irimie, A., & Berindan-Neagoe, I. (2019). Inhibitory effect of CAPE and kaempferol in colon cancer cell lines—possible implications in new therapeutic strategies. International Journal of Molecular Sciences, 20(5), 1199. https://doi.org/10.3390/ijms20051199
- Catalán, M., Rodríguez, C., Olmedo, I., Carrasco-Rojas, J., Rojas, D., MolinaBerríos, A., Díaz-Dosque, M., & Jara, J. A. (2020). Kaempferol induces cell death and sensitizes human head and neck squamous cell carcinoma cell lines to cisplatin. In W. E. Crusio, H. Dong, H. H. Radeke, N. Rezaei, O. Steinlein, & J. Xiao (Eds.), Cell biology and translational medicine (Vol. 12, pp. 95–109). Springer.
- Chen, M., Xiao, J., El-Seedi, H. R., Woźniak, K. S., Daglia, M., Little, P. J., & Xu, S. (2022). Kaempferol and atherosclerosis: From mechanism to medicine. Critical Reviews in Food Science and Nutrition, 1–19. https://doi.org/10.1080/10408398.2022.2121261
- Choi, J. H., Park, S. E., Kim, S. J., & Kim, S. (2015). Kaempferol inhibits thrombosis and platelet activation. Biochimie, 115, 177–186. https://doi.org/10.1016/j.biochi.2015.06.001
- Choi, Y. J., Lee, Y. H., & Lee, S.-T. (2015). Galangin and kaempferol suppress phorbol12-myristate-13-acetate-induced matrix metalloproteinase-9 expression in human fibrosarcoma HT-1080 c. Molecules and Cells, 38(2), 151.
- Chung, M. J., Pandey, R. P., Choi, J. W., Sohng, J. K., Choi, D. J., & Park, Y. I. (2015). Inhibitory effects of kaempferol-3-O-rhamnoside on ovalbumin-induced lung inflammation in a mouse model of allergic asthma. International Immunopharmacology, 25(2), 302–310. https://doi.org/10.1016/j.intimp.2015.01.031
- Da, J., Xu, M., Wang, Y., Li, W., Lu, M., & Wang, Z. (2019). Kaempferol promotes apoptosis while inhibiting cell proliferation via androgen-dependent pathway and suppressing vasculogenic mimicry and invasion in prostate cancer. Analytical Cellular Pathology, 2019, 10.
- Devi, K. P., Malar, D. S., Nabavi, S. F., Sureda, A., Xiao, J., Nabavi, S. M., & Daglia, M. (2015). Kaempferol and inflammation: From chemistry to medicine. Pharmacological Research, 99, 1–10. https://doi.org/10.1016/j.phrs.2015.05.002
- Duan, L., Ding, W., Liu, X., Cheng, X., Cai, J., Hua, E., & Jiang, H. (2017). Biosynthesis and engineering of kaempferol in Saccharomyces cerevisiae. Microbial Cell Factories, 16(1), 1–10. https://doi.org/10.1186/s12934-017-0774-x
- Duračka, M., Debacker, M., Bučko, O., Lukač, N., & Tvrda, E. (2019). The effect of kaempferol and naringenin may improve the in-vitro quality of stored boar semen. Journal of Central European Agriculture, 20(4), 1069–1075. https://doi.org/10.5513/JCEA01/20.4.2294
- Ekalu, A., & Habila, J. D. (2020). Flavonoids: Isolation, characterization, and health benefits. Beni-Suef University Journal of Basic and Applied Sciences, 9(1), 1–14. https://doi.org/10.1186/s43088-020-00065-9
- Estakhri, F., Panjehshahin, M. R., Tanideh, N., Gheisari, R., Mahmoodzadeh, A., Azarpira, N., & Gholijani, N. (2020). The effect of kaempferol and apigenin on allogenic synovial membrane-derived stem cells therapy in knee osteoarthritic male rats. The Knee, 27(3), 817–832. https://doi.org/10.1016/j.knee.2020.03.005
- Fouzder, C., Mukhuty, A., & Kundu, R. (2021). Kaempferol inhibits Nrf2 signaling pathway via downregulation of Nrf2 mRNA and induces apoptosis in NSCLC cells. Archives of Biochemistry and Biophysics, 697, 108700. https://doi.org/10.1016/j.abb.2020.108700
- Haeri, V., Karimi, E., & Oskoueian, E. (2023). Synthesized nanoliposome-encapsulated kaempferol attenuates liver health parameters and gene expression in mice challenged by cadmium-induced toxicity. Biotechnology and Applied Biochemistry, 70(1), 429–438. https://doi.org/10.1002/bab.2368
- Han, X., Zhao, S., Song, H., Xu, T., Fang, Q., Hu, G., & Sun, L. (2021). Kaempferol alleviates LD-mitochondrial damage by promoting autophagy: Implications in Parkinson's disease. Redox Biology, 41, 101911. https://doi.org/10.1016/j.redox.2021.101911
- Hassan, E. S., Hassanein, N. M., & Ahmed, H. M. S. (2021). Probing the chemoprevention potential of the antidepressant fluoxetine combined with epigallocatechin gallate or kaempferol in rats with induced early stage colon carcinogenesis. Journal of Pharmacological Sciences, 145(1), 29–41. https://doi.org/10.1016/j.jphs.2020.10.005
- He, C., Yang, J., Jiang, X., Liang, X., Yin, L., Yin, Z., Geng, Y., Zhong, Z., Song, X., Zou, Y., Li, L., Zhang, W., & Lv, C. (2019). Kaempferol alleviates LPS-ATP mediated inflammatory injury in splenic lymphocytes via regulation of the pyroptosis pathway in mice. Immunopharmacology and Immunotoxicology, 41(5), 538–548. https://doi.org/10.1080/08923973.2019.1666405
- Hosseini, A., Alipour, A., Baradaran Rahimi, V., & Askari, V. R. (2023). A comprehensive and mechanistic review on protective effects of kaempferol against natural and chemical toxins: Role of NF-κB inhibition and Nrf2 activation. BioFactors, 49(2), 322–350. https://doi.org/10.1002/biof.1923
- Hu, W. H., Dai, D. K., Zheng, B. Z. Y., Duan, R., Chan, G. K. L., Dong, T. T. X., Qin Q.-W., & Tsim, K. W. K. (2021). The binding of kaempferol-3-O-rutinoside to vascular endothelial growth factor potentiates anti-inflammatory efficiencies in lipopolysaccharide-treated mouse macrophage RAW264. 7 cells. Phytomedicine, 80, 153400. https://doi.org/10.1016/j.phymed.2020.153400
- Hu, W. H., Wang, H. Y., Xia, Y. T., Dai, D. K., Xiong, Q. P., Dong, T. T. X., Duan, R., Chan, G. K.-L., Qin, Q.-W., & Tsim, K. W. K. (2020). Kaempferol, a major flavonoid in ginkgo folium, potentiates angiogenic functions in cultured endothelial cells by binding to vascular endothelial growth factor. Frontiers in Pharmacology, 11, 526. https://doi.org/10.3389/fphar.2020.00526
- Huang, W. W., Chiu, Y. J., Fan, M. J., Lu, H. F., Yeh, H. F., Li, K. H., Chen, P. Y., Chung, J. G., & Yang, J. S. (2010). Kaempferol induced apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Molecular Nutrition & Food Research, 54(11), 1585–1595. https://doi.org/10.1002/mnfr.201000005
- Imran, M., Rauf, A., Shah, Z. A., Saeed, F., Imran, A., Arshad, M. U., Ahmad, B., Bawazeer, S., Atif, M., Peters, D. G., & Mubarak, M. S. (2019). Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phytotherapy Research, 33(2), 263–275. https://doi.org/10.1002/ptr.6227
- Jamalan, M., Ghaffari, M. A., Hoseinzadeh, P., Hashemitabar, M., & Zeinali, M. (2016). Human sperm quality and metal toxicants: Protective effects of some flavonoids on male reproductive function. International Journal of Fertility & Sterility, 10(2), 215.
- Jan, R., Khan, M., Asaf, S., Asif, S., Lubna, & Kim, K.-M. (2022). Bioactivity and therapeutic potential of kaempferol and quercetin: New insights for plant and human health. Plants, 11(19), 2623. https://doi.org/10.3390/plants11192623
- Jia, Z., Chen, A., Wang, C., He, M., Xu, J., Fu, H., Zhang, X., Lv, W., & Guo, Z. (2019). Amelioration effects of kaempferol on immune response following chronic intermittent cold-stress. Research in Veterinary Science, 125, 390–396. https://doi.org/10.1016/j.rvsc.2019.08.012
- Kashyap, D., Sharma, A., Tuli, H. S., Sak, K., Punia, S., & Mukherjee, T. K. (2017). Kaempferol–A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. Journal of Functional Foods, 30, 203–219. https://doi.org/10.1016/j.jff.2017.01.022
- Khazdair, M. R., Anaeigoudari, A., & Agbor, G. A. (2021). Anti-viral and anti-inflammatory effects of kaempferol and quercetin and COVID-2019: A scoping review. Asian Pacific Journal of Tropical Biomedicine, 11(8), 327. https://doi.org/10.4103/2221-1691.319567
- Kim, B.-W., Lee, E.-R., Min, H.-M., Jeong, H.-S., Ahn, J.-Y., Kim, J.-H., Choi, H.-Y., Choi, H., Kim, E. Y., Park, S. P., & Cho, S.-G. (2008). Sustained ERK activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-D culture condition. Cancer Biology & Therapy, 7(7), 1080–1089. 48. https://doi.org/10.4161/cbt.7.7.6164
- Kim, J. K., & Park, S. U. (2020). Recent studies on kaempferol and its biological and pharmacological activities. EXCLI Journal, 19(2019), 627–634.
- Lee, G. A., Choi, K. C., & Hwang, K. A. (2017). Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environmental Toxicology and Pharmacology, 49, 48–57. https://doi.org/10.1016/j.etap.2016.11.016
- Lee, Y. J., Choi, H. S., Seo, M. J., Jeon, H. J., Kim, K. J., & Lee, B. Y. (2015). Kaempferol suppresses lipid accumulation by inhibiting early adipogenesis in 3T3-L1 cells and zebrafish. Food & Function, 6(8), 2824–2833. https://doi.org/10.1039/C5FO00481K
- Li, S., Yan, T., Deng, R., Jiang, X., Xiong, H., Wang, Y., Yu, Q., Wang, X., Chen, C., & Zhu, Y. (2017). Low dose of kaempferol suppresses the migration and invasion of triple-negative breast cancer cells by downregulating the activities of RhoA and Rac1. OncoTargets and Therapy, 10, 4809–4819. https://doi.org/10.2147/OTT.S140886
- Liu, C., Liu, H., Lu, C., Deng, J., Yan, Y., Chen, H., Wang, Y., Liang, C.-L., Wei, J., Han, L., & Dai, Z. (2019). Kaempferol attenuates imiquimod-induced psoriatic skin inflammation in a mouse model. Clinical & Experimental Immunology, 198(3), 403–415. https://doi.org/10.1111/cei.13363
- Ma, Y., Liu, Y., Sun, A., Du, Y., Ye, M., Pu, X., & Qi, X. (2017). Intestinal absorption and neuroprotective effects of kaempferol-3-O-rutinoside. RSC Advances, 7(50), 31408–31416. https://doi.org/10.1039/C7RA05415G
- MacPherson, L., & Matthews, J. (2010). Inhibition of aryl hydrocarbon receptor dependent transcription by resveratrol or kaempferol is independent of estrogen receptor α expression in human breast cancer cells. Cancer Letters, 299(2), 119–129. https://doi.org/10.1016/j.canlet.2010.08.010
- Mikani, A. (2019). Effect of kaempferol on ecdysteroid titer and oocyte size via tachykinin-4 in cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Journal of Crop Protection, 8(2), 153–162.
- Molitorisova, M., Sutovska, M., Kazimierova, I., Barborikova, J., Joskova, M., Novakova, E., & Franova, S. (2021). The anti-asthmatic potential of flavonol kaempferol in an experimental model of allergic airway inflammation. European Journal of Pharmacology, 891, 173698. https://doi.org/10.1016/j.ejphar.2020.173698
- Murden, K., San, K., Martino, A., & Ezekiel, U. (2016). The effect of phytochemicals on a chemoresistant, epithelial-mesenchymal transitioned, colorectal cancer cell line. The FASEB Journal, 30, 1090–1092. https://doi.org/10.1096/fasebj.30.1_supplement.1090.2
- Nandi, S. K., Chatterjee, N., Roychowdhury, T., Pradhan, A., Moiz, S., Manna, K., Sarkar, D. K., Dhar, P., Dutta, A., Mukhopadhyay, S., & Bhattacharya, R. (2023). Kaempferol with verapamil impeded panoramic chemoevasion pathways in breast cancer through ROS overproduction and disruption of lysosomal biogenesis. Phytomedicine, 113, 154689. https://doi.org/10.1016/j.phymed.2023.154689
- Naz, F., Jyoti, S., & Siddique, Y. H. (2020). Effect of kaempferol on the transgenic Drosophila model of Parkinson’s disease. Scientific Reports, 10(1), 1–14. https://doi.org/10.1038/s41598-019-56847-4
- Nejabati, H. R., & Roshangar, L. (2022). Kaempferol as a potential neuroprotector in Alzheimer's disease. Journal of Food Biochemistry, 46(12), e14375. https://doi.org/10.1111/jfbc.14375
- Noor, N., Jhan, F., Gani, A., Raina, I. A., & Shah, M. A. (2023). Nutraceutical and toxicological evaluation of hydrogels architected using resistant starch nanoparticles and gum acacia for controlled release of kaempferol. Food Structure, 35, 100307. https://doi.org/10.1016/j.foostr.2022.100307
- Pan, X., Liu, X., Zhao, H., Wu, B., & Liu, G. (2020). Antioxidant, anti-inflammatory and neuroprotective effect of kaempferol on rotenone-induced Parkinson’s disease model of rats and SH-S5Y5 cells by preventing loss of tyrosine hydroxylase. Journal of Functional Foods, 74, 104140. https://doi.org/10.1016/j.jff.2020.104140
- Qattan, M. Y., Khan, M. I., Alharbi, S. H., Verma, A. K., Al-Saeed, F. A., Abduallah, A. M., & Al Areefy, A. A. (2022). Therapeutic importance of kaempferol in the treatment of cancer through the modulation of cell signaling pathways. Molecules, 27(24), 8864. https://doi.org/10.3390/molecules27248864
- Quintal Martínez, J. P., & Segura Campos, M. R. (2023). Flavonoids as a therapeutical option for the treatment of thrombotic complications associated with COVID-19. Phytotherapy Research, 37(3), 1092–1114. https://doi.org/10.1002/ptr.7700
- Rakha, A., Umar, N., Rabail, R., Butt, M. S., Kieliszek, M., Hassoun, A., & Aadil, R. M. (2022). Anti-inflammatory and anti-allergic potential of dietary flavonoids: A review. Biomedicine & Pharmacotherapy, 156, 113945. https://doi.org/10.1016/j.biopha.2022.113945
- Ren, J., Lu, Y., Qian, Y., Chen, B., Wu, T., & Ji, G. (2019). Recent progress regarding kaempferol for the treatment of various diseases. Experimental and Therapeutic Medicine, 18(4), 2759–2776.
- Saeed, M., Naseer, S., Hussain, S., & Iqbal, M. (2019). Phytochemical composition and pharmacological effects of cassia fistula. Scientific Inquiry and Review, 4(1), 59–69. https://doi.org/10.32350/sir.41.05
- Santos, J. M. S., Lins, T. L. B. G., Barberino, R. S., Menezes, V. G., Gouveia, B. B., & Matos, M. H. T. (2019). Kaempferol promotes primordial follicle activation through the phosphatidylinositol 3-kinase/protein kinase B signaling pathway and reduces DNA fragmentation of sheep preantral follicles cultured in-vitro. Molecular Reproduction and Development, 86(3), 319–329. https://doi.org/10.1002/mrd.23107
- Seydi, E., Salimi, A., Rasekh, H. R., Mohsenifar, Z., & Pourahmad, J. (2018). Selective cytotoxicity of luteolin and kaempferol on cancerous hepatocytes obtained from rat model of hepatocellular carcinoma: Involvement of ROSmediated mitochondrial targeting. Nutrition and Cancer, 70(4), 594–604. https://doi.org/10.1080/01635581.2018.1460679
- Shahbaz, M., Imran, M., Alsagaby, S. A., Naeem, H., Al Abdulmonem, W., Hussain, M., Abdelgawad, M. A., El-Ghorab, A. H., Ghoneim, M. M., El-Sherbiny, M., Atoki, A. V., & Awuchi, C. G. (2023). Anticancer, antioxidant, ameliorative and therapeutic properties of kaempferol. International Journal of Food Properties, 26(1), 1140–1166. https://doi.org/10.1080/10942912.2023.2205040
- Sharma, A., Sinha, S., & Shrivastava, N. (2022). Apigenin and kaempferol as novel renoprotective agent against cisplatin-induced toxicity: An in-vitro study. Natural Product Research, 36(23), 6085–6090. https://doi.org/10.1080/14786419.2022.2045603
- Sheik, A., Kim, K., Varaprasad, G. L., Lee, H., Kim, S., Kim, E., Shin, J.-Y., Oh, S. Y., & Huh, Y. S. (2021). The anti-cancerous activity of adaptogenic herb Astragalus membranaceus. Phytomedicine, 91, 153698. https://doi.org/10.1016/j.phymed.2021.153698
- Shrivastava, S., Uthra, C., Reshi, M. S., & Shukla, S. (2016). Protective role of kaempferol against acrylamide intoxication. Free Radicals and Antioxidants, 7(1), 36–42. https://doi.org/10.5530/fra.2017.1.6
- Siddique, Y. H. (2021). Neurodegenerative diseases and flavonoids: Special reference to kaempferol. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 20(4), 327–342.
- Siegelin, M. D., Reuss, D. E., Habel, A., Herold-Mende, C., & Von Deimling, A. (2008). The flavonoid kaempferol sensitizes human glioma cells to TRAIL-mediated apoptosis by proteasomal degradation of survivin. Molecular Cancer Therapeutics, 7(11), 3566–3574.
- Simington, L. (2022). Quantification of kaempferol conjugates in watercress juice and methanol extract: A study using HPLC and protein binding.
- Song, W., Dang, Q., Xu, D., Chen, Y., Zhu, G., Wu, K., Zeng, J., Long, Q., Wang, X., He, D., Li L. (2014). Kaempferol induces cell cycle arrest and apoptosis in renal cell carcinoma through EGFR/p38 signaling. Oncology Reports, 31(3), 1350–1356. https://doi.org/10.3892/or.2014.2965
- Suguna, M., & Umesha, S. (2023). HR-LCMS Profiling of phytochemical constituents and evaluation of antioxidant, antibacterial, anti-cancerous and anti-inflammatory potentials, plasma biocompatibility and cytotoxicity of Grewia orbiculata Rottler. Vegetos, 36(3), 1–12. https://doi.org/10.1007/s42535-022-00530-z
- Tahir, M. S., Almezgagi, M., Zhang, Y., Bashir, A., Abdullah, H. M., Gamah, M., Wang, X., Zhu, Q., Shen, X., Ma, Q., Ali, M., Solangi, Z. A., Malik, W. S., & Zhang, W. (2021). Mechanistic new insights of flavonols on neurodegenerative diseases. Biomedicine & Pharmacotherapy, 137, 111253. https://doi.org/10.1016/j.biopha.2021.111253
- Tamer, F., Tullemans, B. M., Kuijpers, M. J., Claushuis, T. A., & Heemskerk, J. W. (2022). Nutrition phytochemicals affecting platelet signaling and responsiveness: Implications for thrombosis and hemostasis. Thrombosis and Haemostasis, 122(06), 879–894.
- Torres-Villarreal, D., Camacho, A., Castro, H., Ortiz-Lopez, R., & De la Garza, A. L. (2019). Anti-obesity effects of kaempferol by inhibiting adipogenesis and increasing lipolysis in 3T3-L1 cells. Journal of Physiology and Biochemistry, 75(1), 83–88. https://doi.org/10.1007/s13105-018-0659-4
- Tu, L. Y., Pi, J., Jin, H., Cai, J. Y., & Deng, S. P. (2016). Synthesis, characterization and anti-cancer activity of kaempferol-zinc(II) complex. Bioorganic & Medicinal Chemistry Letters, 26(11), 2730–2734. https://doi.org/10.1016/j.bmcl.2016.03.091
- Wang, F., Wang, L., Qu, C., Chen, L., Geng, Y., Cheng, C., Yu, S., Wang, D., Yang, L., & Meng, Z. (2021). Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer, 21(1), 1–11. https://doi.org/10.1186/s12885-020-07763-8
- Wang, J., Mao, J., Wang, R., Li, S., Wu, B., & Yuan, Y. (2020). Kaempferol protects against cerebral ischemia reperfusion injury through intervening oxidative and inflammatory stress induced apoptosis. Frontiers in Pharmacology, 11, 424. https://doi.org/10.3389/fphar.2020.00424
- Wang, T., Wu, Q., & Zhao, T. (2020). Preventive effects of kaempferol on high-fat diet-induced obesity complications in C57BL/6 mice. BioMed Research International, 2020. Article ID 4532482. https://doi.org/10.1155/2020/4532482
- Wang, X., Yang, Y., An, Y., & Fang, G. (2019). The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomedicine & Pharmacotherapy, 117, 109086. https://doi.org/10.1016/j.biopha.2019.109086
- Wu, B., Luo, H., Zhou, X., Cheng, C. Y., Lin, L., Liu, B. L., Liu, K., Li, P., & Yang, H. (2017). Succinate-induced neuronal mitochondrial fission and hexokinase II malfunction in ischemic stroke: Therapeutical effects of kaempferol. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1863(9), 2307–2318. https://doi.org/10.1016/j.bbadis.2017.06.011
- Xu, X. H., Zhao, C., Peng, Q., Xie, P., & Liu, Q. H. (2017). Kaempferol inhibited VEGF and PGF expression and in-vitro angiogenesis of HRECs under diabetic-like environment. Brazilian Journal of Medical and Biological Research, 50(3), 1–7. https://doi.org/10.1590/1414-431X20165396
- Yang, G., Xing, J., Aikemu, B., Sun, J., & Zheng, M. (2021a). Kaempferol exhibits a synergistic effect with doxorubicin to inhibit proliferation, migration, and invasion of liver cancer. Oncology Reports, 45(4), 1–10. https://doi.org/10.3892/or.2021.7952
- Yang, L., Gao, Y., Bajpai, V. K., El-Kammar, H. A., Simal-Gandara, J., Cao, H., & Xiao, J. (2021b). Advance toward isolation, extraction, metabolism and health benefits of kaempferol, a major dietary flavonoid with future perspectives. Critical Reviews in Food Science and Nutrition, 63(16), 1–17. https://doi.org/10.1080/10408398.2021.1980762
- Yao, H., Sun, J., Wei, J., Zhang, X., Chen, B., & Lin, Y. (2020). Kaempferol protects blood vessels from damage induced by oxidative stress and inflammation in association with the Nrf2/HO-1 signaling pathway. Frontiers in Pharmacology, 11, 1118. https://doi.org/10.3389/fphar.2020.01118
- Yao, S., Wang, X., Li, C., Zhao, T., Jin, H., & Fang, W. (2016). Kaempferol inhibits cell proliferation and glycolysis in esophagus squamous cell carcinoma via targeting EGFR signaling pathway. Tumor Biology, 37(8), 10247–10256. https://doi.org/10.1007/s13277-016-4912-6
- Yao, X., Jiang, H., NanXu, Y., Piao, X., Gao, Q., & Kim, N. H. (2019). Kaempferol attenuates mitochondrial dysfunction and oxidative stress induced by H2O2 during porcine embryonic development. Theriogenology, 135, 174–180. https://doi.org/10.1016/j.theriogenology.2019.06.013
- Yeon, M. J., Lee, M. H., Kim, D. H., Yang, J. Y., Woo, H. J., Kwon, H. J., Moon, C., Kim, S.-H., & Kim, J. B. (2019). Anti-inflammatory effects of kaempferol on Helicobacter pylori-induced inflammation. Bioscience, Biotechnology, and Biochemistry, 83(1), 166–173. https://doi.org/10.1080/09168451.2018.1528140
- Zabela, V., Sampath, C., Oufir, M., Moradi-Afrapoli, F., Butterweck, V., & Hamburger, M. (2016). Pharmacokinetics of dietary kaempferol and its metabolite 4-hydroxyphenylacetic acid in rats. Fitoterapia, 115, 189–197. https://doi.org/10.1016/j.fitote.2016.10.008
- Zang, Y., Zhang, L., Igarashi, K., & Yu, C. (2015). The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food & Function, 6(3), 834–841. https://doi.org/10.1039/C4FO00844H
- Zeng, Z. C., Jiang, J., Wang, X. J., Wei, K. N., Liang, H. S., Zeng, L. X., Xu, Y., Xie, S.-J., Meng, Z., Yang, X.-J., Guo, A.-W., & Wang, H. L. (2022). Kaempferol ameliorates in-vitro and in-vivo postovulatory oocyte ageing in mice. Reproductive BioMedicine Online, 45(6), 1065–1083. https://doi.org/10.1016/j.rbmo.2022.07.005
- Zhang, K., Gu, L., Chen, J., Zhang, Y., Jiang, Y., Zhao, L., Bi, K., & Chen, X. (2015a). Preparation and evaluation of kaempferol–phospholipid complex for pharmacokinetics and bioavailability in SD rats. Journal of Pharmaceutical and Biomedical Analysis, 114, 168–175. https://doi.org/10.1016/j.jpba.2015.05.017
- Zhang, Q., Wu, D., Wu, J., Ou, Y., Mu, C., Han, B., & Zhang, Q. (2015b). Improved blood–brain barrier distribution: Effect of borneol on the brain pharmacokinetics of kaempferol in rats by in-vivo microdialysis sampling. Journal of Ethnopharmacology, 162, 270–277. https://doi.org/10.1016/j.jep.2015.01.003
- Zhang, S. S., Liu, M., Liu, D. N., Shang, Y. F., Du, G. H., & Wang, Y. H. (2022). Network pharmacology analysis and experimental validation of kaempferol in the treatment of ischemic stroke by inhibiting apoptosis and regulating neuroinflammation involving neutrophils. International Journal of Molecular Sciences, 23(20), 12694. https://doi.org/10.3390/ijms232012694
- Zhang, Z., Guo, Y., Chen, M., Chen, F., Liu, B., & Shen, C. (2021). Kaempferol potentiates the sensitivity of pancreatic cancer cells to erlotinib via inhibition of the PI3K/AKT signaling pathway and epidermal growth factor receptor. Infammopharmacology, 29(5), 1587–1601. https://doi.org/10.1007/s10787-021-00848-1
- Zhou, M., Ren, H., Han, J., Wang, W., Zheng, Q., & Wang, D. (2015). Protective effects of kaempferol against myocardial ischemia/reperfusion injury in isolated rat heart via antioxidant activity and inhibition of glycogen synthase kinase-3. Oxidative Medicine and Cellular Longevity, 2015. Article ID 481405. https://doi.org/10.1155/2015/481405
- Zhu, X., Wang, X., Ying, T., Li, X., Tang, Y., Wang, Y., Yu, T., Sun, M., Zhao, J., Du, Y., & Zhang, L. (2022). Kaempferol alleviates the inflammatory response and stabilizes the pulmonary vascular endothelial barrier in LPS-induced sepsis through regulating the SphK1/S1P signaling pathway. Chemico-Biological Interactions, 368, 110221. https://doi.org/10.1016/j.cbi.2022.110221