ABSTRACT
Since the 1990s the worldwide rate of obesity has escalated significantly. This overflow affects all demographic groups, with a notable impact on older adults and women. Obesity is associated with a variety of serious chronic health conditions. Additionally, it is linked to endocrine disorders such as hypothyroidism and subclinical hypothyroidism. This review analyzes the connection between obesity and micronutrient levels, particularly focusing on vitamin D, and evaluates potential nutritional and supplementation approaches for this population.
Micronutrient imbalances in obesity arise from poor dietary intake, increased nutritional needs, altered pharmacokinetics, and absorption difficulties. These imbalances can lead to critical health and metabolic issues. For example, vitamin D deficiency, which is common in individuals with obesity, is associated with decreased calcium absorption and an incremented risk of type 2 diabetes, metabolic syndrome, and various inflammatory conditions such as cancer.
Effectively addressing micronutrient deficiencies requires dietary modifications and, when necessary, supplementation. While enhancing nutrition is critical, supplementation often becomes essential to meet the nutritional needs of individuals with obesity, particularly those on restrictive diets or undergoing bariatric surgery. Supplementing micronutrients and vitamin D in patients with obesity can improve health related outcomes. Moreover, dietary patterns like the Mediterranean diet can increase vitamin D levels. This review underlines the crucial role of tailored supplementation strategies and the demand for continued research to ascertain optimal dosing and its implications for health outcomes.
KEYWORDS:
Introduction
Worldwide, the prevalence of obesity has increased by almost double during the past three decades (Arroyo-Johnson & Mincey, Citation2016). Kloock et al. suggest it has even tripled since 1975, with two out of three United States (U.S.) citizens being overweight (Kloock et al., Citation2023). In 2014, 11% of males and 15% of women aged 18 or older had obesity (Arroyo-Johnson & Mincey, Citation2016). In 2013, more than 40 million children under the age of five were classified as overweight (Arroyo-Johnson & Mincey, Citation2016). Regardless of geographic location, ethnicity, or financial status, obesity rates have risen across all ages and sexes, with older individuals and women more likely to have obesity (Barrea et al., Citation2023; Chooi et al., Citation2019). This trend is consistent across nations and regions despite significant variations in absolute prevalence rates (Chooi et al., Citation2019).
Obesity is related to several health problems. The most common cause of mortality among the comorbid illnesses associated with obesity is cardiovascular disease (CVD), which is followed by type 2 diabetes mellitus (T2DM), several cancers (particularly of the rectum, esophagus, liver, and colon), and chronic kidney disease (Kloock et al., Citation2023). Increased body fat percentage could be associated with 4-9% of all cancer cases (Kloock et al., Citation2023). Excess weight generally leads to a reduced lifespan, a fact highlighted by the Covid-19 pandemic (Kloock et al., Citation2023).
Obesity is also linked to endocrine alterations, including hypothyroidism and subclinical hypothyroidism. Numerous studies and meta-analyses have examined the correlation between these conditions and adults with obesity, showing that patients with obesity have a higher prevalence of subclinical hypothyroidism, especially in harmful obesity phenotypes (Biondi, Citation2023; Chapela et al., Citation2024).
This review seeks to examine the relationship between obesity and levels of vitamin D and other micronutrients. Additionally, it will discuss possible nutritional and supplementation strategies for this population.
Micronutrient disturbances in obesity
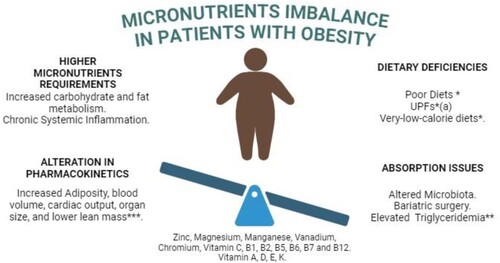
Obesity is a well-known nutritional imbalance that alters micronutrient levels. It is a crucial risk factor for various nutrient deficiencies, more frequently affecting minerals such as zinc, copper, iron, calcium, and magnesium, as well as both fat-soluble and water-soluble vitamins (McKay et al., Citation2020). This situation arises mainly from four different mechanisms (see ): 1) Dietary Deficiency; 2) Higher Requirements; 3) Altered Pharmacokinetics; and 4) Absorption Issues.
: Patients with obesity have several issues that might lead to micronutrients imbalance. This includes changes in the micronutrients requirements due to chronic systemic inflammation and altered macronutrient metabolism. Additionally, these patients have higher cardiac output due to changes in lean body mass and adipose tissue, which leads to alterations in the pharmacokinetics of micronutrients. Finally, changes in diets, or abortion issues due to modified microbiota or bariatric surgery with Y-Roux reconstruction, can lead to micronutrient deficiency. *Nutrient-poor and low in dietary fibre, protein, and micronutrients. ** Increased blood levels of cholesterol, triglycerides, and free fatty acids can alter the distribution of protein-bound micronutrients. UPF: Ultra-processed Foods (Lapik et al., Citation2020; McKay et al., Citation2020; Shahbaz et al., Citation2023)
Dietary deficiency
Micronutrient deficiencies have been observed in individuals with obesity due to poor diet, characterised by decreased intake of vitamins and minerals. An analysis from a nationally representative sample (NHANES 2001‒2008) of adults with normal weight, overweight and obesity shows a high proportion of individuals across all weight categories with inadequate micronutrient intake (Agarwal et al., Citation2015). This issue is exacerbated by diets high in processed foods that are calorie-dense and low in nutrient density (Guardiola-Márquez et al., Citation2022). This situation is generally accompanied by a decreased consumption of foods high in micronutrients, such as fruits and vegetables, which are primary sources of vitamins and minerals in a healthy diet (Guardiola-Márquez et al., Citation2022).
Currently, the most common and accessible type of foods in many societies is ultra-processed foods (UPFs), which are unfortunately nutrient-poor and low in dietary fibre, protein, micronutrients, and phytochemicals (Gupta et al., Citation2019; Havlová et al., Citation2023). These foods are high in energy density and low-cost (Poti et al., Citation2017). Their association with significant adverse health outcomes is well documented in the literature (Del Moral et al., Citation2021; Elizabeth et al., Citation2021). Furthermore, it has been demonstrated that the adult population in the U.S. is deficient in certain micronutrients due to the availability and overconsumption of these high-calories, low-nutrient UPFs. Importantly, poor nutrition is linked to the development of chronic conditions prevalent in adults with obesity, such as T2DM (Chen et al., Citation2023).
However, not only high-density UPFs and poor nutritional habits impact micronutrient levels. Very-low-calorie diets have also been associated with micronutrient deficiencies in some studies. A pilot study conducted by Damms-Machado et al. shows that micronutrient deficiency occurs in individuals with obesity and it is not corrected even by the implementation of a diet including a formula high in protein and containing vitamins and minerals (Damms-Machado et al., Citation2012; Shahbaz et al., Citation2023). In fact, some micronutrient levels remain low or become even lower, which might be explained by the other mechanisms underlying the pathophysiology of these micronutrient deficiencies (Damms-Machado et al., Citation2012), as will be further described in this section.
Higher micronutrients requirements
Another cause of micronutrient deficiencies in individuals with obesity could be related to the higher nutrient requirements present in patients with obesity as a result of the pathophysiological and metabolic changes (Astrup & Bügel, Citation2019). These patients often present higher requirements for zinc, magnesium, chromium, manganese, and vanadium because of their role in carbohydrate (CHO) and fat metabolism (McKay et al., Citation2020). Consequently, these patients are at great risk of developing nutritional deficiencies related to these micronutrients (Astrup & Bügel, Citation2019).
These increased requirements are as a result of the enhanced oxidative stress and inflammation observed in obesity, which can deplete these essential nutrients more rapidly (Fernández-Sánchez et al., Citation2011). Increased adiposity and systemic inflammation associated with obesity have been shown in several studies to disrupt the metabolism, absorption, distribution, and elimination of micronutrients (Milner & Beck, Citation2012). Because patients with obesity have higher levels of adiposity, altered blood composition, increased blood volume, and cardiac output, as well as changes in lean body mass and organ size (particularly the liver and kidneys), these conditions impact protein binding, renal clearance, volume of distribution, and hepatic metabolism (Astrup & Bügel, Citation2019; Gouju & Legeay, Citation2023; Wu et al., Citation2023).
Alteration in pharmacokinetics
Besides the factors mentioned above, micronutrient deficiencies in individuals with obesity may also result from altered pharmacokinetics, including changes in distribution, metabolism, and elimination (Astrup & Bügel, Citation2019). These disturbances are most likely caused by pathophysiological changes associated with increased adiposity, which affect pharmacokinetic parameters such as renal clearance, hepatic metabolism, protein binding, and volume of distribution (Gouju & Legeay, Citation2023).
It can be hypothesised that the same pathophysiological changes that affect the pharmacokinetics of drugs also impact micronutrient metabolism in similar ways (Meng et al., Citation2017). Individuals with obesity typically have increased blood volume, cardiac output, adiposity, organ size, and lower lean mass, all of which can influence the volume of distribution and the effects of drugs and micronutrients (Gouju & Legeay, Citation2023). The distribution of lipophilic compounds is frequently altered in patients with obesity. However, the exact degree to which these physiological changes influence distribution is highly variable and very difficult to predict (Zhang et al., Citation2022).
Absorption issues
Patients with obesity may experience altered absorption as a consequence of several factors. One significant cause is the altered microbiota in these individuals, which can affect the absorption and utilisation of micronutrients, thereby contributing to their deficiencies (Krezalek et al., Citation2017). Additionally, the treatment of morbid obesity through bariatric surgery can increase the risk of micronutrient deficiencies by reducing their consumption and absorption (Ciobârcă et al., Citation2020). The effect and significance of these deficiencies depend on the part of the gastrointestinal tract was bypassed. For instance, selenium, chromium, iron, manganese, zinc, calcium, and a variety of vitamins (both fat-soluble and water-soluble, including A, K, E, folic acid, and the B vitamins: B1, B2, B3, B5, B6, and B7), are assimilated in the duodenum and jejunum. In contrast, other fat-soluble vitamins like vitamin D and water-soluble vitamin C are taken up in the ileum (Isom et al., Citation2014). Vitamin B12 attaches to intrinsic factors in the stomach before being absorbed in the ileum. Patients who have undergone gastric bypass or similar surgeries present an increased risk of micronutrients malabsorption due to their anatomical alterations in the stomach and the upper part of the ileum (Amaya García et al., Citation2012).
Finally, elevated levels of triglycerides, cholesterol, and free fatty acids in the bloodstream of individuals with obesity may impact the distribution of protein-bound micronutrients (Siddiqui et al., Citation2023). Similarly, when minerals share chemical properties with other food ingredients, they may compete with transport proteins or other absorption mechanisms, potentially impairing their absorption and bioavailability (Lapik et al., Citation2020).
Effects on health and metabolic function
These alterations in micronutrient levels can lead individuals with obesity to have metabolic alterations or exacerbate pre-existing conditions. For instance, zinc is essential for numerous biochemical pathways, including those involved in insulin signalling and glucose metabolism. Deficiencies in zinc can impair immune function and wound healing, which are already compromised in individuals with obesity (Amaya García et al., Citation2012; Guardiola-Márquez et al., Citation2022). Magnesium is critical for energy production and muscle function, and its deficiency can lead to insulin resistance and hypertension, both of which are common comorbidities in obesity (Barazzoni et al., Citation2017).
Also, chromium enhances the action of insulin and is vital for maintaining normal glucose tolerance (Berger et al., Citation2022). A chromium deficiency can exacerbate insulin resistance and T2DM, conditions frequently associated with obesity (Iacone et al., Citation2016). Manganese is required for the proper functioning of antioxidant enzymes and the metabolism of amino acids, carbohydrates, and cholesterol (Berger et al., Citation2022; El Assar et al., Citation2013). Vanadium, though required in trace amounts, plays a role in glucose metabolism and lipid regulation, with deficiencies potentially worsening dyslipidemia and glucose intolerance (Treviño et al., Citation2019).
Physiopathology of hypovitaminosis D in obesity
The chemical reaction produced after sun exposure on the skin is as follows: 7-dehydrocholesterol is converted to previtamin D3, which is isomerised to vitamin D3 and finally in the liver converted to calcifediol (25-(OH)D (Barrea et al., Citation2021). It is then transported after binding to vitamin D-binding protein (DBP), to the kidney for hydroxylation to calcitriol (1,25(OH)2D) which is transported to the tissues (Bouillon et al., Citation2019; Song et al., Citation2022).
The 25(OH)D is the circulating form and clinically evaluated to define its plasma values. It has a half-life of 15 days (Bennour et al., Citation2022; Bouillon & Bikle, Citation2019; Jones et al., Citation2012; Mu et al., Citation2022). It circulates bound to a specific protein, DBP, and 19% bound to albumin (Bennour et al., Citation2022; Bouillon & Bikle, Citation2019; Cao et al., Citation2022). The biological action of 1,25(OH)2D is mediated through its binding to the vitamin D receptor (VDR) of the nuclear receptor family, present in cells of various tissues, which explains its involvement in the regulation of numerous genes (Bennour et al., Citation2022; Bouillon & Bikle, Citation2019; Kamei et al., Citation1993). The VDR receptor forms a heterodimer with the retinoid X receptor (RXR) and attaches to DNA at a location known as the vitamin D response element (VDRE), found in the promoter region of genes controlled by vitamin D (Carlberg, Citation2019).
Low circulation concentrations of vitamin 25-hydroxyvitamin D 3 are frequently related to obesity [25(OH)D 3]. Results of an Australian prospective study on diabetes, obesity and lifestyle (AusDiab) (Gagnon et al., Citation2012) evaluated the prospective association between 25(OH)D, metabolic syndrome, and its components in a large population-based cohort of adults (Gagnon et al., Citation2012). Lower 25(OH)D concentrations in adult Australians were linked, at five years, to a higher risk of metabolic syndrome as well as higher waist circumference, serum triglycerides, fasting glucose, and insulin resistance (Gagnon et al., Citation2012). In 2015, a systematic review and meta-analysis aimed to assess the relationship between vitamin D insufficiency and obesity across various age groups. The results of the meta-analysis showed that, regardless of age group, obesity was related to vitamin D deficiency (Pereira-Santos et al., Citation2015).
In specific populations with a high prevalence of patients with obesity, certain associations were observed (Barrea et al., Citation2021). For example, in patients with hypopituitarism, patients had a higher prevalence of hypovitaminosis D, and this deficiency was found to be associated with a higher likelihood of dyslipidemia, hypertension, and multiple sclerosis (Savanelli et al., Citation2016). Furthermore, in Prader-Willi syndrome, nutritional advice is aimed at avoiding obesity, and supplementation with vitamin D is recommended due to the restrictive diets that these patients have (Muscogiuri et al., Citation2021).
Factors contributing to vitamin D deficiency in obesity
Despite the associations between obesity and low vitamin D levels, it has not been determined whether low vitamin D levels are the cause of increased fat mass deposition, or these low levels respond to increased body fat storage (). The hypotheses that explain this association are as follows:
Lifestyle patterns: Factors such as eating habits, sedentary lifestyle, clothing, ethnicity, skin colour, geographic location, season, and age (Bennour et al., Citation2022).
Reduced 25-hydroxylation of vitamin D: Hyperparathyroidism present in individuals with obesity increases 1,25(OH)2D, stimulated by PTH levels, leading to negative feedback and reducing the first vitamin D activation (Bell et al., Citation1985).
Sequestration of 25(OH)D by excess adipose tissue: Wortsman et al. (Wortsman et al., Citation2000) reported a 57% attenuation of vitamin D3 concentration in subjects with obesity after exposure to UVB radiation compared to non-obese subjects. The skin's capacity to produce vitamin D3 remained unchanged, but the ability of the skin in the group with obesity to release vitamin D3 into the bloodstream was altered (Wortsman et al., Citation2000), possibly resulting from the sequestration of newly synthesised vitamin D3 by subcutaneous fat, decreasing the amount of substrate reaching the liver for 25-hydroxylation (Lawson et al., Citation1986; Wortsman et al., Citation2000).
Modifications of Metabolism in Adipose Tissue: A 71% reduction in mRNA expression of the CYP2J2 enzyme was evidenced in the subcutaneous adipose tissue of women with obesity, compromising the 25-hydroxylation of vitamin D3 (Wamberg et al., Citation2013).
Downregulation of Gene Expression of CYP2R1: This enzyme encodes the major hepatic 25-hydroxylase, that may contribute to inter-individual variations in vitamin D homeostasis (Bennour et al., Citation2022; Bouillon & Bikle, Citation2019; Liu et al., Citation2022). Roizen et al. (Roizen et al., Citation2019), reported that obesity secondary to high-fat diets in mice decreased the expression of Cyp2r1 mRNA, reducing the activity of 25-hydroxylation of vitamin D to 25(OH)D. They described a significant reduction of 25-hydroxylase activity in obese mice, as well as hepatic decrease of Cyp2r1 mRNA transcription, showing an effect of obesity or high-fat diet (Roizen et al., Citation2019).
VDR receptor polymorphisms (SNPs): As mentioned above, 1,25(OH)2D binds to the VDR receptor, which functions as a transcription factor, thus modulating the expression of target genes (Bikle, Citation2009; Zhao et al., Citation2022). Ochs-Balcom et al. (Ochs-Balcom et al., Citation2011), found a positive association between the VDR SNP (rs3782905) and adiposity phenotype. Homozygous carriers of the rare genotype (GG) had a 1.7-fold higher BMI and 4.4 cm greater waist circumference than carriers of the wild-type genotype. In addition, for the SNP Cdx-2 (rs11568820), there was a positive association with waist circumference and abdominal sagittal diameter.
There is a strong suspicion that the biological effects of 25(OH)D on health and disease, particularly in the context of obesity, are primarily driven by the genomic mechanism of VD through VDR. This suspicion is supported by several studies indicating a correlation between VDR polymorphism and pathological conditions (Bennour et al., Citation2022). However, extensive assessments of genetic polymorphisms in genes associated with vitamin D metabolism and obesity-related traits, conducted in 2011 and 2013, did not find compelling evidence that low vitamin D levels are a causative factor for obesity. (Drincic et al., Citation2012; Liu et al., Citation2022; Wortsman et al., Citation2000).
| 7. | Consequence of Volumetric Dilution Due to Excess Fat Mass: The volumetric dilution model accounted for all the variability in serum 25(OH)D concentration attributable to obesity. The inverse association between vitamin D levels, body weight and body fat is related to the greater volume distribution of both vitamin D and 25(OH)D in tissue mass (Drincic et al., Citation2012). | ||||
Consequences of hypovitaminosis D on bone health and metabolism
Vitamin D has pleiotropic actions on several tissues, organs, and metabolic processes. Its deficiency may contribute to the pathogenesis of several metabolic disorders (Bikle, Citation2009). Regarding bone health, vitamin D deficiency causes reduced calcium absorption and increased PTH to achieve a positive calcium balance at the expense of bone health (Bouillon et al., Citation2019). This deficit, if prolonged, affects the normal bone growth plate structure seen in rickets, impairs bone mineralisation, and increases the risk of falls and fractures (Bouillon et al., Citation2019; Kuchuk et al., Citation2009).
Vitamin D exerts an anti-inflammatory effect, decreasing the expression of chemokines and cytokines in adipocytes (Bouillon et al., Citation2019). In the liver, it regulates pro-inflammatory cytokines, cell apoptosis and fibrosis (Beard et al., Citation2011; Eliades et al., Citation2013). Vitamin D polarises the adaptive immune system pathway towards an increased T helper type 2 (Th2) cell response, associating anti-inflammatory interleukins (IL), such as IL4 and IL10, with the activation of the humoral response and inhibiting Th1 cells, thereby decreasing the production of pro-inflammatory cytokines (Beard et al., Citation2011; Eliades et al., Citation2013; Song-Xin et al., Citation2022). In macrophages present in visceral adipose tissue, this results in a reduction of local and overall inflammation (Hyppönen & Boucher, Citation2018). Moreover, adiponectin, produced by differentiated adipocytes, is a potent anti-inflammatory agent that decreases NF-kB activity and acts as an insulin sensitiser, preventing lipid accumulation in hepatocytes through stimulating β-oxidation and reducing fatty acid synthesis (Chen et al., Citation2022; Cimini et al., Citation2017). Dysfunctional adipose tissue in people with obesity alters the secretion of adiponectin, which is negatively regulated by pro-inflammatory cytokines such as IL-6 or TNF-α. Vitamin D deficiency also reduces adiponectin expression, affecting insulin sensitivity and fatty acid oxidation (Feghaly et al., Citation2020).
Vitamin D also decreases the overactivity of the renin-angiotensin system (RAS), suppressing the production of renin (Barazzoni et al., Citation2022; Mercola et al., Citation2020). The RAS is a sequence of reactions that play an essential role in the regulation of blood pressure, extracellular volume and sodium-potassium balance (Ferder et al., Citation2013). In the presence of vitamin D deficiency, increased renin and angiotensinogen II mRNA expression was observed in the kidney, leading to elevated diastolic and systolic blood pressure (Li et al., Citation2002). Furthermore, treatment with 1,25(OH)2D, reduced renal renin mRNA expression, confirming a negative regulation between vitamin D and RAS in vivo. Thus, 1,25(OH)2D, directly suppresses renin expression in a VDR-dependent manner (Li et al., Citation2002).
Increased abdominal adiposity in the liver and muscle leads to insulin resistance (Santangeli et al., Citation2024). Vitamin D promotes insulin secretion by healthy pancreatic β-cells by increasing intracellular calcium and activating genes such as VDR receptor ligand (Moore et al., Citation2015). Hypovitaminosis D affects the pancreas's endocrine function, causing dysfunction of β-cells and decreased insulin secretion by pancreatic islets, resulting in hyperglycemia and hyperlipemia, which lead to insulin resistance, a key factor in the development of T2DM (Leung, Citation2016). Chronic hyperglycemia and hyperlipemia are associated with increased lipid accumulation in liver and pancreatic islets, causing endoplasmic reticulum (ER) stress, lipotoxicity (inflammation) and insulin resistance (Hotamisligil, Citation2010). This lipotoxicity at the pancreatic islet level impairs glucose-stimulated insulin secretion, promotes ER stress, and results in cell failure and apoptosis (Leung, Citation2016). Another pathway affected is the activation of serine/threonine protein kinase (Akt), which is associated with the reversal of the toxic effects of glucose and lipids in the β-cell (Cheng et al., Citation2013; Dwijayanti et al., Citation2024). Angiotensin, a component of RAS, modulates the activation of the Akt signalling pathway. Vitamin D, as a ligand of RAS, exerts action on the Akt signalling pathway. Cheng et al. (Cheng et al., Citation2013), reported an increase in RAS components in pancreatic β-cells of vitamin D-deficient mice, confirming that chronic hypovitaminosis D modulates the expression of RAS genes in pancreatic islet cells in vivo, altering insulin function and sensitivity. Pancreatic RAS would be involved in pancreatic islet β-cell dysfunction and increased risk of T2DM (Cheng et al., Citation2013).
Moreover, obesity leads to dyslipidemia with increased triglycerides, free fatty acids, and decreased HDL-C with increased serum LDL-C (Hyppönen & Boucher, Citation2018). The elongation of fatty acids greater than 16 carbons requires the specific activity of seven enzymes called very long chain fatty acid elongases (Elovl) (Ji et al., Citation2016). The regulation of Elovl3 expression is ligand-dependent on vitamin D via VDR, finding greater synthesis of fatty acids of 18–24 carbons and greater activity of this elongase in the presence of vitamin D deficiency in white adipose tissue (Ji et al., Citation2016).
Finally, nonalcoholic fatty liver disease (NAFLD), recently defined as metabolic dysfunction-associated steatotic liver disease (MASLD), comprises a large spectrum of liver conditions (Barrea et al., Citation2023; Santangeli et al., Citation2024; Yu et al., Citation2022). This pathology is a consequence of the hepatic excess glucose, which, after glycogenogenesis and oxidation, is converted into fat (de novo lipogenesis). Excess accumulation of these lipids causes inflammation and insulin resistance (Gregor & Hotamisligil, Citation2011). Hepatic insulin resistance leads to dysregulation of glucose homeostasis, leading to a pathological and dysfunctional state of the liver (Hotamisligil, Citation2010). These metabolic disorders are produced by ER stress in liver cells. Vitamin D deficiency can affect lipid and glucose metabolism in the liver (Hotamisligil, Citation2010). Calcitriol (1,25(OH)2D) mediates the signalling pathway involving AMP-activated protein kinase (AMPK), reducing lipid accumulation (via the transcription factor SREBP1c) and glucose production (via regulation of PEPCK and G6Pase enzyme expression) by increasing calcium concentrations (Leung, Citation2016). Decreased vitamin D levels were linked to the severity of NAFLD or non-alcoholic steatohepatitis (NASH) through liver biopsies (Targher et al., Citation2007). In addition, vitamin D exerts an anti-inflammatory and anti-fibrotic effect in hepatocytes, decreasing the expression of pro-inflammatory cytokines such as IL-6 and TNF-α (Cimini et al., Citation2017; Li et al., Citation2023; Pivonello et al., Citation2022; Santangeli et al., Citation2024). Moreover, vitamin D deficiency was associated with increased portal hypertension and infectious complications, being a predictor of mortality in patients with liver disease (Santangeli et al., Citation2024). A meta-analysis revealed low levels of vitamin D in people with NAFLD (OR 1.26; 95% CI: 1.17, 1.35), which could influence the onset of NAFLD (Eliades et al., Citation2013).
Clinical relevance
There is an association between obesity, metabolic syndrome, and 25(OH)D deficiency. Despite the variety of existing hypotheses, it remains undetermined whether low vitamin D levels cause increased fat mass deposition or if these low levels result from increased body fat storage (Barrea et al., Citation2021; Bennour et al., Citation2022; Bouillon & Bikle, Citation2019). In clinical practice, it is necessary to evaluate circulating 25(OH)D levels to detect and correct a possible deficit, thus preventing the risks associated with its low plasma level in relation to obesity-related metabolic disorders.
This vitamin exerts action on bone health, the immune system, and a wide variety of diseases through the regulation of the expression of target genes. Its anti-inflammatory effect influences the expression of pro-inflammatory cytokines, acting on several cells, including adipose, pancreatic and hepatic cells. Its anti-diabetic effect could be dual, modulating hepatic glucose and lipid metabolism and promoting pancreatic cell function and survival. It also supports electrolyte homeostasis, blood volume, and blood pressure (Kwok et al., Citation2013; Leung, Citation2016; Li et al., Citation2002)
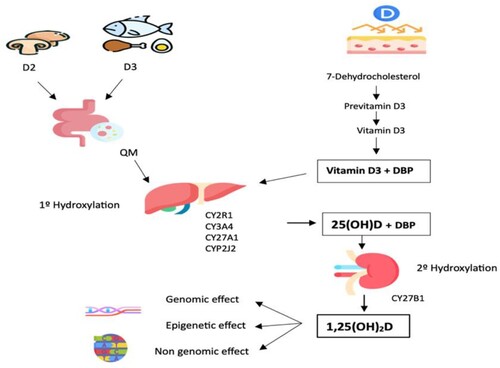
: Vitamin D from food is absorbed and transported by chylomicrons (QM) from the intestine to the liver. Sun exposure on the skin results in the formation of 7-Dehydrocholesterol, which is then converted to previtamin D3 and subsequently to vitamin D3. Vitamin D3 is carried to the liver by the specific protein DBP (Bikle, Citation2009; Lee et al., Citation2022; Ochs-Balcom et al., Citation2011; Sun et al., Citation2023). In the liver, both dietary and skin-derived vitamin D undergo first hydroxylation by hydroxylases enzymes (CY2R1, CY3A4, CY27A1, CYP2J2), producing 25(OH)D (Woranam et al., Citation2022). This 25(OH)D is then delivered by the DBP protein to the kidney for second hydroxylation to 1,25(OH)2D, the active form of vitamin D with genomic, non-genomic, and epigenetic effects (Bikle, Citation2009; Čelakovská et al., Citation2022; Ochs-Balcom et al., Citation2011).
Diet and supplementation of micronutrients in patients with obesity
Role of dietary interventions in correcting micronutrient deficiencies
The literature has shown that patients with severe obesity, compared to normal-weight controls, are exposed to micronutrient deficiencies (MD), including calcium, magnesium, iron, zinc, selenium, and vitamins A, C, and D and E (Bettini et al., Citation2020; Damms-Machado et al., Citation2012; Guardiola-Márquez et al., Citation2022). This demonstrates the complexity of the issue since the individual faces a double burden of malnutrition, with over-nutrition and deficit malnutrition coexisting (Barazzoni & Gortan Cappellari, Citation2020). Multiple factors influence this problem, including a low-quality and poorly varied diet, but this deficiency can also be due to alterations in the absorption, distribution or excretion of nutrients (Kobylińska et al., Citation2022). Additionally, the inflammatory state caused by obesity and the increased expression of the systemic iron-regulating protein hepcidin can affect iron status in the body (Alshwaiyat et al., Citation2021; González-Domínguez et al., Citation2020; Lee-Rangel et al., Citation2022). Likewise, due to various mechanisms, including volumetric dilution in larger volumes of fat, serum, liver, and muscle, vitamin D deficiency is common in individuals with obesity (Vranić et al., Citation2019).
These deficits must first be addressed by acquiring healthy eating habits. The availability and excessive consumption of UPF rich in fat, sugar, and salt and low in nutrients displaces the consumption of natural foods that contain multiple vitamins and minerals (Astrup & Bügel, Citation2019). Studies carried out in the U.S. and Canada indicate a high percentage of the population that does not achieve recommended levels of micronutrients through diet (Ahmed et al., Citation2021; McGuire, Citation2016). A Canadian study found that both males and females had a low frequency of insufficient iron intake. Moreover, poor zinc intakes were highly prevalent (21.1% to 43.5% for men and 29.8% to 34.8% for women) (Ahmed et al., Citation2021; McGuire, Citation2016). With regard to calcium and magnesium, the frequency of insufficient intakes was likewise high with advancing age (Ahmed et al., Citation2021; McGuire, Citation2016).
The first step to alleviating these deficiencies is to address nutritional interventions. It is essential to work on adequate caloric intake from a varied diet that includes foods from different groups (Frias-Toral et al., Citation2022; Muscogiuri et al., Citation2022). Counselling with nutrition professionals is essential, as they will guide and direct the patient in the acquiring and maintening new habits (Astrup & Bügel, Citation2019). At this stage, it is critical to highlight the significance of choosing the appropriate treatment that does not further deepen the deficiencies. Very restrictive diets such as the ketogenic diet, very low-calorie diets, intermittent fasting, among many others, can worsen the situation if not properly guided (Bradley et al., Citation2023). Thus, interventions associated with more gradual weight loss, such as a balanced low-calorie diet, gradual portion control, the Mediterranean diet, or a low-glycemic index diet, tend to be more appropriate for initially addressing MD (Barrea et al., Citation2021; Capurso et al., Citation2019; Murakami & Sasaki, Citation2018).
Below are analyses of some diets and their impact on the contribution of micronutrients, as well as in :
Table 1. Type of diets for losing body weight and possible micronutrient deficiencies.
Ketogenic Diet: This diet is characterised by a high intake of fat, moderate protein, and very restrictive CHO. It causes rapid weight loss over time (McGaugh & Barthel, Citation2022). In this case, adequate advice is essential to carry out this type of diet. By limiting the consumption of CHO, you also reduce the intake of fortified foods, leading to limited thiamine intake (Athanasian et al., Citation2021; Muscogiuri et al., Citation2016).
Intermittent Fasting: This type of intervention requires food consumption within a restricted period, which may involve fasting on alternate days or periodic fasting with varying durations (Varady et al., Citation2022). The modified fasting modality on alternate days is characterised by being low in fibre and micronutrients such as potassium, vitamin A, iron, calcium, iodine, magnesium, linoleic acid and α-linolenic acid (Bradley et al., Citation2023).
Low Carbohydrate Diet: These diets limit CHO intake, generating ketone bodies through fatty acid oxidation and the positive regulation of ketogenic enzymes (Papadopoulou & Nikolaidis, Citation2023). Low-carbohydrate diets often require micronutrient supplementation as they are usually deficient in fibre, folate, potassium, calcium, magnesium, iron, vitamin A, iodine, linoleic acid and α-linolenic acid (Jebeile et al., Citation2020).
Very Low-Calorie Diets: These diets are founded on a calorie restriction of ≤800 kcal per day, usually in the form of meal replacements or food-based diets, generating rapid weight loss (Gow et al., Citation2021). Given the very low caloric intake, and consequently low nutrient intake from food, daily supplementation with multivitamins and mineral supplements is necessary to prevent the risk of malnutrition (Gow et al., Citation2021).
Efficacy and safety of micronutrient supplementation
It is clear that there are potential MD in patients with obesity. Improving nutrition is the first step, but supplementation with micronutrients should also be considered when diet alone cannot meet the requirements. Although more scientific evidence is needed to recommend supplementation broadly, there are cases where it is necessary, such as documented iron deficiency anemia (Astrup & Bügel, Citation2019; Grosso et al., Citation2022). Patients on very restrictive diets, those taking drugs to treat obesity, candidates for bariatric surgery, or those who have undergone surgery may require supplementation, considering the type of procedure and possible malabsorption risks (Bettini et al., Citation2020). Another example of the need for supplementation is in patients who take medications like orlistat for a long time, as they may experience deficiencies in fat-soluble vitamins (Mayer et al., Citation2021).
Multiple studies have demonstrated the importance of supplementation when there is a documented deficiency. Nonetheless, treatment must be individualised. In populations whithout deficiency, supplementation can be counterproductive, potentially exceeding the maximum tolerable intake levels for some micronutrients. Therefore, it is recommended to study each patient, refer them to a nutrition expert if a deficiency is detected, and supplement if necessary (Engle-Stone et al., Citation2019).
Practical recommendations for healthcare professionals
Given the frequent micronutrient deficiency in patients with obesity, healthcare personnel should be aware of this during consultations. Timely detection of the problem and early referral to a nutrition specialist are essential. The specialist will assess the intake and make subsequent recommendations. It is crucial to evaluate the most appropriate treatment for each patient while considering the potential risks (Bradley et al., Citation2023). Healthcare professionals must also assess barriers that patients mention in relation to adopting a healthy lifestyle and diet (Astrup & Bügel, Citation2019). Common obstacles include lack of time and culinary skills (Astrup & Bügel, Citation2019; Barrea et al., Citation2023). Nutritional counselling, providing strategies for planning and organisation, and the use of educational materials are essential (Astrup & Bügel, Citation2019).
Diet and supplementation of vitamin D in patients with obesity
Dietary sources and sunlight exposure considerations
Vitamin D is a hormone with pleiotropic effects, exerting its action through binding to the VDR present in various tissues and systems (Bennour et al., Citation2022). This vitamin can be derived from a dietary source or from endogenous production through skin exposure to sunlight, the latter being the most important source. Since vitamin D is not found in many diets, dermal production is the main natural source of the vitamin (Haddad, Citation1992). Short periods of unplanned sun exposure on the hands, face, and arms are believed to be equivalent to consuming 200 international units (IU) (5 ug) of vitamin D. (Haddad, Citation1992; Muscogiuri et al., Citation2019). There are not many natural exogenous sources of VD (Bennour et al., Citation2022). Cholecalciferol, commonly referred to as Vitamin D3, is present in animal-source products, primarily fish liver oil, fatty fish including salmon, sardines, herring, and mackerel, and egg yolk. Vitamin D2, sometimes referred to as ergocalciferol, is present in plants and mushrooms (Bennour et al., Citation2022). After ingestion and absorption in the small intestine, vitamin D is incorporated into the chylomicrons and transported to the liver through the lymphatic system. In the liver, the first hydroxylation occurs with cytochrome P450 enzymes (CY2R1, CY3A4, CYP27A1 and CYP2J2) with 25-hydroxylase activity converting vitamin D3 to 25-hydroxyvitamin D (25(OH)D), a reaction catalysed by one more specific enzyme, CYP2R1 (Bennour et al., Citation2022; Bouillon & Bikle, Citation2019; Cheng et al., Citation2003). This hepatic hydroxylation is inhibited by parathyroid hormone (PTH) and by 1,25(OH)2D from the second hydroxylation of vitamin D in the kidney by the CYP27B1 enzyme (Akhtar et al., Citation2022; Wamberg et al., Citation2013).
Endogenous production through exposure of the skin to UVB radiation is highly variable, depending on factors such as latitude, pollution, season, use of sunscreen, clothing, skin colour, age, etc (Barrea et al., Citation2017; Bennour et al., Citation2022). Dietary or cutaneously synthesised vitamin D is not physiologically active and must be converted to active metabolites by the liver and kidney through enzymatic processes (Haddad, Citation1992). It is well known that the body can store vitamin D, which enables it to maintain adequate levels over the winter periods, and that sporadic sun exposure delivers sufficient vitamin D. The chemical reaction produced after sun exposure on the skin is as follows: Vitamin D is produced from 7-dehydrocholesterol, which is then converted in the liver into calcifediol (25-(OH)D) (Barrea et al., Citation2021). It then attaches to the DBP and is transported to the kidney for hydroxylation to calcitriol (1,25(OH)2D) (Bouillon et al., Citation2019).
Mediterranean diet
Based on a typical dietary pattern of the Mediterranean region, the Mediterranean diet is a nutritious eating regimen (Barrea et al., Citation2017). This specific diet is known for having very low levels of saturated fat and high levels of micronutrients, such as dietary vitamins and minerals, and polyunsaturated fatty acids (PUFA), which are frequently shown to raise the plasma antioxidant capacity and anti-inflammatory effects (Barrea et al., Citation2017; Muscogiuri et al., Citation2022).
Regarding the Mediterranean diet and its effects on vitamin D levels, there appears to be an association between adherence to this diet and vitamin D levels in patients with obesity. An observational study demonstrated that patients with obesity with higher adherence to this diet had higher levels of vitamin D (Barrea et al., Citation2017). In another study, adherence to Mediterranean diet measured using the PREDIMED score showed a positive correlation between the score and vitamin D levels (Barrea et al., Citation2020). Additionally, in the same study, comparing groups with low and high adherence to the Mediterranean diet, vitamin D levels exhibited the highest OR values in the bivariate proportional odds ratio (OR) model for both genders (OR 1.21 and OR 1.31, respectively) (Barrea et al., Citation2020).
Supplementation guidelines and dosage recommendations
Vitamin D status is assessed through the plasma levels of 25-hydroxyvitamin D [25(OH)D], as described by various scientific societies. The American Geriatric Society, the National and International Osteoporosis Foundation, and the Endocrine Society classify vitamin D insufficiency as having 25-hydroxyvitamin (25 OH D) levels below 30 ng/ml (Chauhan et al., Citation2024). A recommended range of 40–60 ng/ml is suggested by the Endocrine Society (Chauhan et al., Citation2024). The National Institutes of Health, however, describes a vitamin D deficiency as fewer than 20 ng/ml (Chauhan et al., Citation2024). According to several sources, a deficit is less than 12 ng/ml, while an insufficiency is between 12 and 19 ng/ml (Chauhan et al., Citation2024).
Many countries have implemented policies of fortifying foods with vitamin D after multiple studies demonstrated that ingesting 400 IU of vitamin D per day would prevent osteomalacia and rickets (Haddad, Citation1992). Acquiring adequate levels of the vitamin through sun exposure or even through fortifying foods is considered safe (Haddad, Citation1992). Universal screening for vitamin D deficiency should be conducted exclusively in people or groups at risk. It is known that obesity is related to low levels of vitamin D, which is why individuals with obesity may require higher doses than the general population (Walsh et al., Citation2016).
Importance of vitamin D in obesity-related health outcomes
Regarding vitamin D supplementation, a meta-analysis published in 2020 observed that following supplementation, people with obesity had higher serum vitamin D levels (39.83 nmol/L, 95% confidence interval: 34.06-45.61) compared to the control/placebo group. However, obesity in adults reduced the effect of vitamin D supplementation, emphasising the need to evaluate the optimal dose of supplementation in this population (de Oliveira et al., Citation2020). Another systematic review and meta-analysis of randomised and non-randomised controlled trials aimed to determine whether serum 25-hydroxyvitamin D levels rise in response to weight loss as opposed to weight maintenance. The findings suggested that vitamin D levels might improve slightly more with weight loss compared to weight maintenance, given equivalent conditions of supplemental vitamin D consumption (Mallard et al., Citation2016).
Several randomised clinical trials were conducted to determine the reason behind low levels of plasma of vitamin D and its supplementation with obesity. The results were contrasting. Two meta-analyses found no evidence of beneficial effects of vitamin D supplementation on obesity rates (BMI, fat mass, percent fat mass, or lean body mass) (Golzarand et al., Citation2018; Pathak et al., Citation2014). However, a third meta-analysis reported an improvement in BMI and waist ratio after vitamin D supplementation (Perna, Citation2019). In 2018, a double-blind, randomised, placebo-controlled trial assessed the effects of cholecalciferol (25,000 IU/weekly) or placebo for three months in conjunction with a weight loss regimen. Using a hyperinsulinemic-euglycemic clamp to measure insulin sensitivity, individuals who took vitamin D showed a marked improvement in insulin sensitivity (Cefalo et al., Citation2018).
Some authors suggest that people with obesity need higher loading doses of vitamin D to accomplish the same serum 25-hydroxyvitamin D levels as those with normal weight (Walsh et al., Citation2016). Because of the larger volume of distribution, individuals with obesity will require higher loading doses to reach the same serum 25OHD repletion threshold as those with normal weight. (Walsh et al., Citation2016). It may take multiple loading courses for these individuals to reach completion (Walsh et al., Citation2016). A systematic review in 2021 showed that daily vitamin D supplementation between 2000 and 4000 IU is highly recommended for older adults with obesity. Doses equivalent to 1000 IU or less did not significantly affect vitamin D deficiency or associated health problems in older adults with obesity (Rondanelli et al., Citation2022).
Based on the Endocrine Society's clinical practice guidelines, for patients with obesity with vitamin D deficiency, 6,000–10,000 IU/day of vitamin D2 or D3 is recommended to achieve target levels of 25 OH vitamin D, followed by maintenance doses of 3,000-6,000 IU/day (Holick et al., Citation2011). Further research efforts should specifically seek to elucidate whether this group of people could benefit from vitamin D administration in relation to cardiometabolic well-being ().
Table 2. Relationship between vitamin D and obesity.
Conclusions
Obesity is related to micronutrient deficiencies, which can be caused by several factors, including altered pharmacokinetics, poor dietary intake, and disturbed absorption due to factors such as modified microbiota, bariatric surgery, or elevated triglyceridemia. A consensus on the optimal approach to routine supplementation for these deficiencies has not yet been reached.
It has been observed that patients with obesity may have altered vitamin D levels. Although the correlation between obesity and vitamin D deficiency is well established, the exact supplementation dose required for these patients remains unclear. Additionally, there are contradictory results regarding the effects of vitamin D supplementation on body composition and insulin resistance.
Authors contributions
Conceptualisation: Martinuzzi, Andres Luciano Nicolas Methodology: Chapela, Sebastián Supervision: Frias-Toral, Evelyn; Verde, Ludovica; Martha Montalvan Roles/Writing – original draft: Chapela, Sebastián; Writing – review & editing: Martinuzzi, Andres Luciano Nicolas; Ceriani, Florencia; Gonzalez, Victoria; Llobera, Natalia. All authors read and approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Bibliography
- Agarwal, S., Reider, C., Brooks, J. R., & Fulgoni, V. L. (2015). Comparison of prevalence of inadequate nutrient intake based on body weight status of adults in the United States: An analysis of NHANES 2001-2008. Journal of the American College of Nutrition, 34(2), 126–134. https://doi.org/10.1080/07315724.2014.901196
- Ahmed, M., Praneet Ng, A., & L’Abbe, M. R. (2021). Nutrient intakes of Canadian adults: Results from the Canadian community health survey (CCHS)–2015 public use microdata file. The American Journal of Clinical Nutrition, 114(3), 1131–1140. https://doi.org/10.1093/ajcn/nqab143
- Akhtar, J., Abrha, M. G., Teklehaimanot, K., & Gebrekirstos, G. (2022). Cold plasma technology: Fundamentals and effect on quality of meat and its products. Food and Agricultural Immunology, 33(1), 451–478. https://doi.org/10.1080/09540105.2022.2095987
- Alshwaiyat, N. M., Ahmad, A., Wan Hassan, W. M. R., & Al-Jamal, H. A. N. (2021). Association between obesity and iron deficiency (Review). Experimental and Therapeutic Medicine, 22(5), 1268. https://doi.org/10.3892/etm.2021.10703
- Amaya García, M. J., Vilchez López F. J., Campos Martín, C., Sánchez Vera, P., & Pereira Cunill, J. L. (2012). Micronutrients in bariatric surgery. Nutricion Hospitalaria, 27(2), 349–361.
- Arroyo-Johnson, C., & Mincey, K. D. (2016). Obesity epidemiology worldwide. Gastroenterology Clinics of North America, 45(4), 571–579. https://doi.org/10.1016/j.gtc.2016.07.012
- Astrup, A., & Bügel, S. (2019). Overfed but undernourished: Recognizing nutritional inadequacies/deficiencies in patients with overweight or obesity. International Journal of Obesity, 43(2), 219–232. https://doi.org/10.1038/s41366-018-0143-9
- Athanasian, C. E., Lazarevic, B., Kriegel, E. R., & Milanaik, R. L. (2021). Alternative diets among adolescents: Facts or fads? Current Opinion in Pediatrics, 33(2), 252–259. https://doi.org/10.1097/MOP.0000000000001005
- Barazzoni, R., Breda, J., Cuerda, C., Schneider, S., Deutz, N. E., Wickramasinghe, K., Abbasoglu, O., Meijerink, J. B., Bischoff, S., Pelaez, R. B., & Cardenas, D. (2022). COVID-19: Lessons on malnutrition, nutritional care and public health from the ESPEN-WHO Europe call for papers. Clinical Nutrition, 41(12), 2858–2868. https://doi.org/10.1016/j.clnu.2022.07.033
- Barazzoni, R., Deutz, N. E. P., Biolo, G., Bischoff, S., Boirie, Y., Cederholm, T., Cuerda, C., Delzenne, N., Sanz, M. L., Ljungqvist, O., & Muscaritoli, M. (2017). Carbohydrates and insulin resistance in clinical nutrition: Recommendations from the ESPEN expert group. Clinical Nutrition, 36(2), 355–363. https://doi.org/10.1016/j.clnu.2016.09.010
- Barazzoni, R., & Gortan Cappellari, G. (2020). Double burden of malnutrition in persons with obesity. Reviews in Endocrine and Metabolic Disorders, 21(3), 307–313. https://doi.org/10.1007/s11154-020-09578-1
- Barrea, L., Frias-Toral, E., Pugliese, G., Garcia-Velasquez, E., Savastano, S., Colao, A., & Muscogiuri, G. (2021). Vitamin D in obesity and obesity-related diseases: An overview. Minerva Endocrinol (Torino), 46(2), 177–192.
- Barrea, L., Muscogiuri, G., Frias-Toral, E., Laudisio, D., Pugliese, G., Castellucci, B., Garcia-Velasquez, E., Savastano, S., & Colao, A. (2021). Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Critical Reviews in Food Science and Nutrition, 61(18), 3066–3090. https://doi.org/10.1080/10408398.2020.1792826
- Barrea, L., Muscogiuri, G., Laudisio, D., Pugliese, G., de Alteriis, G., Colao, A., & Savastano, S. (2020). Influence of the Mediterranean Diet on 25- hydroxyvitamin D levels in adults. Nutrients, 12(5). https://doi.org/10.3390/nu12051439
- Barrea, L., Savastano, S., Di Somma, C., Savanelli, M. C., Nappi, F., Albanese, L., Orio, F., & Colao, A. (2017). Low serum vitamin D-status, air pollution and obesity: A dangerous liaison. Reviews in Endocrine and Metabolic Disorders, 18(2), 215–214. https://doi.org/10.1007/s11154-017-9410-7
- Barrea, L., Tarantino, G., Somma, C. D., Muscogiuri, G., Macchia, P. E., Falco, A., Colao, A., & Savastano, S. (2017). Adherence to the mediterranean diet and circulating levels of sirtuin 4 in obese patients: A novel association. Oxidative Medicine and Cellular Longevity, 2017, 6101254. https://doi.org/10.1155/2017/6101254
- Barrea, L., Verde, L., Auriemma, R. S., Vetrani, C., Cataldi, M., Frias-Toral, E., Pugliese, G., Camajani, E., Savastano, S., Colao, A., & Muscogiuri, G. (2023). Probiotics and Prebiotics: Any role in menopause-related diseases? Current Nutrition Reports, 12(1), 83–97.
- Barrea, L., Vetrani, C., Caprio, M., El Ghoch, M., Frias-Toral, E., Mehta, R. J., Mendez, V., Moriconi, E., Paschou, S. A., Pazderska, A., & Savastano, S. (2023). Nutritional management of type 2 diabetes in subjects with obesity: An international guideline for clinical practice. Critical Reviews in Food Science and Nutrition, 63(16), 2873–2885. https://doi.org/10.1080/10408398.2021.1980766
- Barrea, L., Vetrani, C., Verde, L., Frias-Toral, E., Ceriani, F., Cernea, S., Docimo, A., Graziadio, C., Tripathy, D., Savastano, S., & Colao, A. (2023, April 10). Comprehensive approach to medical nutrition therapy in patients with type 2 diabetes mellitus: From diet to bioactive compounds. Antioxidants (Basel), 12(4), 904. https://doi.org/10.3390/antiox12040904
- Beard, J. A., Bearden, A., & Striker, R. (2011). Vitamin D and the anti-viral state. Journal of Clinical Virology, 50(3), 194–200. https://doi.org/10.1016/j.jcv.2010.12.006
- Bell, N. H., Epstein, S., Greene, A., Shary, J., Oexmann, M. J., & Shaw, S. (1985). Evidence for alteration of the vitamin D-endocrine system in obese subjects. Journal of Clinical Investigation, 76(1), 370–373. https://doi.org/10.1172/JCI111971
- Bennour, I., Haroun, N., Sicard, F., Mounien, L., & Landrier, J.-F. (2022). Vitamin D and obesity/adiposity-a brief overview of recent studies. Nutrients, 14(10). https://doi.org/10.3390/nu14102049
- Berger, M. M., Shenkin, A., Schweinlin, A., Amrein, K., Augsburger, M., Biesalski, H. K., Bischoff, S. C., Casaer, M. P., Gundogan, K., Lepp, H. L., & De Man, A. M. (2022). ESPEN micronutrient guideline. Clinical Nutrition, 41(6), 1357–1424. https://doi.org/10.1016/j.clnu.2022.02.015
- Bettini, S., Belligoli, A., Fabris, R., & Busetto, L. (2020). Diet approach before and after bariatric surgery. Reviews in Endocrine and Metabolic Disorders, 21(3), 297–306. https://doi.org/10.1007/s11154-020-09571-8
- Bikle, D. (2009). Nonclassic actions of vitamin D. The Journal of Clinical Endocrinology & Metabolism, 94(1), 26–34. https://doi.org/10.1210/jc.2008-1454
- Biondi, B. (2023). Subclinical hypothyroidism in patients with obesity and metabolic syndrome: A narrative review. Nutrients, 16(1). https://doi.org/10.3390/nu16010087
- Bouillon, R., & Bikle, D. (2019). Vitamin D metabolism revised: Fall of dogmas. Journal of Bone and Mineral Research, 34(11), 1985–1992. https://doi.org/10.1002/jbmr.3884
- Bouillon, R., Marcocci, C., Carmeliet, G., Bikle, D., White, J. H., Dawson-Hughes, B., Lips, P., Munns, C. F., Lazaretti-Castro, M., Giustina, A., & Bilezikian, J. (2019). Skeletal and extraskeletal actions of vitamin D: Current evidence and outstanding questions. Endocrine Reviews, 40(4), 1109–1151. https://doi.org/10.1210/er.2018-00126
- Bradley, M., Melchor, J., Carr, R., & Karjoo, S. (2023). Obesity and malnutrition in children and adults: A clinical review. Obesity Pillars, 8, 100087. https://doi.org/10.1016/j.obpill.2023.100087
- Cao, M. Y., Wu, J., Xie, C. Q., Wu, L., Gu, Z., Hu, J. W., & Xiong, W. (2022). Antioxidant and anti-inflammatory activities of Gynura procumbens flowers extract through suppressing LPS-induced MAPK/NF-κB signalling pathways. Food and Agricultural Immunology, 33(1), 511–529. https://doi.org/10.1080/09540105.2022.2098935
- Capurso, C., Bellanti, F., Lo Buglio, A., & Vendemiale, G. (2019). The mediterranean diet slows down the progression of aging and helps to prevent the onset of frailty: A narrative review. Nutrients, 12(1). https://doi.org/10.3390/nu12010035
- Carlberg, C. (2019). Nutrigenomics of vitamin D. Nutrients, 11(3). https://doi.org/10.3390/nu11030676
- Cefalo, C. M., Conte, C., Sorice, G. P., Moffa, S., Sun, V. A., Cinti, F., Salomone, E., Muscogiuri, G., Brocchi, A. A., Pontecorvi, A., & Mezza, T. (2018). Effect of vitamin D supplementation on obesity-induced insulin resistance: A double-blind, randomized, placebo-controlled trial. Obesity (Silver Spring), 26(4), 651–657. https://doi.org/10.1002/oby.22132
- Čelakovská, J., Čermákova, E., Vaňková, R., Andrýs, C., & Krejsek, J. (2022). ALEX2 multiplex examination – results of specific IgE to fish and shrimps in patients suffering from atopic dermatitis. Food and Agricultural Immunology, 33(1), 1–19. https://doi.org/10.1080/09540105.2021.2005546
- Chapela, S. P., Simancas-Racines, A., Ceriani, F., Martinuzzi, A. L. N., Russo, M. P., Zambrano, A. K., Simancas-Racines, D., Verde, L., Muscogiuri, G., Katsanos, C. S., & Frias-Toral, E. (2024, June). Obesity and obesity-related thyroid dysfunction: Any potential role for the very low-calorie ketogenic diet (VLCKD)? Current Nutrition Reports, 13(2), 194–213. https://doi.org/10.1007/s13668-024-00528-w
- Chauhan, K., Shahrokhi, M., & Huecker, M. R. (2024). Vitamin D. StatPearls. StatPearls Publishing.
- Chen, Z., Khandpur, N., Desjardins, C., Wang, L., Monteiro, C. A., Rossato, S. L., Fung, T. T., Manson, J. E., Willett, W. C., Rimm, E. B., & Hu, F. B. (2023). Ultra-processed food consumption and risk of type 2 diabetes: Three large prospective U.S. cohort studies. Diabetes Care, 46(7), 1335–1344. https://doi.org/10.2337/dc22-1993
- Chen, J., Sun, M., Cui, X., & Zhang, X. (2022). Ginsenoside compound K induces mitochondrial apoptosis in human hepatoma cells through Bclaf1-mediated modulation of ERK signaling. Food and Agricultural Immunology, 33(1), 799–816. https://doi.org/10.1080/09540105.2022.2134313
- Cheng, Q., Boucher, B. J., & Leung, P. S. (2013). Modulation of hypovitaminosis D-induced islet dysfunction and insulin resistance through direct suppression of the pancreatic islet renin-angiotensin system in mice. Diabetologia, 56(3), 553–562. https://doi.org/10.1007/s00125-012-2801-0
- Cheng, S., Massaro, J. M., Fox, C. S., Larson, M. G., Keyes, M. J., McCabe, E. L., Robins, S. J., O'Donnell, C. J., Hoffmann, U., Jacques, P. F., & Booth, S. L. (2010). Adiposity, cardiometabolic risk, and vitamin D status: The framingham heart study. Diabetes, 59(1), 242–248. https://doi.org/10.2337/db09-1011
- Cheng, J. B., Motola, D. L., Mangelsdorf, D. J., & Russell, D. W. (2003). De-orphanization of cytochrome P450 2R1: A microsomal vitamin D 25-hydroxilase. Journal of Biological Chemistry, 278(39), 38084–38093. https://doi.org/10.1074/jbc.M307028200
- Chooi, Y. C., Ding, C., & Magkos, F. (2019). The epidemiology of obesity. Metabolism, 92, 6–10. https://doi.org/10.1016/j.metabol.2018.09.005
- Cimini, F. A., Barchetta, I., Carotti, S., Bertoccini, L., Baroni, M. G., Vespasiani-Gentilucci, U., Cavallo, M. G., & Morini, S. (2017). Relationship between adipose tissue dysfunction, vitamin D deficiency and the pathogenesis of non-alcoholic fatty liver disease. World Journal of Gastroenterology, 23(19), 3407–3417. https://doi.org/10.3748/wjg.v23.i19.3407
- Ciobârcă, D., Cătoi, A. F., Copăescu, C., Miere, D., & Crișan, G. (2020). Bariatric surgery in obesity: Effects on gut microbiota and micronutrient status. Nutrients, 12(1). https://doi.org/10.3390/nu12010235
- Damms-Machado, A., Friedrich, A., Kramer, K. M., Stingel, K., Meile, T., Küper, M. A., Königsrainer, A., & Bischoff, S. C. (2012). Pre- and postoperative nutritional deficiencies in obese patients undergoing laparoscopic sleeve gastrectomy. Obesity Surgery, 22(6), 881–889. https://doi.org/10.1007/s11695-012-0609-0
- Damms-Machado, A., Weser, G., & Bischoff, S. C. (2012). Micronutrient deficiency in obese subjects undergoing low calorie diet. Nutrition Journal, 11(1), 34. https://doi.org/10.1186/1475-2891-11-34
- Del Moral, A. M., Calvo, C., & Martínez, A. (2021). Ultra-processed food consumption and obesity-a systematic review. Nutricion Hospitalaria, 38(1), 177–185.
- de Oliveira, L. F., de Azevedo, L. G., da Mota Santana, J., de Sales, L. P. C., & Pereira-Santos, M. (2020). Obesity and overweight decreases the effect of vitamin D supplementation in adults: Systematic review and meta-analysis of randomized controlled trials. Reviews in Endocrine and Metabolic Disorders, 21(1), 67–76. https://doi.org/10.1007/s11154-019-09527-7
- Drincic, A. T., Armas, L. A. G., Van Diest, E. E., & Heaney, R. P. (2012). Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring), 20(7), 1444–1448. https://doi.org/10.1038/oby.2011.404
- Dwijayanti, D. R., Widyananda, M. H., Hermanto, F. E., Soewondo, A., Afiyanti, M., & Widodo, N. (2024). Revealing the anti-inflammatory activity of Euphorbia hirta extract: Transcriptomic and nitric oxide production analysis in LPS-induced RAW 264.7 cells. Food and Agricultural Immunology, 35(1). https://doi.org/10.1080/09540105.2024.2351360
- El Assar, M., Angulo, J., & Rodríguez-Mañas, L. (2013). Oxidative stress and vascular inflammation in aging. Free Radical Biology and Medicine, 65, 380–401. https://doi.org/10.1016/j.freeradbiomed.2013.07.003
- Eliades, M., Spyrou, E., Agrawal, N., Lazo, M., Brancati, F. L., Potter, J. J., Koteish, A. A., Clark, J. M., Guallar, E., & Hernaez, R. (2013). Meta-analysis: Vitamin D and non-alcoholic fatty liver disease. Alimentary Pharmacology & Therapeutics, 38(3), 246–254. https://doi.org/10.1111/apt.12377
- Elizabeth, L., Machado, P., Zinöcker, M., Baker, P., & Lawrence, M. (2021). Effect of fortification with multiple micronutrient powder on the prevention and treatment of iron deficiency and anaemia in Brazilian children: A randomized clinical trial. Nutrients, 13(7), https://doi.org/10.3390/nu13072160
- Engle-Stone, R., Vosti, S. A., Luo, H., Kagin, J., Tarini, A., Adams, K. P., French, C., & Brown, K. H. (2019). Weighing the risks of high intakes of selected micronutrients compared with the risks of deficiencies. Annals of the New York Academy of Sciences, 1446(1), 81–101. https://doi.org/10.1111/nyas.14128
- Feghaly, J., Johnson, P., & Kalhan, A. (2020). Attention deficit hyperactivity disorder in adults: What the non-specialist needs to know. British Journal of Hospital Medicine, 81(1), 1–11. https://doi.org/10.12968/hmed.2019.0188
- Ferder, M., Inserra, F., Manucha, W., & Ferder, L. (2013). The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system. American Journal of Physiology-Cell Physiology, 304(11), C1027–C1039. https://doi.org/10.1152/ajpcell.00403.2011
- Fernández-Sánchez, A., Madrigal-Santillán, E., Bautista, M., Esquivel-Soto, J., Morales-González, Á, Esquivel-Chirino, C., Durante-Montiel, I., Sánchez-Rivera, G., Valadez-Vega, C., & Morales-González, J. A. (2011). Inflammation, oxidative stress, and obesity. International Journal of Molecular Sciences, 12(5), 3117–3132. https://doi.org/10.3390/ijms12053117
- Fiamenghi, V. I., & Mello, E. D. D. (2021). Vitamin D deficiency in children and adolescents with obesity: A meta-analysis. Jornal de Pediatria, 97(3), 273–279. https://doi.org/10.1016/j.jped.2020.08.006
- Frias-Toral, E., Garcia-Velasquez, E., de Los Angeles Carignano, M., Rodriguez-Veintimilla, D., Alvarado-Aguilera, I., & Bautista-Litardo, N. (2022). Polycystic ovary syndrome and obesity: Clinical aspects and nutritional management. Minerva Endocrinol (Torino), 47(2), 215–241.
- Gagnon, C., Lu, Z. X., Magliano, D. J., Dunstan, D. W., Shaw, J. E., Zimmet, P. Z., Sikaris, K., Ebeling, P. R., & Robin, M. (2012). Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: Results from a national, population-based prospective study (The Australian diabetes, obesity and lifestyle study: AusDiab). The Journal of Clinical Endocrinology & Metabolism, 97(6), 1953–1961. https://doi.org/10.1210/jc.2011-3187
- Golzarand, M., Hollis, B. W., Mirmiran, P., Wagner, C. L., & Shab-Bidar, S. (2018). Vitamin D supplementation and body fat mass: A systematic review and meta-analysis. European Journal of Clinical Nutrition, 72(10), 1345–1357. https://doi.org/10.1038/s41430-018-0132-z
- González-Domínguez, Á, Visiedo-García, F. M., Domínguez-Riscart, J., González-Domínguez, R., Mateos, R. M., & Lechuga-Sancho, A. M. (2020). Iron metabolism in obesity and metabolic syndrome. International Journal of Molecular Sciences, 21(15). https://doi.org/10.3390/ijms21155529
- Gouju, J., & Legeay, S. (2023). Pharmacokinetics of obese adults: Not only an increase in weight. Biomedicine & Pharmacotherapy, 166, 115281. https://doi.org/10.1016/j.biopha.2023.115281
- Gow, M. L., Pham-Short, A., Jebeile, H., Varley, B. J., & Craig, M. E. (2021). Current perspectives on the role of very-low-energy diets in the treatment of obesity and type 2 diabetes in youth. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, Volume 14, 215–225. https://doi.org/10.2147/DMSO.S238419
- Gregor, M. F., & Hotamisligil, G. S. (2011). Inflammatory mechanisms in obesity. Annual Review of Immunology, 29(1), 415–445. https://doi.org/10.1146/annurev-immunol-031210-101322
- Grosso, G., Laudisio, D., Frias-Toral, E., Barrea, L., Muscogiuri, G., Savastano, S., & Colao, A. (2022). Anti-inflammatory nutrients and obesity-associated metabolic-inflammation: State of the art and future direction. Nutrients, 14(6). https://doi.org/10.3390/nu14061137
- Guardiola-Márquez, C. E., Santos-Ramírez, M. T., Segura-Jiménez, M. E., Figueroa-Montes, M. L., & Jacobo-Velázquez, D. A. (2022). Fighting obesity-related micronutrient deficiencies through biofortification of agri-food crops with sustainable fertilization practices. Plants, 11(24). https://doi.org/10.3390/plants11243477
- Gupta, S., Hawk, T., Aggarwal, A., & Drewnowski, A. (2019). Characterizing ultra-processed foods by energy density, nutrient density, and cost. Frontiers in Nutrition, 6, 70. https://doi.org/10.3389/fnut.2019.00070
- Haddad, J. G. (1992). Vitamin D–solar rays, the Milky Way, or both? New England Journal of Medicine, 326(18), 1213–1215. https://doi.org/10.1056/NEJM199204303261808
- Havlová, L., Pospiech, M., Javůrková, Z., Bartlová, M., Těšíková, K., Dordevic, D., Dordevic, S., Zemancová, J., & Tremlová, B. (2023). Effect of selected bioactive substances and nanoparticles on the immunoreactivity of edible packages containing chitosan, by the ELISA method. Food and Agricultural Immunology, 34(1). https://doi.org/10.1080/09540105.2023.2222933
- Holick, M. F., Binkley, N. C., Bischoff-Ferrari, H. A., Gordon, C. M., Hanley, D. A., Heaney, R. P., Murad, M. H., & Weaver, C. M. (2011). Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism, 96(7), 1911–1930. https://doi.org/10.1210/jc.2011-0385
- Hotamisligil, G. S. (2010). Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell, 140(6), 900–917. https://doi.org/10.1016/j.cell.2010.02.034
- Hyppönen, E., & Boucher, B. J. (2018). Adiposity, vitamin D requirements, and clinical implications for obesity-related metabolic abnormalities. Nutrition Reviews, 76(9), 678–692. https://doi.org/10.1093/nutrit/nuy034
- Iacone, R., Scanzano, C., Santarpia, L., D’Isanto, A., Contaldo, F., & Pasanisi, F. (2016). Micronutrient content in enteral nutrition formulas: Comparison with the dietary reference values for healthy populations. Nutrition Journal, 15(1), 30. https://doi.org/10.1186/s12937-016-0152-2
- Isom, K. A., Andromalos, L., Ariagno, M., Hartman, K., Mogensen, K. M., Stephanides, K., & Shikora, S. (2014). Nutrition and metabolic support recommendations for the bariatric patient. Nutrition in Clinical Practice, 29(6), 718–739. https://doi.org/10.1177/0884533614552850
- Jebeile, H., Grunseit, A. M., Thomas, M., Kelly, T., Garnett, S. P., & Gow, M. L. (2020). Low-carbohydrate interventions for adolescent obesity: Nutritional adequacy and guidance for clinical practice. Clinical Obesity, 10(4), e12370. https://doi.org/10.1111/cob.12370
- Ji, L., Gupta, M., & Feldman, B. J. (2016). Vitamin D regulates fatty acid composition in subcutaneous adipose tissue through Elovl3. Endocrinology, 157(1), 91–97. https://doi.org/10.1210/en.2015-1674
- Jones, K. S., Schoenmakers, I., Bluck, L. J. C., Ding, S., & Prentice, A. (2012). Plasma appearance and disappearance of an oral dose of 25-hydroxyvitamin D2 in healthy adults. British Journal of Nutrition, 107(8), 1128–1137. https://doi.org/10.1017/S0007114511004132
- Kamei, Y., Kawada, T., Kazuki, R., Ono, T., Kato, S., & Sugimoto, E. (1993). Vitamin D receptor gene expression is up-regulated by 1, 25-dihydroxyvitamin D3 in 3T3-L1 preadipocytes. Biochemical and Biophysical Research Communications, 193(3), 948–955. https://doi.org/10.1006/bbrc.1993.1717
- Kim, J. Y. (2021). Optimal diet strategies for weight loss and weight loss maintenance. Journal of Obesity & Metabolic Syndrome, 30(1), 20–31. https://doi.org/10.7570/jomes20065
- Kloock, S., Ziegler, C. G., & Dischinger, U. (2023). Obesity and its comorbidities, current treatment options and future perspectives: Challenging bariatric surgery? Pharmacology & Therapeutics, 251, 108549. https://doi.org/10.1016/j.pharmthera.2023.108549
- Kobylińska, M., Antosik, K., Decyk, A., & Kurowska, K. (2022). Malnutrition in obesity: Is it possible? Obesity Facts, 15(1), 19–25. https://doi.org/10.1159/000519503
- Konradsen, S., Ag, H., Lindberg, F., Hexeberg, S., & Jorde, R. (2008). Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. European Journal of Nutrition, 47(2), 87–91. https://doi.org/10.1007/s00394-008-0700-4
- Krezalek, M. A., Yeh, A., Alverdy, J. C., & Morowitz, M. (2017). Influence of nutrition therapy on the intestinal microbiome. Current Opinion in Clinical Nutrition & Metabolic Care, 20(2), 131–137. https://doi.org/10.1097/MCO.0000000000000348
- Kuchuk, N. O., Pluijm, S. M. F., van Schoor, N. M., Looman, C. W. N., Smit, J. H., & Lips, P. (2009). Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. The Journal of Clinical Endocrinology & Metabolism, 94(4), 1244–1250. https://doi.org/10.1210/jc.2008-1832
- Kwok, R. M., Torres, D. M., & Harrison, S. A. (2013). Vitamin D and nonalcoholic fatty liver disease (NAFLD): Is it more than just an association? Hepatology, 58(3), 1166–1174. https://doi.org/10.1002/hep.26390
- Lapik, I. A., Galchenko, A. V., & Gapparova, K. M. (2020). Micronutrient status in obese patients: A narrative review. Obesity Medicine, 18, 100224. https://doi.org/10.1016/j.obmed.2020.100224
- Lawson, D. E., Douglas, J., Lean, M., & Sedrani, S. (1986). Estimation of vitamin D3 and 25-hydroxyvitamin D3 in muscle and adipose tissue of rats and man. Clinica Chimica Acta, 157(2), 175–181. https://doi.org/10.1016/0009-8981(86)90223-8
- Lee-Rangel, H. A., Mendoza-Martinez, G. D., Martínez-García, J. A., Espinosa-Ayala, E., Hernández-García, P. A., Cifuentes-López, R. O., Vazquez-Valladolid, A., García-López, J. C., Lara-Bueno, A., & Roque-Jiménez, J. A. (2022). An Indian polyherbal phytogenic source improved blood serum biochemistry and immune response of dairy calves. Food and Agricultural Immunology, 33(1), 97–112. https://doi.org/10.1080/09540105.2021.2024150
- Lee, D. H., Lee, I. H., & Hong, J. T. (2022). Fermented field water-dropwort (Oenanthe javanica) alleviates diet-induced non-alcoholic steatohepatitis. Food and Agricultural Immunology, 33(1), 20–34. https://doi.org/10.1080/09540105.2021.2022603
- Leung, P. S. (2016). The potential protective action of vitamin D in hepatic insulin resistance and pancreatic islet dysfunction in type 2 diabetes mellitus. Nutrients, 8(3), 147. https://doi.org/10.3390/nu8030147
- Li, H., Hu, Z., & Yan, Y. (2023). Litopenaeus vannamei fermentation using selected Lactobacillus spp. to reduce its allergenicity. Food and Agricultural Immunology, 34(1).
- Li, Y. C., Kong, J., Wei, M., Chen, Z.-F., Liu, S. Q., & Cao, L.-P. (2002). 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. Journal of Clinical Investigation, 110(2), 229–238. https://doi.org/10.1172/JCI0215219
- Liu, M., Bai, Y., Dou, L., Kong, Y., Wang, Z., Wen, K., & Shen, J. (2022). A highly salt-tolerant monoclonal antibody-based enzyme-linked immunosorbent assay for the rapid detection of phenylethanolamine A in urine. Food and Agricultural Immunology, 33(1), 575–587. https://doi.org/10.1080/09540105.2022.2084043
- Liu, F.-L., Chen, C.-L., & Huang, C.-H. (2022). Preparation of fermented oat milk and evaluation of its modulatory effect on antigen-specific immune responses in ovalbumin-sensitized mice. Food and Agricultural Immunology, 33(1), 722–735. https://doi.org/10.1080/09540105.2022.2120851
- Mallard, S. R., Howe, A. S., & Houghton, L. A. (2016). Vitamin D status and weight loss: A systematic review and meta-analysis of randomized and nonrandomized controlled weight-loss trials. The American Journal of Clinical Nutrition, 104(4), 1151–1159. https://doi.org/10.3945/ajcn.116.136879
- Mayer, S. B., Graybill, S., Raffa, S. D., Tracy, C., Gaar, E., Wisbach, G., Goldstein, M. G., & Sall, J. (2021). Synopsis of the 2020 U.S. va/dod clinical practice guideline for the management of adult overweight and obesity. Military Medicine, 186(9), 884–896. https://doi.org/10.1093/milmed/usab114
- McGaugh, E., & Barthel, B. (2022). A review of ketogenic diet and lifestyle. Missouri Medicine, 119(1), 84–88.
- McGill, A.-T., Stewart, J. M., Lithander, F. E., Strik, C. M., & Poppitt, S. D. (2008). Relationships of low serum vitamin D3with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutrition Journal, 7(1), 4. https://doi.org/10.1186/1475-2891-7-4
- McGuire, S. (2016). Scientific report of the 2015 dietary guidelines advisory committee. Washington, DC: US departments of agriculture and health and human services, 2015. Advances in Nutrition, 7(1), 202–204. https://doi.org/10.3945/an.115.011684
- McKay, J., Ho, S., Jane, M., & Pal, S. (2020). Overweight & obese Australian adults and micronutrient deficiency. BMC Nutrition, 6(1), 12. https://doi.org/10.1186/s40795-020-00336-9
- Meng, L., Mui, E., Holubar, M. K., & Deresinski, S. C. (2017). Comprehensive guidance for antibiotic dosing in obese adults. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 37(11), 1415–1431. https://doi.org/10.1002/phar.2023
- Mercola, J., Grant, W. B., & Wagner, C. L. (2020). Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients, 12(11). https://doi.org/10.3390/nu12113361
- Milner, J. J., & Beck, M. A. (2012). The impact of obesity on the immune response to infection. Proceedings of the Nutrition Society, 71(2), 298–306. https://doi.org/10.1017/S0029665112000158
- Moore, W. T., Bowser, S. M., Fausnacht, D. W., Staley, L. L., Suh, K.-S., & Liu, D. (2015). Beta cell function and the nutritional state: Dietary factors that influence insulin secretion. Current Diabetes Reports, 15(10), 76. https://doi.org/10.1007/s11892-015-0650-1
- Mu, L., Xu, Y., Li, G., Dai, S., Tong, Q., & Liu, B. (2022). Determination of glucosamine and galactosamine in food by liquid chromatography with pre-column derivatization. Food and Agricultural Immunology, 33(1), 419–437. https://doi.org/10.1080/09540105.2022.2085673
- Murakami, K., & Sasaki, S. (2018). A low-glycemic index and -glycemic load diet is associated with not only higher intakes of micronutrients but also higher intakes of saturated fat and sodium in Japanese children and adolescents: The national health and nutrition survey. Nutrition Research, 49, 37–47. https://doi.org/10.1016/j.nutres.2017.10.015
- Muscogiuri, G., Barrea, L., Altieri, B., Di Somma, C., Laudisio, D., Duval, G. T., Pugliese, G., Annweiler, C., Orio, F., Fakhouri, H., & Savastano, S. (2019). Calcium and vitamin D supplementation. Myths and realities with regard to cardiovascular risk. Current Vascular Pharmacology, 17(6), 610–617. https://doi.org/10.2174/1570161117666190408165805
- Muscogiuri, G., Barrea, L., Caprio, M., Ceriani, F., Chavez, A. O., El Ghoch, M., Frias-Toral, E., Mehta, R. J., Mendez, V., Paschou, S. A., & Pazderska, A. (2022). Nutritional guidelines for the management of insulin resistance. Critical Reviews in Food Science and Nutrition, 62(25), 6947–6960. https://doi.org/10.1080/10408398.2021.1908223
- Muscogiuri, G., Barrea, L., Faggiano, F., Maiorino, M. I., Parrillo, M., Pugliese, G., Ruggeri, R. M., Scarano, E., Savastano, S., Colao, A., & RESTARE. (2021). Obesity in Prader-Willi syndrome: Physiopathological mechanisms, nutritional and pharmacological approaches. Journal of Endocrinological Investigation, 44(10), 2057–2070. https://doi.org/10.1007/s40618-021-01574-9
- Muscogiuri, G., Palomba, S., Laganà, A. S., & Orio, F. (2016). Current insights into inositol isoforms, Mediterranean and ketogenic diets for polycystic ovary syndrome: From bench to bedside. Current Pharmaceutical Design, 22(36), 5554–5557. https://doi.org/10.2174/1381612822666160720160634
- Muscogiuri, G., Verde, L., Sulu, C., Katsiki, N., Hassapidou, M., Frias-Toral, E., Cucalón, G., Pazderska, A., Yumuk, V. D., Colao, A., & Barrea, L. (2022). Mediterranean diet and obesity-related disorders: What is the evidence? Current Obesity Reports, 11(4), 287–304. https://doi.org/10.1007/s13679-022-00481-1
- Ochs-Balcom, H. M., Chennamaneni, R., Millen, A. E., Shields, P. G., Marian, C., Trevisan, M., & Freudenheim, J. L. (2011). Vitamin D receptor gene polymorphisms are associated with adiposity phenotypes. The American Journal of Clinical Nutrition, 93(1), 5–10. https://doi.org/10.3945/ajcn.2010.29986
- Papadopoulou, S. K., & Nikolaidis, P. T. (2023). Low-carbohydrate diet and human health. Nutrients, 15(8). https://doi.org/10.3390/nu15082004
- Parikh, S. J., Edelman, M., Uwaifo, G. I., Freedman, R. J., Semega-Janneh, M., Reynolds, J., & Yanovski, J. A. (2004). The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. The Journal of Clinical Endocrinology & Metabolism, 89(3), 1196–1199. https://doi.org/10.1210/jc.2003-031398
- Pathak, K., Soares, M. J., Calton, E. K., Zhao, Y., & Hallett, J. (2014). Vitamin D supplementation and body weight status: A systematic review and meta-analysis of randomized controlled trials. Obesity Reviews, 15(6), 528–537. https://doi.org/10.1111/obr.12162
- Pereira-Santos, M., Costa, P. R. F., Assis, A. M. O., Santos, C. A. S. T., & Santos, D. B. (2015). Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obesity Reviews, 16(4), 341–349. https://doi.org/10.1111/obr.12239
- Perna, S. (2019, July 12). Is vitamin D supplementation useful for weight loss programs? A systematic review and meta-analysis of randomized controlled trials. Medicina (Kaunas), 55(7), 368. https://doi.org/10.3390/medicina55070368
- Pivonello, C., Negri, M., Patalano, R., Amatrudo, F., Montò, T., Liccardi, A., Graziadio, C., Muscogiuri, G., Pivonello, R., & Colao, A. (2022). The role of melatonin in the molecular mechanisms underlying metaflammation and infections in obesity: A narrative review. Obesity Reviews, 23(3), e13390. https://doi.org/10.1111/obr.13390
- Poti, J. M., Braga, B., & Qin, B. (2017). Ultra-processed food intake and obesity: What really matters for health – processing or nutrient content? Current Obesity Reports, 6(4), 420–431. https://doi.org/10.1007/s13679-017-0285-4
- Roizen, J. D., Long, C., Casella, A., O'Lear, L., Caplan, I., Lai, M., Sasson, I., Singh, R., Makowski, A. J., Simmons, R., & Levine, M. A. (2019). Obesity decreases hepatic 25-hydroxylase activity causing low serum 25-hydroxyvitamin d. Journal of Bone and Mineral Research, 34(6), 1068–1073. https://doi.org/10.1002/jbmr.3686
- Rondanelli, M., Perna, S., Ilyas, Z., Peroni, G., Bazire, P., Sajuox, I., Maugeri, R., Nichetti, M., & Gasparri, C. (2022). Effect of very low-calorie ketogenic diet in combination with omega-3 on inflammation, satiety hormones, body composition, and metabolic markers. A pilot study in class I obese subjects. Endocrine, 75(1), 129–136. https://doi.org/10.1007/s12020-021-02860-5
- Santangeli, E., Abbati, C., Chen, R., Di Carlo, A., Leoni, S., Piscaglia, F., & Ferri, S. (2024). Pathophysiological-based nutritional interventions in cirrhotic patients with sarcopenic obesity: A state-of-the-art narrative review. Nutrients, 16(3). https://doi.org/10.3390/nu16030427
- Savanelli, M. C., Scarano, E., Muscogiuri, G., Barrea, L., Vuolo, L., Rubino, M., Savastano, S., Colao, A., & Di Somma, C. (2016). Cardiovascular risk in adult hypopituitaric patients with growth hormone deficiency: Is there a role for vitamin D? Endocrine, 52(1), 111–119. https://doi.org/10.1007/s12020-015-0779-3
- Seo, M. H., Lee, W. Y., Kim, S. S., Kang, J. H., Kang, J. H., Kim, K. K., Kim, B. Y., Kim, Y. H., Kim, W. J., Kim, E. M., & Kim, H. S. (2019). 2018 Korean society for the study of obesity guideline for the management of obesity in Korea. Journal of Obesity & Metabolic Syndrome, 28(1), 40–45. https://doi.org/10.7570/jomes.2019.28.1.40
- Shahbaz, M., Imran, M., Momal, U., Naeem, H., Alsagaby, S. A., Al Abdulmonem, W., El-Ghorab, A. H., Ghoneim, M. M., Hussain, M., Shaker, M. E., & Abdelgawad, M. A. (2023). Potential effect of kaempferol against various malignancies: Recent advances and perspectives. Food and Agricultural Immunology, 34(1). https://doi.org/10.1080/09540105.2023.2265690
- Siddiqui, S. A., Azmy Harahap, I., Suthar, P., Wu, Y. S., Ghosh, N., & Castro-Muñoz, R. (2023). A comprehensive review of phytonutrients as a dietary therapy for obesity. Foods (basel, Switzerland), 12(21), https://doi.org/10.3390/foods12213908
- Song-Xin, L., Zhi-Man, L., Zi-Jun, S., Yun-Shi, X., Li-Juan, Z., Duo-Duo, R., & Yin-Shi, S. (2022). Effect of velvet antler on the immune activity of cyclophosphamide-induced immunosuppressed mice. Food and Agricultural Immunology, 33(1), 768–779. https://doi.org/10.1080/09540105.2022.2128070
- Song, D., Wang, W., Chen, B., Li, A., Song, G., Cheng, J., Qiao, L., Zhu, R., & Min, Y. (2022). Dietary supplemental synbiotic – yucca extract compound preparation modulates production performance, immune status and faecal microflora diversity in laying hens. Food and Agricultural Immunology, 33(1), 360–376. https://doi.org/10.1080/09540105.2022.2080187
- Sun, F., Ma, Y., Li, F., Li, C., & Yang, Z. (2023). Exosomes derived from berberine-treated bone marrow mesenchymal stem cells ameliorate inflammatory arthritis in rats with collagen-induced rheumatoid arthritis. Food and Agricultural Immunology, 34(1). https://doi.org/10.1080/09540105.2023.2220566
- Targher, G., Bertolini, L., Scala, L., Cigolini, M., Zenari, L., Falezza, G., & Arcaro, G. (2007). Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutrition, Metabolism and Cardiovascular Diseases, 17(7), 517–524. https://doi.org/10.1016/j.numecd.2006.04.002
- Treviño, S., Díaz, A., Sánchez-Lara, E., Sanchez-Gaytan, B. L., Perez-Aguilar, J. M., & González-Vergara, E. (2019). Vanadium in biological action: Chemical, pharmacological aspects, and metabolic implications in diabetes mellitus. Biological Trace Element Research, 188(1), 68–98. https://doi.org/10.1007/s12011-018-1540-6
- Tummolo, A., Carella, R., De Giovanni, D., Paterno, G., Simonetti, S., Tolomeo, M., Leone, P., & Barile, M. (2023). Micronutrient deficiency in inherited metabolic disorders requiring diet regimen: A brief critical review. International Journal of Molecular Sciences, 24(23). https://doi.org/10.3390/ijms242317024
- Varady, K. A., Cienfuegos, S., Ezpeleta, M., & Gabel, K. (2022). Clinical application of intermittent fasting for weight loss: Progress and future directions. Nature Reviews Endocrinology, 18(5), 309–321. https://doi.org/10.1038/s41574-022-00638-x
- Vranić, L., Mikolašević, I., & Milić, S. (2019, August 28). Vitamin D deficiency: Consequence or cause of obesity? Medicina (Kaunas), 55(9), 541. https://doi.org/10.3390/medicina55090541
- Walsh, J. S., Bowles, S., & Evans, A. L. (2017). Vitamin D in obesity. Current Opinion in Endocrinology, Diabetes & Obesity, 24(6), 389–394. https://doi.org/10.1097/MED.0000000000000371
- Walsh, J. S., Evans, A. L., Bowles, S., Naylor, K. E., Jones, K. S., Schoenmakers, I., Jacques, R. M., & Eastell, R. (2016). Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. The American Journal of Clinical Nutrition, 103(6), 1465–1471. https://doi.org/10.3945/ajcn.115.120139
- Wamberg, L., Christiansen, T., Paulsen, S. K., Fisker, S., Rask, P., Rejnmark, L., Richelsen, B., & Pedersen, S. B. (2013). Expression of vitamin D-metabolizing enzymes in human adipose tissue – the effect of obesity and diet-induced weight loss. International Journal of Obesity, 37(5), 651–657. https://doi.org/10.1038/ijo.2012.112
- Woranam, K., Mootsikapun, P., Senawong, G., Prompipak, J., Promdee, L., Pintaraks, K., Ketterman, A. J., & Senawong, T. (2022). Safety and immunomodulatory activity ofHouttuynia cordatafermentation product in healthy volunteers and its effect on antiretroviral-drug level in rats. Food and Agricultural Immunology, 33(1), 80–96. https://doi.org/10.1080/09540105.2021.2024152
- Wortsman, J., Matsuoka, L. Y., Chen, T. C., Lu, Z., & Holick, M. F. (2000). Decreased bioavailability of vitamin D in obesity. The American Journal of Clinical Nutrition, 72(3), 690–693. https://doi.org/10.1093/ajcn/72.3.690
- Wu, Y., Liu, C., Lu, W., Li, S., Chen, Z., Xia, J., Tian, Y., Ma, A., & Jia, Y. (2023). Transcriptomics reveals the potential mechanism of ellagic acid extract from raspberry on wound healing. Food and Agricultural Immunology, 34(1). https://doi.org/10.1080/09540105.2023.2214709
- Yu, J. H., Choi, M. Y., Park, S. J., Geum, N. G., Lee, J. W., Park, G. H., & Jeong, J. B. (2022). Immunostimulatory activity of Hovenia dulcis branches extracts through TLR4/JNK-dependent macrophage activation and TLR4-dependent macrophage autophagy in RAW264. 7 cells. Food Science and Biotechnology, 31(3), 1753–1760.
- Zhang, T., Krekels, E. H. J., Smit, C., & Knibbe, C. A. J. (2022). Drug pharmacokinetics in the obese population: Challenging common assumptions on predictors of obesity-related parameter changes. Expert Opinion on Drug Metabolism & Toxicology, 18(10), 657–674. https://doi.org/10.1080/17425255.2022.2132931
- Zhao, Z., Su, J., Zhao, J., Chen, J., Cui, X., Sun, M., & Zhang, X. (2022). Curcumin inhibits invasion and metastasis of human hepatoma cells through Bclaf1-mediated Wnt/β-catenin signalling. Food and Agricultural Immunology, 33(1), 664–676. https://doi.org/10.1080/09540105.2022.2113864