ABSTRACT
Low ART-adherence amongst adolescents is associated with morbidity, mortality and onward HIV transmission. Reviews find no effective adolescent adherence-promoting interventions. Social protection has demonstrated benefits for adolescents, and could potentially improve ART-adherence. This study examines associations of 10 social protection provisions with adherence in a large community-based sample of HIV-positive adolescents. All 10–19-year-olds ever ART-initiated in 53 government healthcare facilities in a health district of South Africa’s Eastern Cape were traced and interviewed in 2014–2015 (n = 1175 eligible). About 90% of the eligible sample was included (n = 1059). Social protection provisions were “cash/cash in kind”: government cash transfers, food security, school fees/materials, school feeding, clothing; and “care”: HIV support group, sports groups, choir/art groups, positive parenting and parental supervision/monitoring. Analyses used multivariate regression, interaction and marginal effects models in SPSS and STATA, controlling for socio-demographic, HIV and healthcare-related covariates. Findings showed 36% self-reported past-week ART non-adherence (<95%). Non-adherence was associated with increased opportunistic infections (p = .005, B .269, SD .09), and increased likelihood of detectable viral load at last test (>75 copies/ml) (aOR 1.98, CI 1.1–3.45). Independent of covariates, three social protection provisions were associated with reduced non-adherence: food provision (aOR .57, CI .42–.76, p < .001); HIV support group attendance (aOR .60, CI .40–.91, p < .02), and high parental/caregiver supervision (aOR .56, CI .43–.73, p < .001). Combination social protection showed additive benefits. With no social protection, non-adherence was 54%, with any one protection 39–41%, with any two social protections, 27–28% and with all three social protections, 18%. These results demonstrate that social protection provisions, particularly combinations of “cash plus care”, may improve adolescent adherence. Through this they have potential to improve survival and wellbeing, to prevent HIV transmission, and to advance treatment equity for HIV-positive adolescents.
Introduction
The scale-up of HIV-treatment provides an opportunity for the survival and long-term well-being of the 1.6 million HIV-positive adolescents in Southern Africa (UNAIDS, Citation2015b). But studies show that adolescents are the lowest-adherent age group (Hudelson & Cluver, Citation2015; Nachega et al., Citation2009), leading to morbidity, mortality, viral resistance and onward HIV transmission (Pillay, Citation2001; Sherr et al., Citation2010). AIDS is currently the single greatest cause of death amongst adolescents aged 10–19 in Africa (UNAIDS, Citation2015a).
Our understanding of why adherence is so difficult for adolescents remains incomplete (Lowenthal, Bakeera-Kitaka, et al., Citation2014). Studies in the USA and Southern Africa find associations between non-adherence to sexual risk behaviour and mental health distress (Lowenthal et al., Citation2012; Mellins et al., Citation2011). Adolescence is a period of social, familial and emotional change. Adolescence is also a transitional period in health provisions and practices (Ferrand et al., Citation2010), as adolescents may move from paediatric to adolescent services, changing from caregiver-mediated to autonomous adherence.
Three recent systematic reviews find virtually no evidence-based interventions to improve adolescent adherence or retention in care (Hudelson & Cluver, Citation2015; MacPherson et al., Citation2015; Vreeman, Wiehe, Pearce, & Nyandiko, Citation2008). Findings from adult HIV treatment suggest that economic support may be important. This includes grants usually labelled as “cash” (now with very high coverage for children in South Africa due to successful scale-up since 2008 (Hall, Citation2015)) or other “cash in kind” social protections, such as clinic transport subsidies (Emenyonu et al., Citation2012) and food provision (de Pee, Grede, Mehra, & Bloem, Citation2014). Child development literature suggests that psychosocial “care” social protection from caregivers or other adults is important for healthy adolescent behaviours (Bronfenbrenner, Citation1979; Rutter, Citation2007). Regional evidence shows positive effects of social protection on other adolescent outcomes, such as sexual risk behaviours (Baird, Garfein, McIntosh, & Ozler, Citation2012; Cluver, Orkin, Boyes, & Sherr, Citation2014; DSD, SASSA, & UNICEF, Citation2012; Handa, Halpern, Pettifor, & Thirumurthy, Citation2014), mental health distress and family relationships (Bhana et al., Citation2014; Kilburn, Thirumurthy, Halpern, Pettifor, & Handa, Citation2016). Growing evidence of associations between social protection and HIV-risk reduction (Cluver et al., Citation2015; Pettifor, Rosenberg, & Bekker, Citation2016) is reflected in a number of policy documents by UNICEF, UNAIDS and PEPFAR-USAID that focus on paediatric and adolescent HIV-prevention (PEFPAR, Citation2015; UNAIDS, Citation2014; UNICEF, Citation2012).
To date, no known studies have examined associations of social protection (narrowly or broadly defined) with adolescent ART-adherence. Nor does any research examine whether combinations of different types of social protection may be more effective than individual provisions. “Cash” social protections that may be particularly valuable for HIV-positive adolescents include government cash transfers to households, or “cash in kind” (e.g., food to facilitate medication taking or free schooling to obviate resource spending on education). Potential “care” social protections include those provided by clinics and communities: HIV support groups (Grimsrud, Lesosky, Kalombo, Bekker, & Myer, Citation2016), recreational or sports groups. At home, different parenting “care” approaches may potentially support adherence, including positive parenting (praise and support) and parental supervision/monitoring of adolescent activities.
This study asks: (i) Are various forms of cash and/or care social protection provisions associated with adolescent ART-adherence? and (ii) Can combinations of social protection provisions have additive associations with adherence?
Methods
One thousand fifty-nine ART-initiated adolescents were interviewed using clinic sampling with community tracing in a mixed urban, peri-urban and rural health district of the Eastern Cape, South Africa. From 2014–2015, all public health facilities that provided ART to >4 adolescents were identified (n = 53). Within each facility, all adolescents aged 10–19 who had ever initiated ART were identified through paper and computerised records. All adolescents were followed up in their homes or met at clinics, to ensure inclusion regardless of clinic attendance rates or being lost to follow-up. About 90.1% of the eligible sample was interviewed. Of the remainder, 4.1% refused participation (either adolescent or caregiver), 0.9% had such severe cognitive disability that they were unable to participate, 1.2% were unable to be interviewed for safety reasons and 3.7% were unable to be traced.
Voluntary informed consent was obtained from caregivers and adolescents for a 90-minute interview. No incentives were provided, but all adolescents were given a certificate, snack, toothbrush and toothpaste. To prevent identification or stigmatisation through HIV-related research, the study was presented locally as focusing on general needs of adolescents using social and health services. Also with this aim, 467 additional adolescents who were co-resident, or who lived in neighbouring homes, were also interviewed with a version of the questionnaire that did not include items on HIV-medication or HIV-illness (not included in these analyses).
Questionnaires, interview schedules and consent forms were translated and back-translated between English and Xhosa, and used tablets with youth-friendly graphics and interactive games. Adolescents participated in the language of their choice. Interviewers were trained in working with HIV-affected adolescents. Confidentiality was upheld, except in cases of significant harm or when participants requested assistance. Where participants reported recent abuse, rape or risk of significant harm, referrals were made to child protection and health services, with follow-up support. Ethics protocols were approved by the Universities of Cape Town (CSSR 2013/14) and Oxford (SSD/CUREC2/12-21), the Provincial Departments of Health and Education and ethics review committees of participating hospitals.
The study design was developed in collaboration with the South African National Departments of Health, Social Development and Education, UNICEF South Africa, Regional and New York Pediatric HIV teams, PEPFAR-USAID, and NGOs including Pediatric AIDS Treatment for Africa (PATA) and the Regional Psychosocial Support Initiative (REPSSI). Research tools were informed by in-depth qualitative research, and pre-piloted with 25 HIV+ adolescents in the Eastern Cape. Questionnaires, accompanying vignettes, pictures and games were developed in consultation with two Teen Advisory Groups of HIV-infected and affected adolescents from urban and rural areas of the Eastern Cape (n = 20) and Western Cape (n = 18).
Measures
ART adherence was measured by adolescent self-report (Evans et al., Citation2015), using the standardised Patient Medication Adherence Questionnaire (Duong et al., Citation2001), combined with adolescent adherence measures developed in Botswana (Lowenthal, Haruna, et al., Citation2014). After piloting, and in order to reduce social desirability bias, vignettes were added, for example, “Even if Andiwe tries his best sometimes unexpected things get in the way and prevent him from taking his pills … this is not his fault”. Past-week and past-year non-adherence were measured using a 95% adherence cut-off, based on the number of prescribed daily doses (Paterson et al., Citation2000), but past-week adherence was used for all analyses due to evidence of increased reliability for more recent recall. Two validation measures of self-reported adherence were included. Opportunistic infections were measured as sores on the body or face, tuberculosis symptoms (e.g., coughing blood and night sweats), shingles and mouth ulcers in the past six months, using a verbal symptom checklist (Lopman et al., Citation2006), validated in previous studies of adults in South Africa. Additionally, for a 25% subset of adolescents from randomly selected clinics, viral load measures were collected from clinic files.
Social protection provisions included economic “cash” and psychosocial “care” provisions. Within “cash”, cash transfers were any government welfare grant provided to the household (child support, foster child, care dependency, pension or disability grant); food security was measured using items from the National Food Consumption Survey and defined as two meals daily for the past week; school access was capacity to pay for or free access to school, textbooks and uniform. School feeding was measured as daily free provision of a meal at school. Access to sufficient clothing was measured using items from the SA Social Attitudes Survey (Pillay, Roberts, & Rule, Citation2006). Within “care”: access to an HIV-support group was past-month attendance at either a youth-focused or general HIV-support group; access to sports, choir or arts groups was attending past-month extra-curricular organised activities. Positive parenting (i.e., praise and positive reinforcement from any primary caregiver) and parental supervision/monitoring (i.e., primary caregiver’s monitoring of adolescent activities, rules about going out) were measured using adolescent-reported subscales of the Alabama Parenting Questionnaire (Elgar, Waschbusch, Dadds, & Sigvaldason, Citation2007). “Parenting’ referred to any biological or non-biological primary caregiver.
Potential covariates that were controlled for in analyses were socio-economic factors of adolescent age, gender, language, formal/informal (shack) housing, urban/rural location and education level (highest school grade passed) measured using items adapted from the South African census (SSA, Citation2011). Maternal and paternal death were asked using items from a South African national survey of AIDS-affected children (Cluver et al., Citation2013). HIV and medication factors included perinatal/horizontal infection, using modelling data from Southern Africa (Ferrand et al., Citation2009), whether the adolescent lived with a caregiver who was AIDS-symptomatic or on ART, whether the adolescent was aware of their own HIV-positive status (using clinic file data and adolescent report) and duration of time on treatment using patient file data, supported by caregiver report and cross-checked with adolescent self-report. Healthcare factors included general past-month self-reported health and time of travel to clinic, and whether the participant had received care in hospital for illness in the past year.
Analyses
Analyses were conducted in four stages in SPSS 21.0 and STATA 13.1. First, known characteristics (age, gender, urban/rural location) of excluded participants were compared to those included, to check for potential differences, and subsequently descriptive statistics for outcomes, social protection variables, and covariates were calculated, and social protection provisions were excluded from analyses where a comparison group was too small for reliable analysis (). Second (), linear and logistic regressions tested associations of self-reported non-adherence, number of opportunistic infections and detectable viral load, controlling for all potential covariates.
Table 1. Socio-demographic, health and social protection factors for HIV-positive adolescents (n = 1059).
Table 2 Associations of past-week self-reported non-adherence.
Third (), associations between specific social protection provisions and past-week ART non-adherence were assessed, following the sequential approach recommended by Hosmer and Lemeshow (Citation1989). Three logistic regression models were run: (a) with all potential covariates and potential social protection factors to control for potential confounding from non-randomised allocation of social protection provisions, (b) with all covariates and all potential social protection factors significant at .1 or below and (c) with only those covariates and social protection factors significant at .05 or below.
Table 3. Logistic regression of all potential social protection factors and covariates.
Fourth, we tested for potential interactive or additive effects on adolescent ART-adherence of combinations of social protections. To test for interactive effects, a logistic regression included all covariates, significant social protection provisions (using only those significant in Stage 3 above), and all possible two-way and three-way interactions of significant social protections. To identify potential additive effects, all potential combinations of the statistically significant social protection variables were entered into a marginal effects analysis using binary logistic regression, with covariates held at their mean values. This analysis indicated how the predicted probability of the outcome changed when different interventions (and combinations of interventions) were present ().
Results
Descriptives (Table 1)
Outcomes
There were no significant differences between included and excluded participants on known factors of age, gender and urban/rural location. About 36% of adolescents reported ART non-adherence in the past week, and 52% reported non-adherence in the past year. Past-week reporting was used for all further analyses. Adolescents reported a mean of 1.7 current opportunistic infections (median 2.0, SD 1.4, range 0–5). Within the subset of adolescents where we collected viral load measures (n = 266), 45.1% had a detectable viral load (>75 copies/ml).
Socio-demographic, HIV and healthcare covariates
The sample had a mean age of 13.8 (median 13.0, SD 2.8, range 10–19), was 55% female, and 97% first-language Xhosa; 19% lived in informal housing and 81% in formal homes, with 21% in rural areas and the remainder in peri-urban or urban locations. On average, adolescents reported completing nearly 6 grades of school (mean 5.77, SD 2.6, median 6.0, range 0–12); 44% were maternally and 30% paternally orphaned, with a further 3% of mothers and 16% of fathers non-resident; 67% were perinatally infected. Adolescents had been on ART for a mean of 5.9 years (median 5.0, SD 4.5, range 0–19 years), and 59.9% reported poor/not good health in the past 6 months. Mean travel time to healthcare facilities was three quarters of an hour (median 30 minutes, SD 95 minutes, range 1 minute to 3 hours).
Social protection access
In all, 95% of adolescents received a cash transfer grant in their household, and 93% had regular school feeding; 78% had enough food to eat in the past week, 46% had access to school, uniform and textbooks; 81% had enough clothes to stay warm and dry, 13% attended any HIV support group, 13% were part of a sports team or organised sports group and 15% attended a choir or arts group. Forty-two per cent reported high positive parenting and 41% reported high parental supervision.
Validating self-reported adherence (Table 2)
Regression analyses controlling simultaneously for all potential covariates (age, gender, language, housing type, location, education, maternal/paternal orphanhood, perinatal infection, caregiver AIDS-illness, caregiver ART-taking, status knowledge, time on treatment, clinic travel time, health status and hospitalisation) showed that past-week non-adherence was associated with increased rates of current opportunistic infections (B 0.269, p < .006), with adolescents reporting a mean of 1.7 OIs (SD 1.36, range 0–5) in the past six months. In the 25% subsample (n = 266), past-week non-adherence was significantly associated with increased rates of detectable viral load (aOR 1.98, CI 1.13–4.45, p < .05). Median viral load of adolescents with detectable VL was 689 copies/ml.
Independent associations of social protection provisions with non-adherence (Table 3)
Associations of eight potential social protection provisions with ART non-adherence were tested simultaneously (government cash transfers and school feeding were excluded due to less than 100 adolescents not receiving the provision), controlling for all the potential covariates (age, gender, language, housing type, location, education, maternal/paternal orphanhood, perinatal infection, caregiver AIDS-illness, caregiver ART-taking, status knowledge, time on treatment, clinic travel time, health status and hospitalisation, all entered simultaneously). shows the three factors that remained significantly associated with reduced non-adherence, independent of all other social protection factors and covariates. Sufficient food (aOR 0.57, CI 0.42–0.76, p < .001); attending an HIV support group (aOR 0.60, CI 0.40–0.91, p < .05) and high parental supervision/monitoring (aOR 0.56, CI 0.43–0.73, p < .001) were associated with reduced non-adherence.
Associations of combination social protection interventions with non-adherence
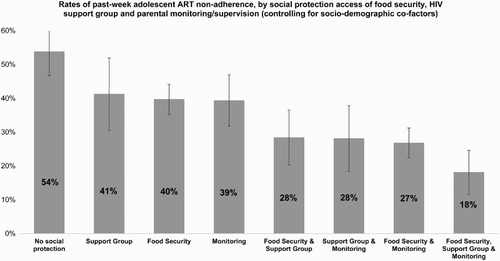
Using only the three social protection provisions significantly associated with adherence, potential multiplicative effects of two-way and three-way combinations (food × support group, supervision × support group, food × supervision, food × support group × supervision) were tested using interaction terms in logistic regression, controlling for all covariates. No statistically significant interactions were shown, indicating no multiplicative effects (). Consequently, to investigate potential additive effects of the three social protections that were all significant independently of each other, we calculated interval estimates of the predicted probability of the outcome when different combinations of social protections are received, whilst controlling for covariates. We found strong additive effects of combining social protection provisions. Predicted probability of past-week non-adherence for adolescents not receiving sufficient food, support group or good parental monitoring was 54%. With any one of these social protections, predicted probability of past-week non-adherence was 39–41%, and with any two, 27–28%. With all three social protection provisions, predicted probability of past-week non-adherence was 18% ().
Table 4. Logistic regression of all significant potential social protection factors, interaction terms and covariates.
Discussion
Findings have three key messages for approaches to treatment rollout in Southern Africa. First, that HIV-positive adolescents are at high risk of ART non-adherence, with no associations of socio-demographic factors such as age, gender and mode of infection. Self-reported data show that more than a third of adolescents were non-adherent in the past week, and half were non-adherent in the past year. Associations of non-adherence with more opportunistic infections and higher viral loads have direct clinical implications for adolescent survival. This is an immediate and urgent challenge.
Second, findings show that specific social protection provisions, “cash plus care”, are associated with reduced non-adherence. Daily provision of at least two meals per day, attending an HIV-support group, and high levels of parental supervision/monitoring may facilitate ART adherence among HIV-positive adolescents. These findings should act as an impetus for programming, as we have evidence-based programmes for all these provisions: feeding programmes, HIV-support groups and parenting interventions (Grimwood et al., Citation2012; Knerr, Gardner, & Cluver, Citation2013; Snyder et al., Citation2014). Scaling up such programming and ensuring access for HIV-positive adolescents are essential.
Third, data show that combinations of social protection provisions are associated with greater reductions in non-adherence than single provisions alone. The combination of cash and care social protections was associated with reductions in past-week non-adherence from 54% with none of these social protections to 18% with all three. It may be that multi-component interventions are more beneficial in combatting the complex challenges that HIV-positive adolescents face simultaneously across different parts of their lives.
This study has a number of limitations. First, data are cross-sectional and causality cannot be determined in non-randomised designs. Inclusion of a wide range of pre-selected covariates in all analyses aims to mitigate the non-randomised allocation of provisions, but these identified programmatic options should be tested in future longitudinal data and randomised trials.
Second, this study aimed to include a real-world sample of adolescents initiated by government healthcare facilities in a low-resource area of Southern Africa – reflecting the majority of the world’s population of ART-initiated adolescents. The sample reached over 90% of eligible adolescents, and rates of perinatal/horizontal transmission parallel recent estimates of the overall HIV+ adolescent population (Stover, Citation2014). The small groups of refusals, untraceable adolescents and adolescents with very severe cognitive delay may represent particularly high-risk subsamples for future research. However, this study is unique in being the only known study to include both clinic-attending and non-attending adolescents, by following up all ART-initiated participants to their homes, and inclusion rates were very high for such a vulnerable group.
Third, it is important to test these findings in other contexts. The study’s location was selected by state and bilateral partners as most closely reflecting poverty and healthcare contexts in other low-income Southern African contexts. The Eastern Cape is South Africa’s poorest province, with 30% antenatal HIV-prevalence, and health provision impacted by historical and current resource factors (National Department of Health, Citation2013).
Fourth, South Africa’s highly successful national scale-up of government cash transfers for adolescents resulted in such high coverage in this sample that government grants were almost universally received and thus not utilised in these analyses. However, economic support encompasses more than cash grants alone, and our data show that wider material provision in the form of food, clothing and school fees can be considered in the category. A broad definition of financial support is vital. Investigation of associations of cash transfers and adherence in countries with lower cash grant coverage will be essential to identify potential effects of this important social protection form on its own as well as in combination with wider financing. It will also be valuable to examine associations of social protection with ART-adherence in adolescent groups who may not have ever accessed targeted health care, including young MSM, adolescents engaged in sex work, and adolescents in institutions or living on the streets.
Finally, this study primarily uses self-reported adherence data, which risks under-reporting due to social desirability and recall bias. However, research on adherence has identified unreliability in all measurement approaches. For example, healthcare provider and parents reporting of adolescent adherence has been shown to be far higher than self-report (Evans et al., Citation2015). Pill counts may be manipulated by youth using techniques such as pill-dumping, and biomarkers such as viral load may be influenced by infections and viral resistance as well as ART non-adherence. In this study, validation checks against opportunistic infections and viral load measures suggest correlations between self-reported non-adherence and clinical outcomes, and support similar findings in multisite studies (Buscher, Hartman, Kallen, & Giordano, Citation2011; Fletcher et al., Citation2005; Usitalo et al., Citation2014).
Despite these limitations, this study has great value for informing programmatic approaches to adolescent HIV-treatment. It uses existing interventions that are currently provided by states, NGOs, or families at a large scale. It demonstrates that social protection combinations remain strongly associated with reductions in non-adherence independently of a wide range of socio-demographic, HIV-related and healthcare factors. This study takes place in real-world, low-resource contexts, with a highly representative sample of adolescents who have ever entered a government ART programme. In doing so, it is the first study of its kind. This study provides promising initial evidence that – even in these highly challenging contexts – combination social protection may have the potential to reduce non-adherence. And this in turn could contribute to reducing inequitable rates of adolescent AIDS deaths, the potential for viral resistance and treatment failure, the need for expensive and complex salvage regimes, and onward transmission of HIV.
Acknowledgements
We thank Tom Fenn, Patricia Lim Ah Ken, Susan Kasedde, Chewe Luo and Chiho Suzuki (UNICEF), David Chipanta (UNAIDS) Rajen Govender (SA MRC and UCT) Stuart Kean (WorldVision), John Miller (Coalition for Children Affected by AIDS), Jason Wolfe (USAID), and Mpumi Zungu (HSRC) for discussion and input. We thank Marija Pantelic for additional analyses. We thank research teams and all participants and their families.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Baird, S. J., Garfein, R. S., McIntosh, C. T., & Ozler, B. (2012). Effect of a cash transfer programme for schooling on prevalence of HIV and herpes simplex type 2 in Malawi: a cluster randomised trial. The Lancet, 379(9823), 1320–1329. doi:10.1016/S0140-6736(11)61709-1
- Bhana, A., Mellins, C. A., Petersen, I., Alicea, S., Myeza, N., Holst, H., … McKay, M. (2014). The VUKA family program: Piloting a family-based psychosocial intervention to promote health and mental health among HIV infected early adolescents in South Africa. AIDS Care, 26(1), 1–11. doi:10.1080/09540121.2013.806770
- Bronfenbrenner, U. (1979). The ecology of human development: Experiments by nature and design. Cambridge: Harvard University Press.
- Buscher, A., Hartman, C., Kallen, M. A., & Giordano, T. P. (2011). Validity of self-report measures in assessing antiretroviral adherence of newly diagnosed, HAART-naive, HIV patients. HIV Clinical Trials, 12(5), 244–254. doi:10.1310/hct1205-244
- Cluver, L., Hodes, R. J., Sherr, L., Orkin, F. M., Meinck, F., Lim Ah Ken, P., … Vicari, M. (2015). Social protection: Potential for improving HIV outcomes among adolescents. Journal of the International AIDS Society, 18(7 Suppl 6), 20260. doi:10.7448/IAS.18.7.20260
- Cluver, L., Orkin, F. M., Boyes, M. E., & Sherr, L. (2014). Cash plus care: social protection cumulatively mitigates HIV-risk behaviour among adolescents in South Africa. AIDS, 28(Suppl 3), S389–397. doi:10.1097/QAD.0000000000000340
- Cluver, L., Orkin, M., Boyes, M. E., Sherr, L., Makasi, D., & Nikelo, J. (2013). Pathways from parental AIDS to child psychological, educational and sexual risk: Developing an empirically-based interactive theoretical model. Social Science & Medicine, 87, 185–193. doi:10.1016/j.socscimed.2013.03.028
- DSD, SASSA and UNICEF (2012). The South African Child Support Grant Impact Assessment: Evidence from a survey of children, adolescents and their households. Pretoria: UNICEF South Africa.
- Duong, M., Piroth, L., Grappin, M., Forte, F., Peytavin, G., Buisson, M., … Portier, H. (2001). Evaluation of the Patient Medication Adherence Questionnaire as a tool for self-reported adherence assessment in HIV-infected patients on antiretroviral regimens. HIV Clinical Trials, 2(2), 128–135. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11590521 doi: 10.1310/M3JR-G390-LXCM-F62G
- Elgar, F., Waschbusch, D., Dadds, M., & Sigvaldason, N. (2007). Development and validation of a short form of the Alabama Parenting Questionnaire. Journal of Child and Family Studies, 16(2), 243–259. Retrieved from http://dx.doi.org/10.1007/s10826-006-9082-5 doi: 10.1007/s10826-006-9082-5
- Emenyonu, N., Muyindike, W., Habayarimana, J., Pops-Eleches, C., Thirumurthy, N., Ragland, K., & Bangsberg, D. (2012). Cash transfers to cover clinic transportation costs improve adherence and retention in care in a HIV treatment program in rural Uganda. Paper presented at the 17th conference on retroviruses and opportunistic infections, Seattle.
- Evans, S. D., Mellins, C. A., Leu, C. S., Warne, P., Elkington, K. S., Dolezal, C., … Abrams, E. J. (2015). HIV treatment adherence measurement and reporting concordance in youth with perinatally acquired HIV infection and their caregivers. AIDS Patient Care and STDs, 29(1), 43–51. doi:10.1089/apc.2014.0058
- Ferrand, R., Corbett, E. L., Wood, R., Hargrove, J., Ndhlovu, C. E., Cowan, F. M., … Williams, B. G. (2009). Aids among older children and adolescents in Southern Africa: Projecting the time course and magnitude of the epidemic. AIDS, 23(15), 2039–2046. doi:10.1097/QAD.0b013e32833016ce
- Ferrand, R., Lowe, S., Whande, B., Munaiwa, L., Langhaug, L., Cowan, F., … Corbett, E. L. (2010). Survey of children accessing HIV services in a high prevalence setting: Time for adolescents to count? Bulletin of the World Health Organization, 88(6), 428–434. doi:10.2471/BLT.09.066126
- Fletcher, C. V., Testa, M. A., Brundage, R. C., Chesney, M. A., Haubrich, R., Acosta, E. P., … Gulick, R. M. (2005). Four measures of antiretroviral medication adherence and virologic response in AIDS clinical trials group study 359. JAIDS Journal of Acquired Immune Deficiency Syndromes, 40(3), 301–306. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16249704 doi: 10.1097/01.qai.0000180078.53321.6a
- Grimsrud, A., Lesosky, M., Mothibi, E., Kalombo, C., Bekker, L. G., & Myer, L. (2016). Implementation and operational research: Community-based adherence clubs for the management of stable antiretroviral therapy patients in Cape Town, South Africa: A cohort study. Journal of Acquired Immune Deficency Syndromes, 71(1), e16–e23. doi:10.1097/QAI.0000000000000863
- Grimwood, A., Fatti, G., Mothibi, E., Malahlela, M., Shea, J., & Eley, B. (2012). Community adherence support improves programme retention in children on antiretroviral treatment: A multicentre cohort study in South Africa. Journal of the International AIDS Society, 15(2), 17381. doi:10.7448/IAS.15.2.17381
- Hall, K. (2015). Children count: Statistics on children in South Africa. Retrieved from Cape Town. http://www.childrencount.org.za/indicator.php?id=2&indicator=10
- Handa, S., Halpern, C. T., Pettifor, A., & Thirumurthy, H. (2014). The government of Kenya’s cash transfer program reduces the risk of sexual debut among young people age 15–25. PLoS ONE, 9(1), e85473. doi:10.1371/journal.pone.0085473
- Hosmer, D., & Lemeshow, S. (1989). Applied logistic regression. New York, NY: John Wiley & Sons.
- Hudelson, C., & Cluver, L. (2015). Factors associated with adherence to antiretroviral therapy among adolescents living with HIV/AIDS in low- and middle-income countries: a systematic review. AIDS Care, 27(7), 805–816. doi:10.1080/09540121.2015.1011073
- Kilburn, K., Thirumurthy, H., Halpern, C. T., Pettifor, A., & Handa, S. (2016). Effects of a large-scale unconditional cash transfer program on mental health outcomes of young people in Kenya. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine, 58(2), 223–229. doi:10.1016/j.jadohealth.2015.09.023
- Knerr, W., Gardner, F., & Cluver, L. (2013). Improving positive parenting skills and reducing harsh and abusive parenting in low- and middle-income countries: A systematic review. Prevention Science: The Official Journal of the Society for Prevention Research. doi:10.1007/s11121-012-0314-1
- Lopman, B., Barnabas, R., Boerma, T., Chawira, J., Gaitskell, K., Harrop, T., … Gregson, S. (2006). Creating and validating an algorithm to measure AIDS mortality in the adult population using verbal autopsy. Public Library of Science Medicine, 3(8), 1273–1281.
- Lowenthal, E., Bakeera-Kitaka, S., Marukutira, T., Chapman, J., Goldrath, K., & Ferrand, R. (2014). Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: A review of emerging challenges. Lancet Infectious Diseases. doi:10.1016/S1473-3099(13)70363-3
- Lowenthal, E., Haruna, B., Mmapula, L., Keofentse, M., Tshume, O., & Anabwani, G. (2014). Disclosure of HIV status to HIV-infected children in a large African treatment center: Lessons learned in Botswana. Child and Youth Services Review, 45, 143–149. doi: 10.1016/j.childyouth.2014.03.031
- Lowenthal, E., Lawler, K., Harari, N., Moamogwe, L., Masunge, J., Masedi, M., … Gross, R. (2012). Rapid psychosocial function screening test identified treatment failure in HIV+ African youth. AIDS Care, 24(6), 722–727. doi:10.1080/09540121.2011.644233
- MacPherson, P., Munthali, C., Ferguson, J., Armstrong, A., Kranzer, K., Ferrand, R., & Ross, D. A. (2015). Service delivery interventions to improve adolescents’ linkage, retention and adherence to antiretroviral therapy and HIV care. Tropical Medicine & International Health : TM & IH, 20(8), 1015–1032. doi:10.1111/tmi.12517
- Mellins, C. A., Tassiopoulos, K., Malee, K., Moscicki, A. B., Patton, D., Smith, R., … Pediatric HIV/AIDS Cohort Study. (2011). Behavioral health risks in perinatally HIV-exposed youth: Co-occurrence of sexual and drug use behavior, mental health problems, and nonadherence to antiretroviral treatment. AIDS Patient Care and STDs, 25(7), 413–422. doi:10.1089/apc.2011.0025
- Nachega, J. B., Hislop, M., Nguyen, H., Dowdy, D. W., Chaisson, R. E., Regensberg, L., … Maartens, G. (2009). Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in Southern Africa. JAIDS Journal of Acquired Immune Deficiency Syndromes, 51(1), 65–71. doi:10.1097/QAI.0b013e318199072e
- National Department of Health. (2013). The 2012 National Antenatal Sentinel HIV and Herpes Simplex type-2 prevalence Survey, South Africa. National Department of Health, Pretoria. http://www.health-e.org.za/wp-content/uploads/2014/05/ASHIVHerp_Report2014_22May2014.pdf. Retrieved from Pretoria.
- Paterson, D. L., Swindells, S., Mohr, J., Brester, M., Vergis, E. N., Squier, C., … Singh, N. (2000). Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine, 133(1), 21–30. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10877736 doi: 10.7326/0003-4819-133-1-200007040-00004
- de Pee, S., Grede, N., Mehra, D., & Bloem, M. W. (2014). The enabling effect of food assistance in improving adherence and/or treatment completion for antiretroviral therapy and tuberculosis treatment: A literature review. AIDS and Behavior, 18(Suppl 5), S531–541. doi:10.1007/s10461-014-0730-2
- PEFPAR. (2015). Preventing HIV in adolescent girls and young women: Guidance for PEPFAR country teams on the DREAMS partnership. Washington, DC: USAID-PEPFAR.
- Pettifor, A., Rosenberg, N., & Bekker, L. G. (2016). Can cash help eliminate mother-to-child HIV transmission? Lancet HIV, 3(2), e60–62. doi:10.1016/S2352-3018(16)00003-5
- Pillay, D. (2001). The emergence and epidemiology of resistance in the nucleoside-experienced HIV-infected population. Antiviral Therapy, 6(Suppl 3), 15–24. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11678470
- Pillay, U., Roberts, B., & Rule, S. (Eds.). (2006). South African social attitudes: Changing times, diverse voices. Cape Town: HSRC Press.
- Rutter, M. (2007). Resilience, competence, and coping. Child Abuse and Neglect, 31, 205–209. doi: 10.1016/j.chiabu.2007.02.001
- Sherr, L., Lampe, F. C., Clucas, C., Johnson, M., Fisher, M., Leake Date, H., … Harding, R. (2010). Self-reported non-adherence to ART and virological outcome in a multiclinic UK study. AIDS Care, 22(8), 939–945. doi:10.1080/09540121.2010.482126
- Snyder, K., Wallace, M., Duby, Z., Aquino, L., Stafford, S., Hosek, S., & Bekker, L. G. (2014). Preliminary results from Hlanganani (coming together): A structured support group for HIV-infected adolescents piloted in Cape Town, South Africa. Children and Youth Services Review, 45, 114–121. doi: 10.1016/j.childyouth.2014.03.027
- Statistics South Africa (2011). Census 2011: Household Questionnaire. Pretoria: Statistics South Africa.
- Stover, J. (2014). The costs and impacts of the global goals for adolescents 10–19 in the All In! initiative. New York: UNICEF.
- UNAIDS. (2014). HIV and social protection guidance note. Retrieved from Geneva.
- UNAIDS. (2015a). 2015 HIV and AIDS estimates. Geneva: UNAIDS.
- UNAIDS. (2015b). UNAIDS 2014 HIV and AIDS estimates, in key regional charts and figures: Eastern and Southern Africa. Retrieved from Geneva.
- UNICEF. (2012). UNICEF social protection strategic framework. Retrieved from New York http://www.unicef.org/socialprotection/framework/
- Usitalo, A., Leister, E., Tassiopoulos, K., Allison, S., Malee, K., Paul, M. E., … Mellins, C. A. (2014). Relationship between viral load and self-report measures of medication adherence among youth with perinatal HIV infection. AIDS Care, 26(1), 107–115. doi:10.1080/09540121.2013.802280
- Vreeman, R. C., Wiehe, S. E., Pearce, E. C., & Nyandiko, W. M. (2008). A systematic review of pediatric adherence to antiretroviral therapy in low- and middle-income countries. The Pediatric Infectious Disease Journal, 27(8), 686–691. doi:10.1097/INF.0b013e31816dd325