ABSTRACT
Uptake of HIV testing remains low among men in South Africa. As part of a trial, we assessed the acceptability of a theoretically derived and adapted tablet-based-application (EPIC-HIV1) in rural South Africa. We conducted 20 in-depth interviews with men aged ≥18 years and offered a tablet-based survey to all men aged ≥15 years who received EPIC-HIV1 (Sep-Dec 2018). We conducted a descriptive analysis of the survey and used Self-Determination Theory (SDT) to guide our thematic analysis. A total of 232/307 (75%) completed the survey, 55% of whom were aged 15–24 years. 96%[ CI: 92.8–98.2%; n = 223] found EPIC-HIV1 acceptable and 77% [95% CI: 71.8–82.6%; n = 179] found it user-friendly. 222 [96%] reported that EPIC-HIV1 motivated them to test; 83% (192/232) tested for HIV, of which 33% (64/192) were first time testers. Those who did not consent (n = 40) were more likely to have had an HIV-positive test result. Participants reported that the app boosted their confidence to test. However, they were unsure that the app would help them overcome barriers to test in local clinics. Given reach and usability, an adapted SDT male-tailored app was found to be acceptable and could encourage positive health-seeking behavioural change among men.
Background
In rural KwaZulu-Natal (KZN) South Africa, men are less likely to test for HIV and are more likely to die of AIDS-related illnesses than women (Iwuji et al., Citation2016; Iwuji et al., Citation2018; Tanser et al., Citation2013). The 2017 UNAIDS report “Reaching Out to Men and Boys: Addressing a Blind Spot in the Response to HIV” highlights the barriers to men’s uptake of HIV testing, prevention and treatment (UNAIDS, Citation2017). A consistent theme, across different settings, is that the social costs of testing and accessing care outweigh the perceived health benefits, particularly for young men (Adams & Zamberia, Citation2017; Adeagbo et al., Citation2018; Adeagbo et al., Citation2019; Chikovore et al., Citation2016; HSRC, Citation2018; Iwuji et al., Citation2018). Structural barriers such as a lack of male-friendly HIV services and privacy, facing anticipated stigma in the clinic, restrictive clinic hours and long waiting times as well as fear of an “HIV identity” and its perceived incompatibility with masculinity, are some factors impeding men’s effective uptake of HIV testing and care services (Bor et al., Citation2015; Chikovore et al., Citation2016; Mathenjwa et al., Citation2019; Tanser et al., Citation2013).
To achieve zero new infections and zero AIDS-related deaths, innovative interventions are needed for men for early diagnosis and treatment. HIV self-testing has shown promise for men (Choko et al., Citation2015; Harichund et al., Citation2019; Krause et al., Citation2013); however, HIV testing needs to be coupled with promoting benefits of HIV treatment for those who test positive and ways to stay negative for the others. Digital interventions such as mobile communication technologies (mHealth) have the potential to fill this health promotion gap and support men’s engagement with HIV care (Adeagbo et al., Citation2019). The World Health Organization has recommended the use of mHealth for HIV and other chronic disease management (Daher et al., Citation2017; WHO, Citation2011; Zhao et al., Citation2018).
We developed a theoretically driven, interactive tablet-based application EPIC-HIV (Empowering People through Informed Choices for HIV) to improve men’s intrinsic motivation to test and link to HIV care services in rural KZN. EPIC-HIV contains two different applications: EPIC-HIV1: a self-administered app offered at the point of HIV test to men to help them make an informed choice about testing and, if necessary, whether to engage with care. EPIC-HIV2: a second app offered to men who tested HIV-positive but not linked to care within a month after diagnosis, was to motivate them to initiate antiretroviral therapy (ART) and stay in care (Mathenjwa et al., Citation2019).
EPIC-HIV messaging is rooted in self-determination theory (SDT) to increase men’s intrinsic motivation to test (EPIC-HIV1) for HIV. SDT is a behavioural change theory that relies on individual psychological needs for autonomy, competence and relatedness to be internally motivated to adopt a new behaviour (Deci & Flaste, Citation1995; Deci & Ryan, Citation2000; Quinlivan et al., Citation2017). Autonomy entails people’s ability to make individual decisions associated with their identity, beliefs and values, while competence requires an individual to be able to act (Deci & Ryan, Citation2000; Johnson, Citation2007; Johnston & Finney, Citation2010; Quinlivan et al., Citation2013). Relatedness reflects individuals feelings of connectedness and being cared for (Quinlivan et al., Citation2017; Ryan & Deci, Citation2000). The SDT components combined support for a target population to become intrinsically motivated to adopt new behaviours. Studies that adopted SDT in developing behavioural change interventions have shown effect across a variety of behaviours such as diabetes, smoking cessation, ART adherence, and hepatitis C treatment (Houston et al., Citation2012; Igreja et al., Citation2000; Morse et al., Citation2012; Quinlivan et al., Citation2017; Toth et al., Citation2013; Williams et al., Citation2011). There is, however, limited literature on the use of SDT to promote positive health behaviours in the HIV care continuum. In this article, we report on the acceptability of EPIC-HIV1.
Methods
Trial overview
EPIC-HIV1 is a self-administered tablet-based app developed as part of a larger factorial design cluster randomised controlled trial: Home-based Intervention to Test and Start (HITS- ClinicalTrials.gov #NCT03757104). The HITS trial was designed to assess the effectiveness of financial micro-incentives (R50[$3] food vouchers) and/or a SDT informed decision support application (EPIC-HIV1) delivered on tablet to increase the uptake of home-based HIV testing in men. Detailed information about HITS and EPIC-HIV development are described elsewhere (Mathenjwa et al., Citation2019).
The intervention
EPIC-HIV1 was a tablet-based application that was designed iteratively using human computer interaction person-based approach and informed by SDT (Yardley et al., Citation2015). The app was designed to be administered in 5–10 min and enable the user to hear the “story” of a chosen character portrayed on the app.
Study setting
The study was conducted in uMkhanyakude district in rural KZN, South Africa. Study participants were recruited from a large population-based HIV surveillance cohort in an HIV hyperendemic area.
Study participants
All male participants (≥15 years) who received EPIC-HIV1 in two (EPIC-HIV arms) of the three intervention arms were asked to consent and complete a satisfaction survey between September and December 2018. A sub-sample of adult males aged ≥18 years were selected to participate in the in-depth interview (IDI) based on the following criteria (a) participants must have received EPIC-HIV1; (b) completed the satisfaction survey; (c) tested for HIV or not after their engagement with EPIC-HIV app; (d) and must have consented to be followed up during the home-based testing visit. Of those (n = 84) who agreed to be interviewed, not all were available due to time, inactive phone numbers (n = 30), work and other personal reasons. Of the 40 available, 20 men (10 from each arm as planned) were randomly selected and interviewed face-to-face from the clusters in the two EPIC-HIV intervention arms. Separate informed written consent was completed for participating in IDIs and satisfaction surveys.
Study procedures
We conducted a mixed-method process evaluation to assess the acceptability of and satisfaction with EPIC-HIV1 among males using both quantitative surveys and qualitative interviews. First, an anonymised tablet-based ten-item satisfaction survey was developed and completed inResearch Electronic Data Capture (REDCap- developed by Vanderbilt University, USA) to assess EPIC-HIV1 users’ satisfaction and acceptability of the app using a 4-point Likert scale of 1–4 (Harris et al., Citation2009). While the higher score (4) was associated with greater satisfaction, the lower score (1) indicated no satisfaction. The survey was administered by fieldworkers to consenting participants immediately after they had completed EPIC-HIV1.
Semi-structured IDIs were conducted by experienced social science research assistants fluent in the local language (IsiZulu). The interviews were audio recorded and conducted at participants’ place of choice in the local language and lasted 30–60 min. Participants were asked about their experience using the application, the importance of the messaging as well as the impact of the app on their decision to test or not for HIV during home visits. The data were transcribed into IsiZulu and later translated to English. All data were de-identified to protect confidentiality. The data were checked and compared to the recordings by a senior social scientist (OA) and two senior research assistant supervisors for quality control and to make sure that important meanings were not lost during translation process.
Data analysis
Quantitative data were analysed using STATA 14. Proportions with exact confidence intervals (CI) are reported in a descriptive analysis for the satisfaction survey items. The English translations of interviews were analysed using thematic analysis. Transcripts were coded iteratively and emerging themes identified. The coding was double-checked for consistency across coders before it was analysed. Data analysis was guided by the SDT framework focussed on understanding whether the app did or did not support participants’ motivation to test, through the theorised pathways of autonomy, relatedness and competence.
Ethical considerations
This study was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal, South Africa (Reference Number: BFC398/16). The data were collected with the informed consent (including assent and parental/guardian consent where necessary) of participants prior to their participation in the study. The survey was anonymized. Pseudonyms are used when reporting qualitative data.
Results
Baseline characteristics
Of 307 men invited to complete the satisfaction survey, 232 (75%) completed. About half of the participants (n = 128, 55%) were young men aged 15–24 years. Only 11% (n = 26/232) were married while 23.7% (n = 55/232) were in an informal union. Participant characteristics were provided in .
Table 1. Baseline characteristics among men who participated in the EPIC-HIV 1 satisfaction survey (n = 232)
Satisfaction survey
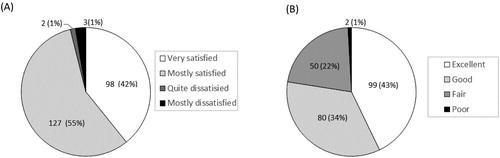
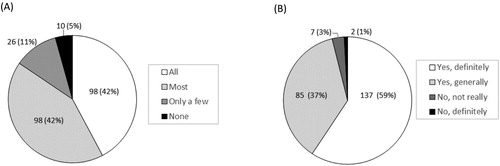
Eight-seven percent (202/232) of participants evaluated that the quality of the EPIC-HIV1 app as good or excellent, while 13% (29/232) indicated the quality was fair, and 96% [95% CI: 92.8–98.2%; n = 223] were satisfied with EPIC-HIV1 app (A). Seventy-seven percent [95% CI: 71.8–82.6%; n = 179] found the user-friendliness of app excellent or good while 22% [95% CI: 16.5–27.5%; n = 50] said it was fair (B). About 84% [95% CI: 79.2–88.9%; n = 196] reported that the app met most or all their needs with regards to information on HIV (A). Nearly everyone [96%, n = 222] reported that EPIC-HIV1 motivated them to test for HIV after they had listened to and watched it (B). Almost everyone [97%, 95% CI: 94.5–99.0%; n = 226] felt empowered in managing their health with the information from the app. Over 93% (n = 215) reported that they would use EPIC-HIV1 again or recommend it to a friend seeking HIV-related information. There was no significant difference among young adolescents and adults (aged 15–24 years), compared to older males (aged ≥25 years), except that fewer young adolescents and adults expressed that they would use the app again for HIV information (88%), compared to the older males (99%, p-value = 0.001) (Table S1).
Uptake of HIV testing
Of 232 men who accepted the EPIC-HIV1 app, and completed the satisfaction survey, 83% (192/232) consented and tested for a home-based rapid HIV test, and 33% (64/192) received HIV testing for the first time in the annual HIV surveillance. Those who did not consent (n = 40) were more likely to have had HIV-positive test result. Detailed HIV testing uptake is described elsewhere (Tanser et al., Citation2019).
Qualitative findings
Twenty men (18–73 years) who had received EPIC-HIV1 agreed to the interviews. Participants’ felt that the application motivated them to test for HIV because it promoted HIV awareness that was relevant to them. However, the men also gave the reasons why they may not test, even when using the app alongside free confidential home-based testing.
Factors beyond the application: exploring men’s autonomy for HIV testing
Most men interviewed tested for HIV during home visits, one tested later, while three did not test. Even among those who tested, there were different accounts of the impact of EPIC-HIV1 in motivating participants to test. Several men described the app as bolstering their existing motivation to test. These were often men who had tested before, as illustrated by this young man who describes how the app motivated him to test because it resonated with his existing experience and practice:
“I lost my parents to HIV/AIDS. Although this happened when I was young, I understood everything that was happening, but it terrified me. That is what led me to test for HIV for the first time. This year I have tested twice already within a period of 6 months, because I now understand the significance of testing for HIV and knowing your status.” (31 years)
For others, the app whilst reinforcing their intrinsic motivation to test was only “sufficient” to prompt uptake of testing because it was coupled with the convenience and privacy of home-based testing:
… It [EPIC-HIV] also taught me about protecting myself in that way I am not only protecting myself but also my partner … Yes I did test! … What I see as important is for you to come more often and visit our homes [Home-based testing …] (28 years).
Positive messaging alleviating fear: men feeling competent to make choices
Some men reflected on how the positive message that the app provided alleviated their fears and re-enforced their plans to adopt new behaviours to improve their health outcomes:
… As I have already stated that I stopped the things that I used to do. I ended up doing this because I got a fright after listening to the app about people’s risky sexual behaviour … (31 years).
… I felt that the App was very good … I think it would bring safety to the community … would help people to commit themselves to testing regularly, we should listen and adhere to the things that are discussed in the App … (23 years).
Feeling connected to the app contents: men’s feelings of care and motivation
Participants described the ways in which the local narratives in the app resonated with their reality and were told in a way that they could understand and relate to, which helped them decide to test:
You will know how to behave and what life-style you should live that’s why I am saying it helps to listen to health messages … it’s the one that helps a lot … Yes, there are things I learned and the way they were explaining even the person who finds it hard to understand can hear it (27 years).
For some, this also helped them decide to change their behaviour:
… I think I first listened to it and then tested for HIV … I managed to change the things that I used to do before … I now do things the way I was taught after it had been explained to me and watching the pictures that were showing what happens to a person once he is infected … (24 years).
Some men suggested that similar content should be created to get men into all other healthcare services, not just HIV care.
Discussion
Our study shows that a male-tailored tablet-based application was acceptable among men, particularly those aged 15–24 years. Most men reported the app was easy to use, and the HIV information provided in the app met their individual needs. Men across different age groups related to the characters and the messages in the app resonated with their lived experiences and helped motivate them to test. However, factors such as the convenience and privacy of home-based testing and sexual partners’ encouragement and support also played an important role in motivating some men to test. Following engagement with EPIC-HIV1, most men in our sample were able to initiate a new behaviour, such as HIV testing, to achieve positive health outcomes (Quinlivan et al., Citation2017; Ryan & Deci, Citation2000). This suggests that offering EPIC-HIV1 with other interventions such as HIV self-testing, and support partner-initiated testing, has the potential to encourage men to exercise their autonomy to test.
Most men, including a substantial number of first-time testers, claimed the app gave them a “nudge” to test for HIV. Other studies have shown the potential of a digital intervention to support early HIV diagnosis in men (Rawat et al., Citation2018; Wood et al., Citation2019; Zhao et al., Citation2018). However, individuals’ autonomy to test for HIV was shaped by factors other than the app, suggesting that digital applications like EPIC-HIV1 may be most effective for men when used to support effective decentralised HIV care (Li et al., Citation2017; Liu et al., Citation2016; Nakanwagi et al., Citation2016; Nhassengo et al., Citation2018).
The positive messaging of the app was particularly important and appealed to men. It made participants feel empowered to take charge of their life without fear. There were also suggestions that the “counselling app” will promote “safety” within the community if it could be made available to all men since most men find it difficult to access HIV care and other health services. Safety in this context means improving men’s health outcomes as well as that of their sexual partners since it is likely that most men will know their HIV status and take charge of their health.
In summary, an adapted SDT based male-tailored intervention that incorporated local narratives was found to be acceptable and supported early HIV testing and could bring about positive health-seeking behavioural change among men. However, such app should be used alongside other interventions as it is insufficient to overcome some barriers to accessing HIV testing and care services at public health clinics.
Study limitations and strengths
The generalizability outside of our setting of KZN may be limited, due to the locally tailored design of the EPIC-HIV1 app, the sampling method, sample size and specific study sites. The survey was implemented by the same fieldworkers that delivered the app and so may be open to social desirability bias. EPIC-HIV1 and HIV testing were offered to men who were found at home during annual surveillance; some men may have chosen to be absent for those visits to avoid being offered HIV testing. Also, we did not interview those who were found by the fieldworkers but chose not to receive EPIC-HIV1. The home-based testing delivered by fieldworkers may have impacted on men’s ability to exercise their full autonomy in the study. Also, due to the fluid nature of mobile technology and user expectations in app-based communications, EPIC-HIV1 may have time-limited relevance. Nevertheless, the evaluation of the uptake, acceptability and the impact of the app in motivating men to test for HIV in a rural HIV hyperendemic area. Highlight the strength of the study as it gave us the opportunity to triangulate the findings from a range of methods. The theoretical stance adopted for the analysis adds to our understanding of the uptake and impact of a male-tailored digital intervention in a resource-constrained setting.
Conclusion
An SDT-informed male-tailored tablet-based application that includes local narratives to support early HIV testing was acceptable among men in our sample. Given its potential reach and usability with HIV self-testing, EPIC-HIV1 could increase men’s intrinsic motivation to test in a setting with low HIV testing rates in men. Our findings suggest that coupled with partner testing, HIV self-testing and/or with decentralised, non-judgmental and convenient HIV testing services, a male-tailored intervention may improve men’s uptake of HIV testing in the cascade of care.
Acknowledgements
The authors wish to thank the colleagues at AHRI supporting day-to-day project operations; Anya Zeitlin, who led the user-centred design of EPIC-HIV1; the fieldworkers and all the study participants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adams, A. K., & Zamberia, A. M. (2017). “I will take ARVs once my body deteriorates”: an analysis of Swazi men’s perceptions and acceptability of test and start. African Journal of AIDS Research, 16(4), 295–303. https://doi.org/10.2989/16085906.2017.1362015
- Adeagbo, O., Carina, H., Blandford, A., McKendry, R., Estcourt, C., Seeley, J., & Shahmanesh, M. (2019). Exploring people’s candidacy for mobile health–supported HIV testing and care services in rural KwaZulu-Natal, South Africa: Qualitative study. Journal of Medical Internet Research, 21(11), e15681–13. https://doi.org/10.2196/15681
- Adeagbo, O., Herbst, C., Dlamini, N., Mhlongo, T., Blandford, A., Valerian, T., … Shahmanesh, M. (2018). The ‘Missing Middle’ in HIV Treatment Cascade: Ensuring Men’s and Young People’s access to HIV Care in KwaZulu-Natal, South Africa [Paper presentation]. Association of Social Science and Humanities in HIV, 2018 International AIDS Society Pre-conference Amsterdam.
- Bor, J., Rosen, S., Chimbindi, N., Haber, N., Herbst, K., Mutevedzi, T., Tanser, F., Pillay, D., Bärnighausen, T., & Tsai, A. C. (2015). Mass HIV treatment and sex disparities in life expectancy: Demographic surveillance in rural South Africa. PLoS Medicine, 12(11), e1001905. https://doi.org/10.1371/journal.pmed.1001905
- Chikovore, J., Gillespie, N., McGrath, N., Orne-Gliemann, J., Zuma, T., & Group, A. T. S. (2016). Men, masculinity, and engagement with treatment as prevention in KwaZulu-Natal, South Africa. AIDS Care, 28(sup3), 74–82. https://doi.org/10.1080/09540121.2016.1178953
- Choko, A. T., MacPherson, P., Webb, E. L., Willey, B. A., Feasy, H., Sambakunsi, R., Mdolo, A., Makombe, S. D., Desmond, N., Hayes, R., Maheswaran, H., Corbett, E. L., & Bangsberg, D. R. (2015). Uptake, accuracy, safety, and linkage into care over two years of promoting annual self-testing for HIV in Blantyre, Malawi: A community-based prospective study. PLoS Medicine, 12(9), e1001873. https://doi.org/10.1371/journal.pmed.1001873
- Daher, J., Vijh, R., Linthwaite, B., Dave, S., Kim, J., Dheda, K., Peter, T., & Pai, N. P. (2017). Do digital innovations for HIV and sexually transmitted infections work? Results from a systematic review (1996–2017). BMJ Open, 7(11), e017604. https://doi.org/10.1136/bmjopen-2017-017604
- Deci, E. L., & Flaste, R. (1995). Why we do what we do: Understanding self-motivation. Penguins Books.
- Deci, E. L., & Ryan, R. M. (2000). The” what” and” why” of goal pursuits: Human needs and the self-determination of behavior. Psychological Inquiry, 11(4), 227–268. https://doi.org/10.1207/S15327965PLI1104_01
- Harichund, C., Karim, Q. A., Kunene, P., Simelane, S., & Moshabela, M. (2019). Exploring factors that influence the integration of HIVST with HCT using a qualitative comparative cross-over design in KwaZulu-Natal, South Africa. Global Public Health, 1–13.
- Harris, P. A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., & Conde, J. G. (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. https://doi.org/10.1016/j.jbi.2008.08.010
- Houston, E., McKirnan, D. J., Cervone, D., Johnson, M. S., & Sandfort, T. G. (2012). Assessing treatment motivation among patients receiving antiretroviral therapy: A multidimensional approach. Psychology & Health, 27(6), 674–687.
- HSRC. (2018). South African National HIV Prevalence, Incidence, Behaviour and Communication Survey 2017.
- Igreja, I., Zuroff, D. C., Koestner, R., Saltaris, C., Brouillette, M. J., & Lalonde, R. (2000). Applying self-determination theory to the prediction of distress and well-being in gay men with HIV and AIDS 1. Journal of Applied Social Psychology, 30(4), 686–706. https://doi.org/10.1111/j.1559-1816.2000.tb02819.x
- Iwuji, C. C., Orne-Gliemann, J., Larmarange, J., Balestre, E., Thiebaut, R., Tanser, F., Okesola, N., Makowa, T., Dreyer, J., Herbst, K., McGrath, N., Bärnighausen, T., Boyer, S., De Oliveira, T., Rekacewicz, C., Bazin, B., Newell, M.-L., Pillay, D., Dabis, F., … Rekacewicz, C. (2018). Universal test and treat and the HIV epidemic in rural South Africa: A phase 4, open-label, community cluster randomised trial. The Lancet HIV, 5(3), e116–e125. https://doi.org/10.1016/S2352-3018(17)30205-9
- Iwuji, C. C., Orne-Gliemann, J., Larmarange, J., Okesola, N., Tanser, F., Thiebaut, R., Rekacewicz, C., Newell, M.-L., Dabis, F., & Low, N. (2016). Uptake of home-based HIV testing, linkage to care, and community attitudes about ART in rural KwaZulu-Natal, South Africa: Descriptive results from the first phase of the ANRS 12249 TasP cluster-randomised trial. PLoS Medicine, 13(8), e1002107. https://doi.org/10.1371/journal.pmed.1002107
- Johnson, V. D. (2007). Promoting behavior change: Making healthy choices in wellness and healing choices in illness—Use of self-determination theory in nursing practice. Nursing Clinics of North America, 42(2), 229–241. https://doi.org/10.1016/j.cnur.2007.02.003
- Johnston, M. M., & Finney, S. J. (2010). Measuring basic needs satisfaction: Evaluating previous research and conducting new psychometric evaluations of the basic needs satisfaction in General scale. Contemporary Educational Psychology, 35(4), 280–296. https://doi.org/10.1016/j.cedpsych.2010.04.003
- Krause, J., Subklew-Sehume, F., Kenyon, C., & Colebunders, R. (2013). Acceptability of HIV self-testing: A systematic literature review. BMC Public Health, 13(1), 735. https://doi.org/10.1186/1471-2458-13-735
- Li, H., Wei, C., Tucker, J., Kang, D., Liao, M., Holroyd, E., Zheng, J., Qi, Q., & Ma, W. (2017). Barriers and facilitators of linkage to HIV care among HIV-infected young Chinese men who have sex with men: A qualitative study. BMC Health Services Research, 17(1), 214. https://doi.org/10.1186/s12913-017-2158-7
- Liu, Y., Osborn, C. Y., Qian, H.-Z., Yin, L., Xiao, D., Ruan, Y., Simoni, J. M., Zhang, X., Shao, Y., Vermund, S. H., & Amico, K. R. (2016). Barriers and facilitators of linkage to and engagement in HIV care among HIV-positive men who have sex with men in China: A qualitative study. AIDS Patient Care and STDs, 30(2), 70–77. https://doi.org/10.1089/apc.2015.0296
- Mathenjwa, T., Kim, H.-Y., Zuma, T., Shahmanesh, M., Seeley, J., Matthews, P., Wyke, S., McGrath, N., Sartorius, B., Yapa, H. M., Adeagbo, O., Blandford, A., Dobra, A., Bäernighausen, T., & Tanser, F. (2019). Home-based intervention to test and start (HITS) protocol: A cluster-randomized controlled trial to reduce HIV-related mortality in men and HIV incidence in women through increased coverage of HIV treatment. BMC Public Health, 19(1), 969. https://doi.org/10.1186/s12889-019-7277-0
- Morse, D. S., Schiff, M., Levit, S., Cohen-Moreno, R., Williams, G. C., & Neumark, Y. (2012). A pilot training program for a motivational enhancement approach to hepatitis C virus treatment among individuals in Israeli methadone treatment centers. Substance Use & Misuse, 47(1), 56–66. https://doi.org/10.3109/10826084.2011.628735
- Nakanwagi, S., Matovu, J. K., Kintu, B. N., Kaharuza, F., & Wanyenze, R. K. (2016). Facilitators and barriers to linkage to HIV care among female sex workers receiving hiv testing services at a community-based organization in Periurban Uganda: A qualitative study. Journal of Sexually Transmitted Diseases, 2016, 1–8. https://doi.org/10.1155/2016/7673014
- Nhassengo, P., Cataldo, F., Magaço, A., Hoffman, R. M., Nerua, L., Saide, M., Cuco, R., Hoek, R., Mbofana, F., Couto, A., Gudo, E., Chicumbe, S., Dovel, K., & Garcia, J. (2018). Barriers and facilitators to the uptake of test and Treat in Mozambique: A qualitative study on patient and provider perceptions. PloS one, 13(12), e0205919. https://doi.org/10.1371/journal.pone.0205919
- Quinlivan, E. B., Messer, L. C., Adimora, A. A., Roytburd, K., Bowditch, N., Parnell, H., Seay, J., Bell, L., & Pierce, J. K. (2013). Experiences with HIV testing, entry, and engagement in care by HIV-infected women of color, and the need for autonomy, competency, and relatedness. AIDS Patient Care and STDs, 27(7), 408–415. https://doi.org/10.1089/apc.2012.0434
- Quinlivan, E., Messer, L. C., Roytburd, K., & Blickman, A. (2017). Unmet core needs for self-determination in HIV-infected women of color in medical care. AIDS Care, 29(5), 603–611. https://doi.org/10.1080/09540121.2016.1243788
- Rawat, S., Wilkerson, J. M., Lawler, S. M., Patankar, P., Rosser, B. S., Shukla, K., Butame, S., & Ekstrand, M. L. (2018). Recommendations for the development of a mobile HIV prevention intervention for men who have sex with men and Hijras in Mumbai: Qualitative study. JMIR Public Health and Surveillance, 4(2), e46. https://doi.org/10.2196/publichealth.9088
- Ryan, R. M., & Deci, E. L. (2000). Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American Psychologist, 55(1), 68–78. https://doi.org/10.1037/0003-066X.55.1.68
- Tanser, F., Bärnighausen, T., Grapsa, E., Zaidi, J., & Newell, M.-L. (2013). High Coverage of ART associated with Decline in Risk of HIV Acquisition in rural KwaZulu-Natal, South Africa. Science, 339(6122), 966–971. https://doi.org/10.1126/science.1228160
- Tanser, F., Hae-Young, K., Mathenjwa, T., Shahmanesh, M., Seeley, J., Matthews, P., … Barnighausen, T. (2019.
- Toth, M., Messer, L. C., & Quinlivan, E. B. (2013). Barriers to HIV care for women of color living in the Southeastern US are associated with physical symptoms, social environment, and self-determination. AIDS Patient Care and STDs, 27(11), 613–620. https://doi.org/10.1089/apc.2013.0030
- UNAIDS. (2017). Blind Spot - Reaching Out to Men and Boys: Addressing a Blind Spot in the Response to HIV. http://www.unaids.org/sites/default/files/media_asset/blind_spot_en.pdf
- WHO. (2011). The Ottawa Charter for Health Promotion. https://www.who.int/healthpromotion/conferences/previous/ottawa/en/
- Williams, G. C., Patrick, H., Niemiec, C. P., Ryan, R. M., Deci, E. L., & Lavigne, H. M. (2011). The smoker's health project: A self-determination theory intervention to facilitate maintenance of tobacco abstinence. Contemporary Clinical Trials, 32(4), 535–543. https://doi.org/10.1016/j.cct.2011.03.002
- Wood, C. S., Thomas, M. R., Budd, J., Mashamba-Thompson, T. P., Herbst, K., Pillay, D., … Stevens, M. M. (2019). Taking connected mobile-health diagnostics of infectious diseases to the field. Nature, 566(7745), 467–474. https://doi.org/10.1038/s41586-019-0956-2
- Yardley, L., Morrison, L., Bradbury, K., & Muller, I. (2015). The person-based approach to intervention development: Application to digital health-related behavior change interventions. Journal of Medical Internet Research, 17(1), e30. https://doi.org/10.2196/jmir.4055
- Zhao, Y., Zhu, X., Pérez, A. E., Zhang, W., Shi, A., Zhang, Z., Gao, P., Wang, J., Yang, C., Zaller, N., Sun, Y., Operario, D., & Zhang, H. (2018). MHealth approach to promote Oral HIV self-testing among men who have sex with men in China: A qualitative description. BMC Public Health, 18(1), 1146. https://doi.org/10.1186/s12889-018-6046-9