ABSTRACT
HIV remains elevated among female sex workers (FSW) globally, with a number of structural (e.g., poverty, access to care) factors driving these persistently high rates. Pre-exposure prophylaxis (PrEP), a user-controlled prevention method, is a promising means of empowering vulnerable populations to protect themselves and enhance agency. Yet there is a dearth of PrEP research and interventions targeting cisgender women in the United States, and even fewer aimed to reach FSW. We developed and implemented a multifaceted PrEP pilot intervention, the Promoting Empowerment And Risk Reduction (PEARL) study, to meet this gap. This paper describes the development process and nature of a community-informed intervention for tenofovir/emticitrabine (TDF/FTC) pre-exposure prophylaxis engagement among street-based cisgender FSW in Baltimore, Maryland, U.S. In the course of the study’s implementation, structural, programmatic, and medical barriers have already posed significant barriers to full engagement. PEARL implemented a number of strategies in an effort to counter barriers and facilitate increased success of PrEP uptake and maintenance. The study will provide critical insights into the nature of intervention components that could help FSW to initiate PrEP and reduce PrEP care cascade gaps.
Introduction
HIV rates have been elevated among female sex workers (FSW) globally for over three decades. It is estimated that cisgender women who exchange sex for money, drugs, or goods have 14 times the risk of HIV infection as compared to cisgender women who do not exchange sex (Baral et al., Citation2012). Among the few studies of HIV among FSW in the U.S., HIV prevalence estimates have ranged 3.7%–10.9%, yet few prevention efforts have targeted this population (Miles et al., Citation2013; Parvez et al., Citation2013; Raifman & Sherman, Citation2018; Sherman et al., Citation2019; Tomko et al., Citation2019). Sex work criminalization exacerbates FSWs’ HIV risk through a complex array of socio-structural vulnerabilities (e.g., poverty, housing instability, stigma, egregious police behaviors) that shape risk behaviors (e.g., unprotected sex, high-risk sex partners) as well as foster violence against women, problematic substance use, and poor health outcomes (Abad et al., Citation2015; Buttram et al., Citation2014; Campbell & Kinnell, Citation2000; Decker et al., Citation2017; Footer et al., Citation2016, Citation2019; Goldenberg et al., Citation2015; Shannon et al., Citation2008; Sherman et al., Citation2019; Surratt et al., Citation2012). Tailored PrEP interventions are needed for FSW.
Pre-exposure prophylaxis (PrEP) is a user-controlled HIV prevention method that offers a means of empowering individuals to protect themselves from HIV even in the context of enduring socio-structural vulnerability (Abdool Karim et al., Citation2012; Glick et al., Citation2019). Four clinical trials that included women demonstrated reductions in HIV acquisition by 49%–79% in intent-to-treat analyses (Baeten et al., Citation2012; Choopanya et al., Citation2013; Donnell et al., Citation2014; Marrazzo et al., Citation2013; Murnane et al., Citation2013; Thigpen et al., Citation2012). Two other large trials ended early due to suboptimal adherence, highlighting the need to support adherence in future interventions (Van Damme et al., Citation2012; Van Der Straten et al., Citation2016).
Studies have found high PrEP interest and ease in taking a pill daily amongst FSW (Eakle et al., Citation2018; Garfinkel et al., Citation2017; Glick et al., Citation2019; Koechlin et al., Citation2017; Peitzmeier et al., Citation2017; Shea et al., Citation2019; Tomko et al., Citation2019). In one Baltimore-based study, while only 20% of FSW had heard of PrEP, the vast majority (74%) were interested upon learning about it (Tomko et al., Citation2019). While domestic and international PrEP policy recommendations have resulted in increased FSWs’ PrEP engagement (Cowan et al., Citation2018; Eakle et al., Citation2017), little has been done in the U.S. to include FSW in current PrEP initiatives (Raifman & Sherman, Citation2018; WHO, Citation2015).
In response to this gap, we developed and delivered a multifaceted PrEP intervention, the Promoting Empowerment And Risk Reduction (PEARL) study. PEARL had three aims: (1) to employ community-engaged formative research to inform the development of an intervention tailored for cisgender FSW (hereafter referred to as “FSW”); (2) to assess PEARL’s acceptability, feasibility, and preliminary efficacy on PrEP uptake and adherence; and (3) to explore patterns and predictors of PrEP adherence. Here, we outline PEARL study protocols and explore emergent challenges to study implementation to inform future PrEP interventions among this understudied population.
Methods
Study design
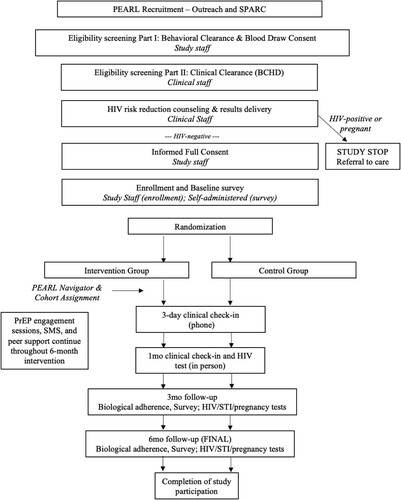
To optimize the relevance and effectiveness of the study (Aim 1), the intervention was informed by systematic literature reviews, (Glick et al., Citation2019, Citation2020) focus groups with FSW, and key informant interviews with providers. Incorporating these perspectives enhanced the relevance of PEARL to the needs of the target population. Aim 2 involved intervention and retention components (daily text message reminders, peer navigation, and small group sessions), and assessed the intervention’s feasibility, acceptability, and preliminary efficacy on PrEP uptake and adherence among intervention versus control participants at 6-month follow-up. Aim 3 explored correlates (e.g., homelessness, violence) of PEARL’s efficacy among intervention participants on drug adherence measured at baseline, 3, and 6 months by plasma and dried blood spots (DBS). The study was reviewed and approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Setting and participants
In June 2019, PEARL was implemented at the SPARC (Sex Workers Promoting Action, Risk Reduction, and Community Mobilization) Center, a harm reduction drop-in center that provides low-barrier medical (e.g., reproductive health, STI clinic, wound care), social, legal, and drop-in services at no cost. While SPARC is open to anyone who does not identify as a man, services are specifically promoted to women who use drugs and/or trade sex in Baltimore. Locating PEARL at SPARC provided additional opportunities to encounter participants in person, provide adherence counseling, and remind participants about PEARL study visits.
Recruitment
Recruitment occurred from June 2019 to March 2020 at SPARC and on foot and mobile outreach, offering bus tokens to those in need. Participants were also recruited through promotional flyers at partnering organizations and relevant research studies. Due to shut-down mandates from the Maryland Governor in March 2020, all research activities were suspended.
Inclusion and exclusion criteria
Women were screened using a brief socio-demographic questionnaire and clinical testing. Eligibility criteria were: (1) age 18+; (2) cisgender woman; (3) HIV negative; (4) not pregnant or planning to become pregnant in the next 6 months (per BCHD policy); and (5) traded sex for money or drugs at least three times in the past three months. Women were excluded if they were: (1) taking PrEP; (2) did not agree to share data with the BCHD; and (c) unable to provide informed consent (e.g., language barriers, cognitive impairment). We initially excluded women if they did not own a cell phone; however, this criterion was a significant recruitment barrier and was thus lifted to recruit a more representative sample of FSWs.
Screening
Enrollment was conducted at SPARC two days a week, five hours a day. Interested women met with study staff in a private office to learn about the study and if interested, be screened for study inclusion. If tentatively eligible, participants consented to health screening, which included blood draws and test results sharing with BCHD.
Potential participants then met with a Certified Registered Nurse Practitioner (CRNP) as part of standard BCHD protocol, which included but was not limited to: (1) pre-test HIV counseling; (2) a rapid HIV Ab/Ag screening test; (3) bloodwork including a complete metabolic panel and hepatitis B serology; (4) a pregnancy test, and (5) HIV and pregnancy test results. All potential participants were offered STI and hepatitis C virus screening. Counseling, treatment, and linkage to care was offered to participants with positive or abnormal results. If a woman was HIV negative and not pregnant, she was offered PEARL enrollment and consented if interested.
Once enrolled, the CRNP provided medication and adherence counseling, reviewed the follow-up plan, and provided a 30-day prescription for TDF/FTC so the participant was able to start PrEP the same day as study enrollment while waiting for lab results. When lab results returned, the CRNP contacted the participant to review results and provided counseling on continuation, or if medically necessary, discontinuation of PrEP and linkage to care. Participants received prescriptions at a pharmacy of their choice and were informed of a partnered pharmacy within walking distance that covered co-pays.
Randomization and control condition
Participants were randomized to control and intervention after completion of the baseline survey. The control condition was “standard of care” comprised of HIV risk reduction counseling and PrEP referral. All participants were able to receive PrEP standard of care with a CRNP using Baltimore City Health Department (BCHD) standard guidelines for PrEP care. An overview of the study design is shown in ; intervention components are described below.
Intervention
The intervention arm included control conditions and several components that have proved effective among other populations but have yet to be piloted among FSWs: peer navigators, mHealth, and small group sessions.
Peer navigators
Peer navigators (PNs) were selected based on FSW preferences (e.g., shared lived experience, respectful demeanor), uncovered through formative research. Peers are defined here as individuals with relevant lived experience who could empathize with experiences of stigma, marginalization, drug use, and HIV risk. Previous research has demonstrated that PNs can be instrumental to connect underserved populations to such resources as health education, harm reduction programs (i.e., syringe services programs (SSPs)), and linkage to care (e.g., HIV, HCV) (Cunningham et al., Citation2018; Latkin, Citation1998; Latkin et al., Citation2003; Mayer et al., Citation2018; Purcell et al., Citation2007; Tobin et al., Citation2011; Weeks et al., Citation2009). PNs were matched with participants at enrollment and supported them throughout their intervention engagement. PNs provided: face-to-face and phone counseling; appointment reminders; service coordination; referrals; harm reduction supplies, and accompanying participants to medical and social service appointments at the study location and elsewhere.
Mhealth
mHealth is the use of mobile and wireless technologies to support health promotion and has been used with numerous populations, yet use with FSW is limited (Catalani et al., Citation2013; Cooper et al., Citation2017). We designed a two-way text messaging system that sent automated daily messages, including appointment and daily medication reminders for intervention participants with cell phones. Intervention participants also received weekly messages requesting their self-reported PrEP adherence. PNs helped participants without phones apply for one through a free government cell phone program. Study participation was not predicated on cell phone ownership.
Small group sessions
We developed four 2-hour guided sessions to foster a sense of community and supportive norms around PrEP uptake and adherence, emphasizing the self-care and empowerment potential PrEP affords. Sessions positioned PrEP in a broader framework of access and barriers to care, provider/patient communication, sexual health, HIV/STI education, harm reduction, stigma, and health/science literacy. The bi-weekly sessions occurred at the SPARC Center and were co-led by the PNs, CRNP, and study coordinator. Lunch was provided and participants were compensated $25 per session. Participants were encouraged to attend four sessions and received a certificate of completion upon doing so.
Data collection
PEARL consisted of baseline, 3-, and 6-month study visits, each of which included a survey, bloodwork, case management services, and compensation for control and intervention participants. Baseline visits were the most intensive and lasted approximately 2 h. The audio computer-assisted self-interview (A-CASI) survey lasted 45–60 min. Survey items, informed by the literature and our prior research, included: socio-demographics and structural vulnerabilities; health service access and utilization; HIV/STI history and risk behaviors; PrEP use, interest, barriers, and facilitators; sex work history; sexual and physical violence; arrest and prison history; stigma; police encounters; social support; resilience; and mental health history (Decker et al., Citation2017; Sherman et al., Citation2019). A whole blood sample was collected at baseline and both follow-up visits for tenofovir diphosphate testing through dried blood spot and plasma, conducted at the Hopkins’ Clinical Pharmacology Analytical Laboratory. At the last study visit, intervention participants were invited to participate in a 45-minute qualitative interview led by an experienced ethnographer to discuss PrEP and the acceptability of study content and delivery. These interviews gauged participants’ exposure to and perceptions of novel intervention components (e.g., mHealth, small group sessions, PNs).
Participant incentives
If participants completed the initial screener but were ineligible due to HIV or pregnancy status, they received a $15 gift card for their time. Participants received $50 for their baseline visit, $25 for their 1- and 3-month visits, and $50 for their 6-month visit. Intervention participants who attended the small group sessions received $25 for each session. Intervention participants who participated in qualitative interviews after study completion received an additional $25.
Results
Recruitment and enrollment
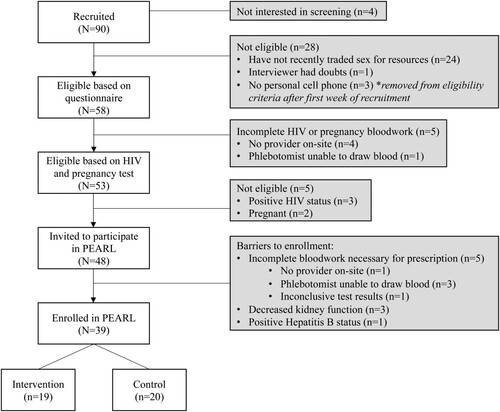
A total of 90 FSWs were recruited between June 2019 to March 2020, at which point the COVID-19 pandemic disrupted recruitment and data collection. 43% (N = 39) of the women recruited were enrolled. A CONSORT diagram () describes the flow of FSWs from recruitment to enrollment.
Two-thirds of those screened met all eligibility criteria. 28% had not recently traded sex and 6% were pregnant or living with HIV, thus excluding them from participation. Five women were lost to follow-up before health eligibility could be established as the on-site provider was unavailable or unable to draw blood, so women were directed to an off-site blood draw location but never returned to complete enrollment.
Forty-eight eligible women were invited to participate in PEARL and underwent further bloodwork to determine PrEP candidacy. Again, 10% of eligible women were lost to follow-up due to inability to draw blood on-site the same day. Three of the women who completed the necessary bloodwork were found to have decreased kidney function that needed to be addressed before the CRNP could prescribe PrEP. None of these women returned to re-enroll. Ultimately, 39 women were enrolled in PEARL and randomized into intervention (n = 19) and control groups (n = 20). The study was not powered to detect differences in drug adherence between the two arms, yet the smaller than expected sample size limited a modest examination of the intervention’s impact.
Baseline socio-demographics
Baseline socio-demographic information can be seen in . The study sample contained a balance of non-Hispanic Black and White women and had a median age of 40 (interquartile range: 33–48). Most participants reported recent drug use (97%). Participants lived in a median of four places and 92% experienced homelessness in the past six months. Analyses of relevant demographic data show that there are very few significant differences between the intervention and control groups.
Table 1. Descriptive statistics of socio-demographic information for participants (N = 39).
Challenges to implementation
Substantial barriers impacted the ease of PEARL recruitment, efficacy of intervention components (mHealth, small group sessions, PNs), and the study’s ability to quickly adapt to unexpected challenges in real time. PEARL implementation was hindered by structural constraints beyond individual control, medical complications and mistrust, and programmatic hurdles inherent in the system itself. By discussing these obstacles, we aim to better inform future interventions to promote PrEP uptake and adherence among FSWs.
Structural barriers, including unstable transportation, criminalization, and financial constraints reduced participation over time. Intermittent phone access and housing instability challenged follow-up and PN’s ability to support adherence. Several participants had their medication stolen or lost. Although SPARC offered access to secure lockers for client use, lockers were unavailable during evenings and weekends. Further, follow-up was challenged by participants moving, becoming incarcerated, or entering inpatient rehab.
There were significant barriers to obtaining the necessary bloodwork and samples for PrEP prescription and maintenance. Many participants had limited and complex vasculature (e.g., collapsed and inaccessible veins) due to long-term injection drug use, dehydration, and malnourishment that made it difficult to draw blood. Some participants were withdrawn from the study for various clinical reasons, including decreased kidney function (n = 3), positive HIV status (n = 3), and positive Hepatitis B status, per BCHD policy (n = 1). Some participants reported side effects with taking TDF/FTC, such as nausea and headache, which resulted in skipping pills or stopping the medication. Moreover, medical mistrust and concern regarding the class action lawsuit against Gilead regarding TDF/FTC obstructed PrEP uptake among some participants.
Efforts to overcome these structural and medical challenges, discussed in full below, resulted in additional programmatic barriers. Though partnerships with BCHD and the lab enabled the provision of more comprehensive health services, emergent and necessary changes to study procedures (e.g., distributing bus tokens) were impeded by the extensive internal approval processes and memorandums of understanding associated with collaboration.
Given provider availability, PEARL recruitment and follow-up was limited to two days a week with one phlebotomist and one CRNP who could prescribe PrEP, providing a narrow window for study activities. When the phlebotomist or CRNP were unavailable, recruitment paused. Furthermore, the phlebotomist was newly certified at study commencement and was challenged by many “hard sticks” due to the compromised vasculature of many of the participants discussed above.
Overcoming implementation challenges
PEARL implemented various strategies to counter these barriers and to facilitate PrEP uptake and maintenance among FSWs in real time. For example, we began issuing bus tokens to address transportation barriers. Payment for various study components motivated participation, yet many obstacles persisted.
To counter blood draw issues, the phlebotomist continuously worked to hone her skills through continuing education training and extensive mentorship and guidance from phlebotomists at BCHD. Further, she utilized single use heating packs and an LED vein finder. When the phlebotomist was unable to successfully perform venipuncture, participants were brought to an outside facility to more experienced phlebotomists. Additionally, SPARC had signage in the waiting room encouraging clients to drink water. SPARC also provided electrolyte replacement fluids and protein shakes as needed. SPARC clients could receive daily snacks, weekly emergency food bags, and weekly fresh vegetables, all of which helped counter malnourishment and made it easier to access veins and, therefore, improve PrEP persistence and study engagement.
To counter medical barriers, all participants who reported side effects were offered countermeasures, including over-the-counter analgesics and prescription anti-nausea medication. Medical concerns were mitigated by counseling at baseline on the basic pharmacology, safety, efficacy, side effects, and box warnings of TDF/FTC for all participants. All SPARC staff were informed about the latest PrEP research and were able to dispel myths about the class action lawsuit against Gilead and offer guidance about side effects (e.g., nausea and headache are temporary and do not pose a serious risk, over-the-counter medications can help mitigate side effects). The CRNP was involved in training staff and met with participants regularly to answer any questions.
Discussion
PEARL was the first randomized pilot intervention aimed to develop a comprehensive PrEP adherence program and to examine its impact of PrEP uptake against standard of care among FSW in the US. It was informed by extensive formative research to build a nuanced approach to support PrEP adherence and persistence (Glick et al., Citation2020; Tomko et al., Citation2019). The study provided critical insights into the nature of novel intervention components (i.e., mHealth, small group sessions, PNs) that could help FSWs to initiate PrEP and reduce PrEP care cascade gaps. The PEARL intervention targeted adherence and focused on bolstering women’s peer support and provider engagement, which could substantially strengthen PrEP efforts within the FSW community in Baltimore and elsewhere. Results from PEARL will inform future integrated interventions targeting similar populations.
PrEP care at SPARC provided FSWs an opportunity to positively engage with the healthcare that was tailored to their needs in an affirming space (Cowan & Delany-Moretlwe, Citation2016; Ortblad & Oldenburg, Citation2018). In addition to existing legal and social services at SPARC, PEARL’s partnership with BCHD expanded clinical services beyond PrEP and included STI screening and treatment, HIV testing and linkage to care, expanded contraception services, wound care, and HCV treatment. These services improved the health and wellbeing of FSWs and also supported participant recruitment and retention as participants visited SPARC for a range of health and social services regularly, not solely for PrEP. However, this pilot intervention revealed challenges to developing and implementing PrEP interventions for FSW.
Challenges to recruitment and follow-up predated the COVID-19 pandemic; however, COVID-19 and mandated shutdowns aggravated existing vulnerabilities in this population and disrupted access to essential services (e.g., increased arrests, fines, accessing health services) (Platt et al., Citation2020). SPARC was forced to temporarily restrict access to on-site resources and reduce the number of services provided to reduce the risk of COVID-19 infection for staff and clients, while expanding the outreach program. While this disruption hindered PEARL’s progress and impact, it also underscores the precarity of service access for FSWs, including PrEP, and the need for tailored interventions. The experiences of implementing this pilot intervention highlight the importance of flexibility as interventions identify barriers and adapt in real time to better reach FSWs and reduce HIV transmission worldwide.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abad, N., Baack, B. N., O’Leary, A., Mizuno, Y., Herbst, J. H., & Lyles, C. M. (2015). A systematic review of HIV and STI behavior change interventions for female sex workers in the United States. AIDS and Behavior, 19, 1701–1719. https://doi.org/https://doi.org/10.1007/s10461-015-1013-2
- Abdool Karim, Q., Humphries, H., & Stein, Z. (2012). Empowering women in human immunodeficiency virus prevention. Best Practice and Research: Clinical Obstetrics and Gynaecology, 26, 487–493. https://doi.org/https://doi.org/10.1016/j.bpobgyn.2012.01.006
- Baeten, J. M., Donnell, D., Ndase, P., Mugo, N. R., Campbell, J. D., Wangisi, J., Tappero, J. W., Bukusi, E. A., Cohen, C. R., Katabira, E., Ronald, A., Tumwesigye, E., Were, E., Fife, K. H., Kiarie, J., Farquhar, C., John-Stewart, G., Kakia, A., Odoyo, J., … Celum, C. (2012). Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. New England Journal of Medicine, 367, 399–410. https://doi.org/https://doi.org/10.1056/NEJMoa1108524
- Baral, S., Beyrer, C., Muessig, K., Poteat, T., Wirtz, A. L., Decker, M. R., Sherman, S. G., & Kerrigan, D. (2012). Burden of HIV among female sex workers in low-income and middle-income countries: A systematic review and meta-analysis. The Lancet Infectious Diseases, 12, 538–549. https://doi.org/https://doi.org/10.1016/S1473-3099(12)70066-X
- Buttram, M. E., Surratt, H. L., & Kurtz, S. P. (2014). Resilience and syndemic risk factors among African-American female sex workers. Psychology, Health and Medicine, 19, 442–452. https://doi.org/https://doi.org/10.1080/13548506.2013.824595
- Campbell, R., & Kinnell, H. (2000). “We shouldn’t have to put up with this”: Street sex work and violence. Criminal Justice Matters, 42, 12–13. https://doi.org/https://doi.org/10.1080/09627250008552877
- Catalani, C., Philbrick, W., Fraser, H., Mechael, P., & Israelski, D. M. (2013). Mhealth for HIV treatment & prevention: A systematic review of the literature. The Open AIDS Journal, 7, 17–41. https://doi.org/https://doi.org/10.2174/1874613620130812003
- Choopanya, K., Martin, M., Suntharasamai, P., Sangkum, U., Mock, P. A., Leethochawalit, M., Chiamwongpaet, S., Kitisin, P., Natrujirote, P., Kittimunkong, S., Chuachoowong, R., Gvetadze, R. J., McNicholl, J. M., Paxton, L. A., Curlin, M. E., Hendrix, C. W., & Vanichseni, S. (2013). Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir study): A randomised, double-blind, placebo-controlled phase 3 trial. The Lancet, 381, 2083–2090. https://doi.org/https://doi.org/10.1016/S0140-6736(13)61127-7
- Cooper, V., Clatworthy, J., Whetham, J., & Consortium, E. (2017). Mhealth interventions to support self-management in HIV: A systematic review. The Open AIDS Journal, 11, 119–132. https://doi.org/https://doi.org/10.2174/1874613601711010119
- Cowan, F. M., Davey, C., Fearon, E., Mushati, P., Dirawo, J., Chabata, S., Cambiano, V., Napierala, S., Hanisch, D., Wong-Gruenwald, R., Masuka, N., Mabugo, T., Hatzold, K., Mugurungi, O., Busza, J., Phillips, A., & Hargreaves, J. R. (2018). Targeted combination prevention to support female sex workers in Zimbabwe accessing and adhering to antiretrovirals for treatment and prevention of HIV (SAPPH-IRe): A cluster-randomised trial. The Lancet HIV, 5, e417–e426. https://doi.org/https://doi.org/10.1016/S2352-3018(18)30111-5
- Cowan, F. M., & Delany-Moretlwe, S. (2016). Promise and pitfalls of pre-exposure prophylaxis for female sex workers. Current Opinion in HIV and AIDS, 11, 27–34. https://doi.org/https://doi.org/10.1097/COH.0000000000000215
- Cunningham, W. E., Weiss, R. E., Nakazono, T., Malek, M. A., Shoptaw, S. J., Ettner, S. L., & Harawa, N. T. (2018). Effectiveness of a peer navigation intervention to sustain viral suppression among HIV-positive men and transgender women released from jail the LINK la randomized clinical trial. JAMA Internal Medicine, 178, 542. https://doi.org/https://doi.org/10.1001/jamainternmed.2018.0150
- Decker, M. R., Nail, J. E., Lim, S., Footer, K., Davis, W., & Sherman, S. G. (2017). Client and partner violence among urban female exotic dancers and intentions for seeking support and justice. Journal of Urban Health, 94, 637–647. https://doi.org/https://doi.org/10.1007/s11524-017-0195-5
- Donnell, D., Baeten, J. M., Bumpus, N. N., Brantley, J., Bangsberg, D. R., Haberer, J. E., Mujugira, A., Mugo, N., Ndase, P., Hendrix, C., & Celum, C. (2014). HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. JAIDS Journal of Acquired Immune Deficiency Syndromes, 66, 340–348. https://doi.org/https://doi.org/10.1097/QAI.0000000000000172
- Eakle, R., Bourne, A., Mbogua, J., Mutanha, N., & Rees, H. (2018). Exploring acceptability of oral PrEP prior to implementation among female sex workers in South Africa. Journal of the International AIDS Society, 21, e25081. https://doi.org/https://doi.org/10.1002/jia2.25081
- Eakle, R., Gomez, G. B., Naicker, N., Bothma, R., Mbogua, J., Cabrera Escobar, M. A., Saayman, E., Moorhouse, M., Venter, W. D. F., & Rees, H. (2017). HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project. PLoS Medicine, 14, e1002444. https://doi.org/https://doi.org/10.1371/journal.pmed.1002444
- Footer, K. H. A., Park, J. N., Allen, S. T., Decker, M. R., Silberzahn, B. E., Huettner, S., Galai, N., & Sherman, S. G. (2019). Police-related correlates of client-perpetrated violence among female sex workers in Baltimore City, Maryland. American Journal of Public Health, 109, 289–295. https://doi.org/https://doi.org/10.2105/AJPH.2018.304809
- Footer, K. H. A., Silberzahn, B. E., Tormohlen, K. N., & Sherman, S. G. (2016). Policing practices as a structural determinant for HIV among sex workers: A systematic review of empirical findings. Journal of the International AIDS Society, 19, 20883. https://doi.org/https://doi.org/10.7448/IAS.19.4.20883
- Garfinkel, D. B., Alexander, K. A., McDonald-Mosley, R., Willie, T. C., & Decker, M. R. (2017). Predictors of HIV-related risk perception and PrEP acceptability among young adult female family planning patients. AIDS Care – Psychological and Socio-Medical Aspects of AIDS/HIV, 29, 751–758. https://doi.org/https://doi.org/10.1080/09540121.2016.1234679
- Glick, J. L., Huang, A., Russo, R., Jivapong, B., Ramasamy, V., Rosman, L., Pelaez, D., Footer, K. H. A., & Sherman, S. G. (2020). ART uptake and adherence among women who use drugs globally: A scoping review. Drug and Alcohol Dependence, 215, 108218. https://doi.org/https://doi.org/10.1016/j.drugalcdep.2020.108218
- Glick, J. L., Russo, R., Jivapong, B., Rosman, L., Pelaez, D., Footer, K. H. A., & Sherman, S. G. (2019). The PrEP care continuum among cisgender women who sell sex and/or use drugs globally: A systematic review. AIDS and Behavior, 24, 1312–1333. https://doi.org/https://doi.org/10.1007/s10461-019-02733-z
- Goldenberg, S. M., Duff, P., & Krusi, A. (2015). Work environments and HIV prevention: A qualitative review and meta-synthesis of sex worker narratives. BMC Public Health, 15. https://doi.org/https://doi.org/10.1186/s12889-015-2491-x
- Koechlin, F. M., Fonner, V. A., Dalglish, S. L., O’Reilly, K. R., Baggaley, R., Grant, R. M., Rodolph, M., Hodges-Mameletzis, I., & Kennedy, C. E. (2017). Values and preferences on the use of oral pre-exposure prophylaxis (PrEP) for HIV prevention among multiple populations: A systematic review of the literature. AIDS and Behavior, 21, 1325–1335. https://doi.org/https://doi.org/10.1007/s10461-016-1627-z
- Latkin, C. A. (1998). Outreach in natural settings: The use of peer leaders for HIV prevention among injecting drug users’ networks. Public Health Reports, 113, 151–159.
- Latkin, C. A., Sherman, S., & Knowlton, A. (2003). HIV prevention among drug users: Outcome of a network-oriented peer outreach intervention. Health Psychology, 22, 332–339. https://doi.org/https://doi.org/10.1037/0278-6133.22.4.332
- Marrazzo, J. M., Ramjee, G., Nair, G., Palanee, T., Mkhize, B., Nakabiito, C., Taljaard, M., Piper, J., Gomez, K., & Chirenje, M. (2013). In Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE study (MTN 003) (pp. 3–6). 20th conference on retroviruses and opportunistic infections.
- Mayer, K. H., Chan, P. A., Patel, R., Flash, C. A., & Krakower, D. S. (2018). Evolving models and ongoing challenges for HIV preexposure prophylaxis implementation in the United States. JAIDS Journal of Acquired Immune Deficiency Syndromes, 77, 119–127. https://doi.org/https://doi.org/10.1097/QAI.0000000000001579
- Miles, I. J., Le, B. C., Wejnert, C., Oster, A., DiNenno, E., & Paz-Bailey, G. (2013). HIV infection among heterosexuals at increased risk – United States, 2010. Morbidity and Mortality Weekly Report, 62(10), 183–188.
- Murnane, P. M., Celum, C., Mugo, N., Campbell, J. D., Donnell, D., Bukusi, E., Mujugira, A., Tappero, J., Kahle, E. M., Thomas, K. K., & Baeten, J. M. (2013). Efficacy of preexposure prophylaxis for HIV-1 prevention among high-risk heterosexuals: Subgroup analyses from a randomized trial. Aids (London, England), 27, 2155–2160. https://doi.org/https://doi.org/10.1097/QAD.0b013e3283629037
- Ortblad, K. F., & Oldenburg, C. E. (2018). Tailoring combination HIV prevention for female sex workers. The Lancet HIV, 5, e406–e407. https://doi.org/https://doi.org/10.1016/S2352-3018(18)30136-X
- Parvez, F., Katyal, M., Alper, H., Leibowitz, R., & Venters, H. (2013). Female sex workers incarcerated in New York City jails: Prevalence of sexually transmitted infections and associated risk behaviors. Sexually Transmitted Infections, 89, 280–284. https://doi.org/https://doi.org/10.1136/sextrans-2012-050977
- Peitzmeier, S. M., Tomko, C., Wingo, E., Sawyer, A., Sherman, S. G., Glass, N., Beyrer, C., & Decker, M. R. (2017). Acceptability of microbicidal vaginal rings and oral pre-exposure prophylaxis for HIV prevention among female sex workers in a high-prevalence US city. AIDS Care – Psychological and Socio-Medical Aspects of AIDS/HIV, 29, 1453–1457. https://doi.org/https://doi.org/10.1080/09540121.2017.1300628
- Platt, L., Elmes, J., Stevenson, L., Holt, V., Rolles, S., & Stuart, R. (2020). Sex workers must not be forgotten in the COVID-19 response. The Lancet, 396, 9–11. https://doi.org/https://doi.org/10.1016/S0140-6736(20)31033-3
- Purcell, D. W., Latka, M. H., Metsch, L. R., Latkin, C. A., Gómez, C. A., Mizuno, Y., Arnsten, J. H., Wilkinson, J. D., Knight, K. R., Knowlton, A. R., Santibanez, S., Tobin, K. E., Rose, C. D., Valverde, E. E., Gourevitch, M. N., Eldred, L., & Borkowf, C. B. (2007). Results from a randomized controlled trial of a peer-mentoring intervention to reduce HIV transmission and increase access to care and adherence to HIV medications among HIV-seropositive injection drug users. JAIDS Journal of Acquired Immune Deficiency Syndromes, 46, S35–S47. https://doi.org/https://doi.org/10.1097/QAI.0b013e31815767c4
- Raifman, J., & Sherman, S. G. (2018). US guidelines that empower women to prevent HIV with preexposure prophylaxis. Sexually Transmitted Diseases, 45, e38–e39. https://doi.org/https://doi.org/10.1097/OLQ.0000000000000811
- Shannon, K., Kerr, T., Allinott, S., Chettiar, J., Shoveller, J., & Tyndall, M. W. (2008). Social and structural violence and power relations in mitigating HIV risk of drug-using women in survival sex work. Social Science and Medicine, 66, 911–921. https://doi.org/https://doi.org/10.1016/j.socscimed.2007.11.008
- Shea, J., Bula, A., Dunda, W., Hosseinipour, M. C., Golin, C. E., Hoffman, I. F., Miller, W. C., Go, V. F., Lungu, T., & Lancaster, K. E. (2019). “The drug will help protect my tomorrow”: Perceptions of integrating prep into HIV prevention behaviors among female sex workers in Lilongwe, Malawi. AIDS Education and Prevention, 31, 421–432. https://doi.org/https://doi.org/10.1521/aeap.2019.31.5.421
- Sherman, S. G., Park, J. N., Galai, N., Allen, S. T., Huettner, S. S., Silberzahn, B. E., Decker, M. R., Poteat, T. C., & Footer, K. H. A. (2019). Drivers of HIV infection among cisgender and transgender female sex worker populations in Baltimore city: Results from the SAPPHIRE study. JAIDS Journal of Acquired Immune Deficiency Syndromes, 80, 513–521. https://doi.org/https://doi.org/10.1097/QAI.0000000000001959
- Surratt, H. L., Kurtz, S. P., Chen, M., & Mooss, A. (2012). HIV risk among female sex workers in Miami: The impact of violent victimization and untreated mental illness. AIDS Care – Psychological and Socio-Medical Aspects of AIDS/HIV, 24, 553–561. https://doi.org/https://doi.org/10.1080/09540121.2011.630342
- Thigpen, M. C., Kebaabetswe, P. M., Paxton, L. A., Smith, D. K., Rose, C. E., Segolodi, T. M., Henderson, F. L., Pathak, S. R., Soud, F. A., Chillag, K. L., Mutanhaurwa, R., Chirwa, L. I., Kasonde, M., Abebe, D., Buliva, E., Gvetadze, R. J., Johnson, S., Sukalac, T., Thomas, V. T., … Brooks, J. T. (2012). Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. New England Journal of Medicine, 367, 423–434. https://doi.org/https://doi.org/10.1056/NEJMoa1110711
- Tobin, K. E., Kuramoto, S. J., Davey-Rothwell, M. A., & Latkin, C. A. (2011). The STEP into action study: A peer-based, personal risk network-focused HIV prevention intervention with injection drug users in Baltimore, Maryland. Addiction, 106, 366–375. https://doi.org/https://doi.org/10.1111/j.1360-0443.2010.03146.x
- Tomko, C., Park, J. N., Allen, S. T., Glick, J., Galai, N., Decker, M. R., Footer, K. H. A., & Sherman, S. G. (2019). Awareness and interest in HIV pre-exposure prophylaxis among street-based female sex workers: Results from a US context. AIDS Patient Care and STDs, 33, 49–57. https://doi.org/https://doi.org/10.1089/apc.2018.0182
- Van Damme, L., Corneli, A., Ahmed, K., Agot, K., Lombaard, J., Kapiga, S., Malahleha, M., Owino, F., Manongi, R., Onyango, J., Temu, L., Monedi, M. C., Mak’Oketch, P., Makanda, M., Reblin, I., Makatu, S. E., Saylor, L., Kiernan, H., Kirkendale, S., … Taylor, D. (2012). Preexposure prophylaxis for HIV infection among African women. New England Journal of Medicine, 367, 411–422. https://doi.org/https://doi.org/10.1056/NEJMoa1202614
- Van Der Straten, A., Brown, E. R., Marrazzo, J. M., Chirenje, M. Z., Liu, K., Gomez, K., Marzinke, M. A., Piper, J. M., & Hendrix, C. W. (2016). Divergent adherence estimates with pharmacokinetic and behavioural measures in the MTN-003 (VOICE) study. Journal of the International AIDS Society, 19, 20642. https://doi.org/https://doi.org/10.7448/IAS.19.1.20642
- Weeks, M. R., Li, J., Dickson-Gomez, J., Convey, M., Martinez, M., Radda, K., & Clair, S. (2009). Outcomes of a peer HIV prevention program with injection drug and crack users: The risk avoidance partnership. Substance Use and Misuse, 44, 253–281. https://doi.org/https://doi.org/10.1080/10826080802347677
- WHO. (2015). Pre-exposure prophylaxis (PReP): Who expands recommendation on oral pre-exposure prophylaxis of HIV infection. World Health Organization.