ABSTRACT
The DUALIS study demonstrated efficacy and safety of switching to dolutegravir plus ritonavir-boosted darunavir (DRV/r) (2DR) as compared to standard-of-care-therapy with two nucleoside reverse transcriptase inhibitors + DRV/r (3DR) in pretreated people living with HIV (PLWH), 48 weeks after switching. This DUALIS sub-study investigates health-related-quality-of-life (HrQoL) in this study-population. The Hospital Anxiety and Depression Scale (HADS) and the Medical Outcome Survey-HIV (MOS-HIV) were used assessing anxiety and depression symptoms, respectively HrQoL. Data were collected at baseline, 4, 24, and 48 weeks after randomization. Outcome scores were dichotomized and used as criteria in longitudinal models identifying differential developments. Odds ratios (ORs) with 95% confidence intervals (CIs) were computed as main measures of effects. ORs<1 indicate better results for HADS, and worse for MOS-HIV scores in the 2DR compared to 3DR group. In total, 263 subjects were randomized and treated (2DR n=131, 3DR n=132; median age 48 years). Significant different progressions could only be found for HADS-Depression scores (OR=.87, 95% CI: .78, .98, p=.02). While HADS-Depression scores decreased in the 2DR group, they increased in 3DR group. This sub-study showed no disadvantages regarding HrQoL in PLWH after switching to DTG+DRV/r. Considering lifelong requirements for antiretroviral medication, close attention to HrQL is required.
Introduction
Modern antiretroviral therapy (ART) has led to a normalization of life expectancy in people living with HIV (PLWH) (Hasse et al., Citation2011; Obel et al., Citation2011; Smit et al., Citation2015). A combination therapy consisting of two nucleoside reverse transcriptase inhibitors (NRTI) plus one additional substance from three different classes (protease inhibitor (PI), integrase strand transfer inhibitor (INSTI), and non-nucleoside reverse transcriptase inhibitor (NNRTI), is recommended by current guidelines (EACS, Citation2020). As a result of a higher barrier to resistance and improved viral efficacy in combination with favorable safety and tolerability profiles, dual therapy combinations have recently been licensed.
Dolutegravir (DTG), a second-generation INSTI, is a potent anchor drug, providing a high barrier to resistance compared to that of other INSTIs (Blanco et al., Citation2015; Song et al., Citation2011; Stellbrink et al., Citation2013). In randomized controlled trials examining treatment-naïve and treatment-experienced patients, DTG showed high effectiveness as a once-daily formulation as well as a good safety profile (Cahn et al., Citation2013; Clotet et al., Citation2014; Hsu et al., Citation2018; Keeshin & Feinberg, Citation2015; Patel et al., Citation2014; Raffi et al., Citation2013; Song et al., Citation2011; Stellbrink et al., Citation2013; Walmsley et al., Citation2013). Moreover, the potency of DTG has enabled two drug combinations, such as DTG with the NRTI lamivudine or DTG with the NNRTI rilpivirine (Aboud et al., Citation2019). A switch to DTG + rilpivirine also emerged as a well-tolerated treatment alternative in patients who had previously taken a 3- or 4-drug antiretroviral regimen and thus continued to demonstrate low symptom burden and high treatment satisfaction (Oglesby et al., Citation2019). Darunavir (DRV), a non-peptidic HIV-1 PI, has also demonstrated high effectiveness and a favorable safety profile (Clotet et al., Citation2007). The combination of DTG/DRV might be an effective and well-tolerated combination option, mainly for treatment experienced PLWH.
In addition to demonstrating the effectiveness and safety of a drug, the evaluation of health-related quality-of-life (HrQoL) has become of major interest in clinical trials. This investigation is of high importance, remarkably because PLWH can be considered as a particularly vulnerable group with regard to psychiatric comorbidities and psycho-social challenges (Degroote et al., Citation2013; Elliott et al., Citation2002; Lazarus et al., Citation2016; Ronel et al., Citation2018).
Several studies have reported neuropsychiatric adverse events (AEs) under DTG, mainly in real-life settings (Hoffmann et al., Citation2017; Hoffmann & Llibre, Citation2019; Kheloufi et al., Citation2015; Scheper et al., Citation2018; Van den Berk et al., Citation2016). In their retrospective analysis of >1700 PLWH, Hoffmann et al. (Citation2017) found that almost 6% of antiretroviral regimens containing DTG were withdrawn due to neuropsychiatric AEs (such as sleep disturbances, poor concentration, dizziness, or depression) within the first year after initiation. Todd et al. (Citation2017) reported side effects associated with the central nervous system (such as low mood, anxiety, or sleep disturbances) in 25% of naïve and non-naïve PLWH on DTG therapy, with 8% discontinuing treatment. In addition, de Boer et a.l (Citation2016) found a comparatively high discontinuation rate in PLWH, who started DTG (remarkably in combination with abacavir) due to psychiatric symptoms such as anxiety, psychosis, and depression (4.3%) or insomnia and sleep disturbances (5.6%). Cohort studies with >6400 patients from different countries observed discontinuation rates due to neuropsychiatric AEs in approximately 3.5% of the participants receiving DTG. These rates were higher than those observed in randomized clinical trials and also higher than in studies investigating other INSTIs e.g., Elvitegravir or raltegravir (Hoffmann & Llibre, Citation2019). However, in a study conducted as part of the Swedish National Registry, side effects of 4186 PLWH, captured by means of patient-reported outcomes were investigated. It was shown that with increasing use of DTG, side effects halved (Mellgren et al., Citation2020).
The open-label DUALIS phase III study assessed the efficacy and safety of a switch to DTG plus ritonavir-boosted darunavir (DRV/r) (2DR) as compared to standard-of-care therapy with two NRTI + DRV/r (3DR) in virologically controlled and successfully pretreated PLWH 48 weeks after switching. The aim of this sub-study is to assess depression and anxiety symptoms as well as HrQoL in PLWH after switching to DTG + DRV/r within the DUALIS study. For this purpose the German versions of the Hospital Anxiety and Depression Scale (HADS) (Herrmann-Lingen et al., Citation2011; Petermann, Citation2015) and the Medical Outcome Survey-HIV (MOS-HIV) (Wu et al., Citation1997; Zander et al., Citation1994) were used. Both internationally used questionnaires showed good psychometric properties (Henderson et al., Citation2010; Herrmann & Buss, Citation1994; Kemmler et al., Citation2003; Wu et al., Citation1997; Zander et al., Citation1994).
Materials and methods
Data were collected prospectively from PLWH participating in the DUALIS study using standardized psychometric instruments at 27 German outpatient HIV treatment centers. Data collection took place between June 2015 and June 2017. ART was made accessible by specialized physicians at all study centers.
Sample
In total, 269 PLWH receiving ART with fully suppressed HIV-RNA (< 50 cps/mL) for at least 24 weeks prior to randomization were screened, and 263 patients were included and randomized in the study (2DR=131, 3DR=132). All subjects received at least one study dose. Patients younger than 18 years, with known drug allergy, or with severe renal illness, as well as patients with current AIDS-events or documented major genotypic DRV- or integrase inhibitor-related HIV resistance were excluded. Further, pregnant women or nursing mothers could also not be enrolled. Subjects had to provide signed written informed consent and needed all screening evaluations performed prior to the first dose of the study drug.
Procedure
All subjects were identified by screening local patient databases and by recruitment from participating study centers. Subjects had been on a stable and fully suppressive ART with DRV/r in combination with two NRTIs for at least 28 days prior to randomization. The present analysis is embedded in an extensive investigation of the safety, tolerability, and efficacy of 2DR treatment (Spinner et al., Citation2020). The trial was performed in accordance with the Declaration of Helsinki and approved by the institutional review board of the Technical University of Munich, Munich, Germany (approval number: 162/15); it was registered before the enrollment of the first subject (Eudra-CT: 2015-000360-34).
After obtaining informed consent, the subjects were randomized to one of the two study groups. Subjects in the control arm continuously received darunavir boosted with ritonavir (800 + 100 mg) in combination with either tenofovir disoproxil/emtricitabine (245/200 mg), tenofovir alafenamide/emtricitabine 10/200 mg, or abacavir/lamivudine (600/300 mg) orally, once daily for 48 weeks. Subjects in the study arm received darunavir boosted with ritonavir (800 + 100 mg) in combination with dolutegravir (50 mg) once daily for 48 weeks. All medications were provided as tablets. In case of missed study doses, tablets had to be taken up to 12 h after the scheduled time; otherwise, the medication had to be continued with the subsequent regularly scheduled dose. Adherence, assessed via “pill count,” was predominantly considered as good.
Examinations took place at each study center at four time points: before randomization (baseline; BL) as well as after 4 weeks (W4), 24 weeks (W24), and 48 weeks (W48). Depression and anxiety symptoms, as well as HrQoL outcomes, were collected using the standardized questionnaires, HADS (Herrmann-Lingen et al., Citation2011; Petermann, Citation2015) and MOS-HIV (Wu et al., Citation1997; Zander et al., Citation1994). Further sociodemographic data (such as age, sex, or years since diagnosis) were collected at baseline by means of a detailed questionnaire.
Measurements
The HADS serves as an economical, reliable, and sufficiently valid psychological screening tool originally developed by Zigmond and Snaith (Citation1983) to identify anxiety and depression as important psychological symptom complexes in non-psychiatric clinics (Bjelland et al., Citation2002; Hinz & Brähler, Citation2011). Both dimensions, anxiety and depression, are captured by means of seven items each; their symptom severity is rated on a four-point Likert scale, with higher ratings indicating higher symptom intensity. The MOS-HIV is a brief and widely used instrument to measure HrQoL in PLWH (Henderson et al., Citation2010; Kemmler et al., Citation2003; Wu et al., Citation1997; Zander et al., Citation1994). The MOS-HIV consists of 30 items capturing the following 11 dimensions: “general health perceptions,” “physical functioning,” “role functioning,” “bodily pain,” “social functioning,” “mental health,” “energy,” “health distress,” “cognitive functioning,” “quality-of-life,” and “health transition.” Subscales are scored on a 0–100 scale, with higher scores indicating better perceived health.
Statistical analysis
Data were analyzed by means of “R” software (Version 3.6.1) (R Core Team, Citation2019), with a p-value ≤0.05 considered statistically significant. HADS and MOS-HIV scores at BL, W4, W24, and W48 were dichotomized at the median and used as criteria in the longitudinal models. First, differences between sub-samples in the evaluated parameters (HADS and MOS-HIV scores) at baseline and the later measurements were tested using t-tests or Mann-Whitney U tests for independent groups for continuous variables and chi-squared tests or Fisher’s exact test for categorical variables.
Stepwise logistic regression analyses with a combined forward and backward selection strategy based on the Akaike information criterion were completed to support the identification of the most relevant predictors. The final set of predictors for further modeling combined the most successful variables from the stepwise regressions with others that showed to be relevant in the literature. Cross-sectional logistic models were computed to detect differences between treatments at all follow-up measurement times (W4, W24, W48). For each criterion a logistic regression with treatment as a group factor and the other predictors was computed using the function “glm.” Model fit was examined graphically by Q-Q plots with local regression of the residual.
Finally, as the main outcome of the study, longitudinal modeling was applied to identify differential developments between arms for the criteria over time. In addition to the set of predictors used in the previous steps, a time variable representing the measurements BL to W48 and a subject factor as a random effect were included. Models were computed as generalized mixed-effect models with case as a random effect, using the “glmer” function in the R package “lme4” (Bates et al., Citation2015) and for optimization of the estimations the package “optimx” (Nash & Varadhan, Citation2011). Intercepts and slopes were allowed to vary at different time points to control the variation from different cases (Schober & Vetter, Citation2018). Odds ratios (ORs) with 95% confidence intervals (CIs) were computed as the main measure of effects (Charpentier, Citation2013; Lüdecke, Citation2018a, Citation2018b). The primary result was the interaction between measurement time and treatment group adjusted for predictors. In regard to HADS scores, an OR < 1 indicates a better result of the study group (2DR) (i.e., Lower HADS scores) in comparison to 3DR, and in regard to MOS-HIV scores an OR > 1 indicates a better result of 2DR (i.e., Higher MOS-HIV scores) compared to 3DR.
Results
Overall, 263 randomized and treated subjects within the DUALIS study were included in this sub-analysis, with a median age of 48 years (IQR 39–54) and a median time since HIV diagnosis of 7.2 years (IQR 4.3–12.3); 90.1% of subjects were male (237/263). Sociodemographic data and baseline characteristics are presented in . No significant differences were found for sociodemographic data between the two study arms at baseline.
Table 1. Baseline and sociodemographic characteristics.
Subjects within the 2DR study arm showed significantly lower ratings, indicating higher impairment at baseline regarding the subscales MOS-HIV-“mental health” (p≤0.00) and MOS-HIV-“social functioning” (p=0.02). At W4, the 2DR and 3DR group showed significant differences with regard to the unadjusted HADS-Anxiety (p=0.02) and MOS-HIV-“mental health” scores (p=0.01), with the 2DR group showing higher anxiety ratings but better perceived “mental health.” At the end of study (W48), subjects in the 2DR group reported significantly greater impairment with regard to the MOS-HIV-“mental health” scale (p=0.04).
Stepwise regression analyses was conducted to select suitable predictors for the outcome parameters. Most of the potential predictors did not (or only very weakly) correlate with the outcome parameters. Based on the results of the stepwise regression analyses as well as content-related aspects, the following predictors were selected for further logistic analyses: baseline of the criterion, age, current occupation, psychiatric, psychotherapeutic or psychopharmacological treatment, group, sex, and population size of the area of residence. ().
Table 2. Results of stepwise analyses of predictors.
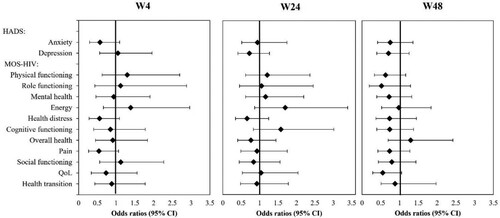
Cross-sectional logistic regression models with the group (study arm vs. control arm) as factors and the previously selected predictors as independent variables were used for the analysis of every outcome criterion at each study visit. ORs for the group factor were calculated to investigate the adjusted differences between groups. The group factor did not reach statistical significance in any of the models (see ). These results indicate that none of the chosen variables could predict significant differences between groups with regard to the outcome criteria at any study visit.
Figure 1. Cross-sectional logistic regression models for the study arm vs. control arm.
Note: shows OR and 95% CI for the effect of group (study vs control arm, i.e., OR<1 indicate better outcomes in the study group on the outcome criteria HADS scales and worse on the outcomes criteria for MOS-HIV scales 4 weeks, 24 weeks, and 48 weeks after randomization. CI: confidence intervals, HADS: Hospital Anxiety and Depression Scale, MOS-HIV: Medical Outcome Survey-HIV, OR: Odds Ratios.
Linear mixed regression models were used to investigate the interaction effects between measurement time and study arm in relation to the outcome parameters, which could be interpreted as a differential effect of the two treatments over time. ORs (95% CIs) of HADS-Anxiety scores and all MOS-HIV scores did not show significant differences: HADS-Anxiety (0.97; 0.85–1.11), MOS-HIV-“physical functioning” (0.92; 0.79–1.07), MOS-HIV-“role functioning” (0.93; 0.77–1.11), MOS-HIV-“mental health” (1.00; 0.88–1.15), MOS-HIV-“energy” (1.04; 0.90–1.20), MOS-HIV-“health distress” (0.85; 0.65–1.10), MOS-HIV-“cognitive functioning” (0.99; 0.85–1.16), MOS-HIV-“overall health” (1.07; 0.93–1.23), MOS-HIV-“pain” (1.00; 0.86–1.15), MOS-HIV-“social functioning” (1.02; 0.87–1.18), MOS-HIV-“quality-of-life” (0.97; 0.84–1.12), and MOS-HIV-“health transition” (1.06, 0.93–1.20). Statistically significant differences in progression over measurement points between the intervention and control arm could only be found for the HADS-Depression scores (b=-.030, p=.02, OR=.87, 95% CI: .78, .98). The significant treatment effect over time on HADS-Depression is shown in and . While HADS-Depression scores in the treatment group (2DR) continuously decreased from baseline to the last follow-up in W48, they increased in the control group (3DR).
Figure 2. Effects of study vs. control group over time.
Note: shows OR and 95% CI for the interaction between study arm and study visits for the outcome criteria (HADS and MOS-HIV scores) over the course of the study visits. CI: confidence intervals, HADS: Hospital Anxiety and Depression Scale, MOS-HIV: Medical Outcome Survey-HIV, OR: Odds Ratios.
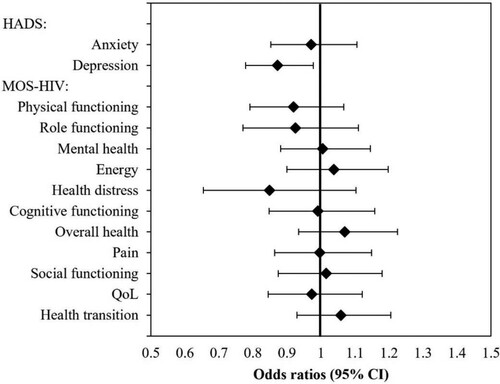
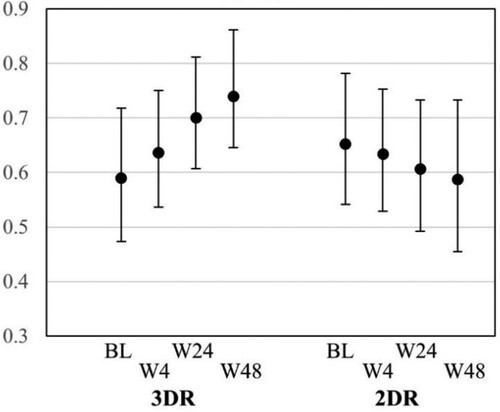
Figure 3. Marginal (adjusted) effects of treatment on HADS scores over time.
Note: shows the predicted probabilities with 95% CI of the HADS-Depression for the study group (2DR) and control group (3DR) across the study visits from baseline until the end of study (week 48). BL: baseline, CI: confidence intervals, HADS: Hospital Anxiety and Depression Scale.
Discussion
This study aimed to compare DRV/r plus DTG (2DR) and DRV/r combined with two NRTIs (3DR) in PLWH with regard to anxiety and depression symptoms as well as HrQoL over 48 weeks within the DUALIS study. Predictors for logistic models were selected, considering content-related aspects as well as the results of stepwise regression analyses. None of the cross-sectional models demonstrated significant effects of the treatment on the outcome parameters at any study visit. A significant longitudinal effect was found for the HADS-Depression score. While the HADS-Depression score decreased in the 2DR group, an increase could be observed in the 3DR group, although the subjects had previously been treated with DRV-containing regimens.
Indications of increased mental distress after switching from 2 NRTI + DRV/r to DTG + DRV/r in the 2DR group, as reported in previous cohort studies, could not be confirmed by our data (Hoffmann et al., Citation2017; Hoffmann & Llibre, Citation2019; Kheloufi et al., Citation2015; Scheper et al., Citation2018; Van den Berk et al., Citation2016). Interestingly, switching to DTG + DRV/r seemed to have a positive impact on depression ratings within the sub-sample.
The following limitations need to be discussed. Since this study was designed as an open-label study, subjects were not blinded, and the study was not controlled for expectation biases. Therefore, positive expectations or negative emotions might have contributed to the differences in HADS-Depression scores (Rief et al., Citation2008). Furthermore, the limited sample size should be addressed. It should also be noted that the selection of subjects in randomized controlled trials is highly selective; this population may differ significantly from the broader population due to explicit exclusion criteria and recruitment biases. Mainly men (90%) with a median age of 48 years were enrolled into the study, indicating that the results are not representative of a more diverse population of PLWH. However, Hoffmann et al. (Citation2017) reported higher termination rates due to neuropsychiatric AEs in patients receiving DTG-containing ART, mainly among women and elderly subjects. It should be considered that 20% of the total sample reported psychiatric or psychotherapeutic treatment, and the total sample showed increased HADS scores at baseline (cutoffs: 0–7 [non-cases], 8–10 [doubtful cases], and 11–21 [cases]) (Hinz & Brähler, Citation2011). It is also critical to note that subjects in the 2DR arm showed significantly worse values regarding the MOS-HIV-“social functioning” and MOS-HIV-“mental health” scales at baseline; this negative initial status could have had an influence on the differences in the HADS-Depression scores.
In conclusion, the DUALIS study confirmed that DTG in combination with boosted DRV can be considered to be effective with an acceptable safety profile. With regard to anxiety and depression symptoms as well as HrQoL, no disadvantages associated with the intake of the 2DR combination could be observed. However, the 2DR arm showed a more favorable course concerning depression symptoms during the 48-week study period than the control group. Considering the lifelong requirement for antiretroviral medication and mental and social challenges, close attention to physical as well as mental quality-of-life is required in PLWH (Degroote et al., Citation2013; Elliott et al., Citation2002). Randomized controlled trials with large sample sizes using double-blind designs and comprehensive standardized data collection procedures, including HrQoL-related endpoints, are needed.
Acknowledgment
We thank the involved participants, sites, and staff as well as the clinical research organizations Munich Study Center (Helen Bidner for study coordination) and MUC Research (Annamaria Balogh for data management and statistical analyses), Munich, Germany. Parts of this study were presented at the International AIDS Society Conference in Mexico 2019 and the European AIDS Society Conference in Basel 2019. We thank the members of the Data Safety Monitoring Board (Georg Behrens, Armin Koch, and Christian Hoffmann), as well as the study coordinators Marcus Kosch and Helen Bidner (both Technical University Munich) for their overall assistance.
Disclosure statement
Christoph Bösecke received honoraria for lectures and/or consultancies from AbbVie, Gilead, Janssen, MSD and ViiV, funding from Deutsche Leberstiftung, DZIF, Hector Stiftung and NEAT ID. Jochen Schneider reports personal fees from Gilead Sciences and from AbbVie outside the submitted work. Simon Weidlich reports travel grants and speaker honoraria from Gilead Sciences as well as personal fees from Takeda, Jansen-Cilag and AbbVie. Hans Heiken reports honoraria for lectures and advisory boards received from AbbVie, Gilead, Janssen, MSD and ViiV Healthcare. These honoraria were not related to the conduct of the study. Hans-Jürgen Stellbrink has received honoraria for scientific advice and presentations from GILEAD Sciences, Janssen-Cilag, MSD, and Theratechnologies. He reports no other conflicts of interest. Stefan Scholten received honoraria for consultancy from Abbvie, Gilead, GSK, Janssen, MSD, ViiV, a sponsorship for congress participation from Abbvie, Gilead, Janssen, MSD, ViiV and honoraria for talks and presentations from Abbvie, Gilead, Janssen, MSD, ViiV. Björn Erik-Ole Jensen reports personal fees from Gilead, personal fees from ViiV, and personal fees from Janssen-Cilag outside the submitted work. Eva Wolf reports fees for consulting or lectures at educational events from AbbVie, Gilead Sciences, GlaxoSmithKline, Janssen-Cilag, MSD Sharp & Dohme, Roche, and ViiV Healthcare. Christoph D. Spinner reports grants and personal fees from Jansen-Cilag, grants and personal fees from ViiV Healthcare, during the conduct of the study; grants and personal fees from Gilead Sciences, personal fees from AbbVie, personal fees from MSD, outside the submitted work. All other authors have nothing disclosed.
Additional information
Funding
References
- Aboud, M., Orkin, C., Podzamczer, D., Bogner, J. R., Baker, D., Khuong-Josses, M. A., Parks, D., Angelis, K., Kahl, L. P., Blair, E. A., Adkison, K., Underwood, M., Matthews, J. E., Wynne, B., Vandermeulen, K., Gartland, M., & Smith, K. (2019). Efficacy and safety of dolutegravir–rilpivirine for maintenance of virological suppression in adults with HIV-1: 100-week data from the andomized, open-label, phase 3 SWORD-1 and SWORD-2 studies. The Lancet. HIV, 6(9), e576–e587. https://doi.org/https://doi.org/10.1016/S2352-3018(19)30149-3
- Bates, D., Maechler, M., & Bolker, B. (2015). Walker., S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. https://doi.org/https://doi.org/10.18637/jss.v067.i01
- Bjelland, I., Dahl, A. A., Haug, T. T., & Neckelmann, D. (2002). The validity of the Hospital Anxiety and Depression Scale: An updated literature review. Journal of Psychosomatic Research, 52(2), 69–77. https://doi.org/https://doi.org/10.1016/S0022-3999(01)00296-3
- Blanco, J. L., Whitlock, G., Milinkovic, A., & Moyle, G. (2015). Hiv integrase inhibitors: A new era in the treatment of HIV. Expert Opinion on Pharmacotherapy, 16(9), 1313–1324. https://doi.org/https://doi.org/10.1517/14656566.2015.1044436
- Cahn, P., Pozniak, A. L., Mingrone, H., Shuldyakov, A., Brites, C., Andrade-Villanueva, J. F., Richmond, G., Buendia, C. B., Fourie, J., Ramgopal, M., Hagins, D., Felizarta, F., Madruga, J., Reuter, T., Newman, T., Small, C. B., Lombaard, J., Grinsztejn, B., Dorey, D., Underwood, M., … extended SAILING Study Team. (2013). Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-I adults with HIV: Week 48 results from the andomized, double-blind, non-inferiority SAILING study. The Lancet, 382(9893), 700–708. https://doi.org/https://doi.org/10.1016/S0140-6736(13)61221-0
- Charpentier, A. (2013). Residuals from a logistic regression. Freakonometrics. An Open lab-notebook experiment. Hypotheses. Org/8210; Retrieved November 21, 2016, from. http://freakonometrics
- Clotet, B., Bellos, N., Molina, J. M., Cooper, D., Goffard, J. C., Lazzarin, A., Wöhrmann, A., Katlama, C., Wilkin, T., Haubrich, R., Cohen, C., Farthing, C., Jayaweera, D., Markowitz, M., Ruane, P., Spinosa-Guzman, S., Lefebvre, E., & POWER 1 and 2 study groups. (2007). Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: A pooled subgroup analysis of data from two andomized trials. The Lancet, 369(9568), 1169–1178. https://doi.org/https://doi.org/10.1016/S0140-6736(07)60497-8
- Clotet, B., Feinberg, J., Van Lunzen, J., Khuong-Josses, M. A., Antinori, A., Dumitru, I., Pokrovskiy, V., Fehr, J., Ortiz, R., Saag, M., Harris, J., Brennan, C., Fujiwara, T., Min, S., & ING114915 Study Team. (2014). Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-I adults with HIV-1 infection (FLAMINGO): 48 week results from the andomized open-label phase 3b study. The Lancet, 383(9936), 2222–2231. https://doi.org/https://doi.org/10.1016/S0140-6736(14)60084-2
- de Boer, M. G., van den Berk, G. E., van Holten, N., Oryszcyn, J. E., Dorama, W., Moha, D. A., & Brinkman, K. (2016). Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. AIDS, 30(18), 2831–2834. https://doi.org/https://doi.org/10.1097/QAD.0000000000001279
- Degroote, S., Vogelaers, D. P., Vermeir, P., Mariman, A., De Rick, A., Van Der Gucht, B., Pelgrom, J., Van Wanzeele, F., Verhofstede, C., & Vandijck, D. M. (2013). Socio-economic, behavioural,(neuro) psychological and clinical determinants of HRQoL in people living with HIV in Belgium: A pilot study. Journal of the International AIDS Society, 16(1), 18643. https://doi.org/https://doi.org/10.7448/IAS.16.1.18643
- Elliott, A. J., Russo, J., & Roy-Byrne, P. P. (2002). The effect of changes in depression on health related quality of life (HRQoL) in HIV infection. General Hospital Psychiatry, 24(1), 43–47. https://doi.org/https://doi.org/10.1016/s0163-8343(01)00174-8
- European AIDS Clinical Society. (2020). European AIDS Clinical Society guidelines 10.1. Retrieved October 12, 2020, from https://www.eacsociety.org/files/guidelines-10.1.finalsept2020.pdf
- Hasse, B., Ledergerber, B., Furrer, H., Battegay, M., Hirschel, B., Cavassini, M., Bertisch, B., Bernasconi, E., Weber, R., & Swiss HIV Cohort Study. (2011). Morbidity and aging in HIV-infected persons: The Swiss HIV cohort study. Clinical Infectious Diseases, 53(11), 1130–1139. https://doi.org/https://doi.org/10.1093/cid/cir626
- Henderson, W. A., Schlenk, E. A., Kim, K. H., Hadigan, C. M., Martino, A. C., Sereika, S. M., & Erlen, J. A. (2010). Validation of the MOS-HIV as a measure of health-related quality of life in persons living with HIV and liver disease. AIDS Care, 22(4), 483–490. https://doi.org/https://doi.org/10.1080/09540120903207292
- Herrmann-Lingen, C., Buss, U., & Snaith, P. (2011). Hospital anxiety and depression scale-deutsche version (HADS-D). Huber.
- Herrmann, C., & Buss, U. (1994). Vorstellung und Validierung einer Deutschen Version der”(HAD-Skala) [Hospital anxiety and depression scale”(HAD-Skala)]. Ein Fragebogen zur Erfassung des psychischen Befindens bei Patienten mit körperlichen Beschwerden. Diagnostica.
- Hinz, A., & Brähler, E. (2011). Normative values for the hospital anxiety and depression scale (HADS) in the general German population. Journal of Psychosomatic Research, 71(2), 74–78. https://doi.org/https://doi.org/10.1016/j.jpsychores.2011.01.005
- Hoffmann, C., & Llibre, J. M. (2019). Neuropsychiatric adverse events with dolutegravir and other integrase strand transfer inhibitors. AIDS Reviews, 21(1), 4–10. https://doi.org/https://doi.org/10.24875/AIDSRev.19000023
- Hoffmann, C., Welz, T., Sabranski, M., Kolb, M., Wolf, E., Stellbrink, H. J., & Wyen, C. (2017). Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Medicine, 18(1), 56–63. https://doi.org/https://doi.org/10.1111/hiv.12468
- Hsu, R., Fusco, J., Henegar, C., Mounzer, K., Wohlfeiler, M., Vannappagari, V., Aboud, M., Curtis, L., & Fusco, G. (2018). Psychiatric outcomes observed in patients living with HIV using six common core antiretrovirals in the observational pharmaco-epidemiology research and analysis database. Therapeutic Advances in Drug Safety, 9(12), 675–686. https://doi.org/https://doi.org/10.1177/2042098618798350
- Keeshin, S. W., & Feinberg, J. (2015). Evaluation of dolutegravir safety for the treatment of HIV-1. Expert Opinion on Drug Safety, 14(1), 141–147. https://doi.org/https://doi.org/10.1517/14740338.2015.973845
- Kemmler, G., Schmied, B., Shetty-Lee, A., Zangerle, R., Hinterhuber, H., Schüssler, G., & Mumelter, B. (2003). Quality of life of HIV-infected patients: Psychometric properties and validation of the German version of the MQOL-HIV. Quality of Life Research, 12(8), 1037–1050. https://doi.org/https://doi.org/10.1023/A:1026114004548
- Kheloufi, F., Allemand, J., Mokhtari, S., & Default, A. (2015). Psychiatric disorders after starting dolutegravir: Report of four cases. AIDS, 29(13), 1723–1725. https://doi.org/https://doi.org/10.1097/QAD.0000000000000789
- Lazarus, J. V., Safreed-Harmon, K., Barton, S. E., Costagliola, D., Dedes, N., del Amo Valero, J., Gatell, J. M., Baptista-Leite, R., Mendão, L., & Porter, K. (2016). Beyond viral suppression of HIV – the new quality of life frontier. BMC Medicine, 14(1), 1–5. https://doi.org/https://doi.org/10.1186/s12916-016-0640-4
- Lüdecke, D. (2018a). Ggeffects: Tidy data frames of marginal effects from regression models. Journal of Open Source Software, 3(26), 772. https://doi.org/https://doi.org/10.21105/joss.00772
- Lüdecke, D. (2018b). sjPlot: Data Visualization for Statistics in Social Science. R package version, 2 (1). Retrieved September 22, 2020, from https://cran.r-project.org/web/packages/sjPlot/index.html
- Mellgren, Å, Eriksson, L. E., Reinius, M., Marrone, G., & Svedhem, V. (2020). Longitudinal trends and determinants of patient-reported side effects on ART-a Swedish national registry study. PLoS ONE, 15(12), e0242710. https://doi.org/https://doi.org/10.1371/journal.pone.0242710
- Nash, J. C., & Varadhan, R. (2011). Unifying optimization algorithms to aid software system users: Optimx for R. Journal of Statistical Software, 43(9), 1–14. https://doi.org/http://doi.org/10.18637/jss.v043.i09
- Obel, N., Omland, L. H., Kronborg, G., Larsen, C. S., Pedersen, C., Pedersen, G., Sørensen, H. T., & Gerstoft, J. (2011). Impact of non-HIV and HIV risk factors on survival in HIV-infected patients on HAART: A population-based nationwide cohort study. PLoS ONE, 6(7), e22698. https://doi.org/https://doi.org/10.1371/journal.pone.0022698
- Oglesby, A., Angelis, K., Punekar, Y., Chounta, V., Antela, A., Matthews, J., Kahl, L., Gartland, M., Wynne, B., Murray, M., & Van Wyk, J. A. (2019). 2484. Patient reported outcomes after switching to a 2-drug regimen of dolutegravir+rilpivirine: Week 148 results from the sword-1 and sword-2 studies. Open Forum Infectious Diseases, 6(Suppl. 2), S861–S861. https://doi.org/https://doi.org/10.1093/ofid/ofz360.2162
- Patel, D. A., Snedecor, S. J., Tang, W. Y., Sudharshan, L., Lim, J. W., Cuffe, R., Pulgar, S., Gilchrist, K. A., Camejo, R. R., Stephens, J., & Nichols, G. (2014). 48-week efficacy and safety of dolutegravir relative to commonly used third agents in treatment-I HIV-1–infected patients: A systematic review and network meta-analysis. PLoS ONE, 9(9), e105653. https://doi.org/https://doi.org/10.1371/journal.pone.0105653
- Petermann, F. (2015). Hospital anxiety and depression scale, deutsche version (HADS-D). Zeitschrift für Psychiatrie Psychologie und Psychotherapie.
- Raffi, F., Jaeger, H., Quiros-Roldan, E., Albrecht, H., Belonosova, E., Gatell, J. M., Baril, J. G., Domingo, P., Brennan, C., Almond, S., Min, S., & extended SPRING-2 Study Group. (2013). Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-I adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomized, double-blind, non-inferiority trial. The Lancet Infectious Diseases, 13(11), 927–935. https://doi.org/https://doi.org/10.1016/S1473-3099(13)70257-3
- R Core Team. (2019). R: A language and environment for statistical computing. Vienna, Austria.
- Rief, W., Hofmann, S. G., & Nestoriuc, Y. (2008). The power of expectation - understanding the placebo and nocebo phenomenon. Social and Personality Psychology Compass, 2(4), 1624–1637. https://doi.org/https://doi.org/10.1111/j.1751-9004.2008.00121.x
- Ronel, J., Dinkel, A., Wolf, E., Marten-Mittag, B., Mueck, B., Mayr, C., Hoffmann, C., Karwat, M., Schewe, K., Baumgarten, A., & Jaeger, H. (2018). Anxiety, depression, and health-related quality of life in aging people living with HIV compared to diabetes patients and patients with minor health conditions: A longitudinal study. Psychology, Health and Medicine, 23(7), 823–830. https://doi.org/https://doi.org/10.1080/13548506.2018.1437276
- Scheper, H., Van Holten, N., Hovens, J., & De Boer, M. (2018). Severe depression as a neuropsychiatric side effect induced by dolutegravir. HIV Medicine, 19(4), e58–e59. https://doi.org/https://doi.org/10.1111/hiv.12538
- Schober, P., & Vetter, T. R. (2018). Repeated measures designs and analysis of longitudinal data: If at first you do not succeed—try, try again. Anesthesia and Analgesia, 127(2), 569–575. https://doi.org/https://doi.org/10.1213/ANE.0000000000003511
- Smit, M., Brinkman, K., Geerlings, S., Smit, C., Thyagarajan, K., van Sighem, Av, de Wolf, F., Hallett, T. B., & ATHENA observational cohort. (2015). Future challenges for clinical care of an ageing population infected with HIV: A modelling study. The Lancet Infectious Diseases, 15(7), 810–818. https://doi.org/https://doi.org/10.1016/S1473-3099(15)00056-0
- Song, I., Min, S. S., Borland, J., Lou, Y., Chen, S., Patel, P., Ishibashi, T., & Piscitelli, S. C. (2011). The effect of lopinavir/ritonavir and darunavir/ritonavir on the HIV integrase inhibitor S/GSK1349572 in healthy participants. The Journal of Clinical Pharmacology, 51(2), 237–242. https://doi.org/https://doi.org/10.1177/0091270010371113
- Spinner, C. D., Kümmerle, T., Schneider, J., Cordes, C., Heiken, H., Stellbrink, H., Krznaric, I., Scholten, S., Jensen, B., Wyen, C., Viehweger, M., Lehmann, C., Sprinzl, M., Stoehr, A., Bickel, M., Jessen, H., Obst, W., Spornraft-Ragaller, P., Khaykin, P., & Boesecke, C. (2020). Efficacy and safety of switching to dolutegravir with boosted darunavir in virologically suppressed adults with HIV-1: A randomized, open-label, multicenter, phase 3, non-inferiority trial: The DUALIS study. Open Forum Infectious Diseases, 7(9). https://doi.org/https://doi.org/10.1093/ofid/ofaa356
- Stellbrink, H. J., Reynes, J., Lazzarin, A., Voronin, E., Pulido, F., Felizarta, F., Almond, S., St Clair, M., Flack, N., Min, S., & SPRING-1 Team. (2013). Dolutegravir in antiretroviral-I adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS (London, England), 27(11), 1771–1778. https://doi.org/https://doi.org/10.1097/QAD.0b013e3283612419
- Todd, S. E. J., Rafferty, P., Walker, E., Hunter, M., Dinsmore, W. W., Donnelly, C. M., McCarty, E. J., Quah, S. P., & Emerson, C. R. (2017). Early clinical experience of dolutegravir in an HIV cohort in a larger teaching hospital. International Journal of STD and AIDS, 28(11), 1074–1081. https://doi.org/https://doi.org/10.1177/0956462416688127
- Van den Berk, G., Oryszczyn, J., Blok, W., Van der Meche, N., Regez, R., Moha, D., & Brinkman, K. (2016). Unexpectedly high rate of intolerance for dolutegravir in a real-life setting. (Ed.), (Eds.). Retroviruses and Opportunistic Infections.
- Walmsley, S. L., Antela, A., Clumeck, N., Duiculescu, D., Eberhard, A., Gutiérrez, F., Hocqueloux, L., Maggiolo, F., Sandkovsky, U., Granier, C., Pappa, K., Wynne, B., Min, S., Nichols, G., & Investigators SINGLE. (2013). Dolutegravir plus Abacavir–lamivudine for the treatment of HIV-1 infection. New England Journal of Medicine, 369(19), 1807–1818. https://doi.org/https://doi.org/10.1056/NEJMoa1215541
- Wu, A. W., Revicki, D. A., Jacobson, D., & Malitz, F. E. (1997). Evidence for reliability, validity, and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV). Quality of Life Research, 6(6), 481–493. https://doi.org/https://doi.org/10.1023/a:1018451930750
- Zander, K., Palitzsch, M., Kirchberger, I., Poppinger, J., & Jägel-Guedes, E. (1994). HIV-Infektion und gesundheitsbezogene Lebensqualität: psychometrische Prüfund der deutschsprachigen Version Des “MOS-HIV"-Fragebogens zur Therapieerfolgskontrolle. AIDS-Forschung, 9(5), 241–252.
- Zigmond, A. S., & Snaith, R. P. (1983). The Hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. https://doi.org/https://doi.org/10.1111/j.1600-0447.1983.tb09716.x
