ABSTRACT
Rapid initiation of antiretroviral therapy (ART) has been proven efficacious and safe, but more investigations are needed to define feasibility of rapid ART approach in real-life settings.We conducted a retrospective, observational study on newly HIVdiagnosed patients referred to our Infectious Diseases Department from September 1st, 2015, to July 31st, 2019. According to the timing of ART initiation, we distinguished 3 groups of patients (rapid, intermediate and late group) and represented the trend of virological response during a 400-days-period. The hazard ratios of each predictor on viral suppression were estimated through the Cox proportional hazard model.The median time from HIV diagnosis to the first medical referral was 15 days and the median time from the first care access to therapy start was 24 days. Among patients, 37.6% started ART within 7 days, 20.6% between 8 and 30 days, and 41.8% after 30 days. Longer time to ART start and higher baseline viral load were associated with a lower probability of viral suppression. After one year, all groups showed a high viral suppression rate (99%). In a high-income setting the rapid ART approach seems useful to accelerate viral suppression which is great over time regardless of ART initiation timing.
| List of abbreviations | ||
| ART | = | antiretroviral therapy |
| TB | = | tuberculous |
| PLWH | = | people live with HIV |
| IRIS | = | immune reconstitution inflammatory syndrome |
| ID | = | infectious diseases |
| AEHI | = | acute or early HIV infections |
| MSM | = | male who have sex with male bisexual |
| IVDU | = | intravenous drug user |
| NRTIs | = | reverse transcriptase inhibitors |
| NNRTIs | = | non-nucleoside reverse transcriptase inhibitors |
| INSTIs | = | integrase strand transfer inhibitors |
| PIs | = | boosted protease inhibitors |
| LTFU | = | lost to follow up |
| HR | = | hazard ratios |
| CI | = | confidence intervals |
| IQR | = | interquartile range |
| SD | = | standard deviation |
| FTC/TAF | = | emtricitabine/tenofovir alafenamide |
| ABC/3TC | = | abacavir/lamivudine |
| FTC/TDF | = | emtricitabine/tenofovir disoproxil |
| DTG | = | dolutegravir |
| RPV | = | rilpivirine |
| ATV | = | atazanavir |
| DRV | = | darunavir |
| ELV | = | elvitegravir |
| RAL | = | raltegravir |
Introduction
Since the introduction of the “treat all” recommendation by WHO in 2015, an emerging debate has focused on the best timing of antiretroviral therapy (ART) initiation in people living with HIV (PLWH) after a confirmed diagnosis. In 2017 WHO took a pass forward, recommending the so-called “rapid ART initiation” (World Health Organization, Citation2017), defined as a start within seven days following an HIV diagnosis and clinical assessment, including the possibility of “same-day” initiation for people ready to be treated and without clinical contraindications. According to the latest WHO recommendations even among PLWH with signs and symptoms of tuberculous (TB) disease (except for meningitis), ART might be initiated cautiously while rapidly investigating TB disease (World Health Organization, Citation2021).
However, benefits and concerns must be considered when starting rapidly ART. On one hand, accelerated ART initiation may lead to undeniable benefits: faster time to viral load suppression, higher therapy uptake together with durable viral control, reduction in HIV-related and non-HIV-related morbidity and mortality, and decreased risk of HIV onward transmission (Coffey et al., Citation2019; Cohen et al., Citation2011; Cohen et al., Citation2016; Hoenigl et al., Citation2016; Koenig et al., Citation2017; Labhardt et al., Citation2018; Lapadula et al., Citation2015; Lundgren et al., Citation2015; Pilcher et al., Citation2017; Rosen et al., Citation2016). On the other hand, a rapid ART start could be related to disadvantages, such as immune reconstitution inflammatory syndrome (IRIS), the transmission of drug resistance according to a sub-optimal adherence and premature loss to care, especially among those people who do not feel psychologically ready to accept the diagnosis and a lifelong treatment (Cuzin et al., Citation2019; Ford et al., Citation2018; Rosen et al., Citation2016; Working Group of the Office of AIDS Research Advisory Council [OARAC], Citation2021).
To our best knowledge, most clinical trials supporting rapid ART initiation strategy were conducted in resource-limited settings where a large part of PLWH often experience disengagement from health services after diagnosis and ART initiation delays as a result of complex socio-cultural factors and poor healthcare infrastructure (Boyd et al., Citation2019; Ford et al., Citation2018; Koenig et al., Citation2017; Labhardt et al., Citation2018; Mateo-Urdiales et al., Citation2019; Rosen et al., Citation2016). The generalizability of these findings might be limited if adapted to high-income settings (OARAC, Citation2021). In this context little is still known about the best timing and the impact of rapid ART initiation beyond those circumstances where it is already considered essential (i.e., AIDS-defining conditions, primary HIV infection, pregnancy, HBV and/or HCV co-infection) (Boyd et al., Citation2019; D'Arminio Monforte et al., Citation2020; Girometti et al., Citation2017). However, the feasibility and acceptability of initiating ART as soon as possible after diagnosis in resource-rich countries was highlighted by Coffey and colleagues through the San Francisco RAPID ART program (Coffey et al., Citation2019). In recent years a few more studies have been conducted in high-income countries to investigate the potential benefits of accelerated ART but without univocal results. For example, one cohort from London (UK) and one cohort from San Diego (CA, USA) of patients with recent HIV infection showed the efficaciousness of the rapid ART approach in reducing time to undetectable viral load and reaching high rate (90-99%) of viral suppression by 6 months of therapy (Girometti et al., Citation2017; Martin et al., Citation2021). Differently, in 2019 an Italian experience from ICONA Foundation Study Cohort did not observe a clear utility of rapid ART initiation in terms of virological response and retention in care at 1 year of follow-up (D'Arminio Monforte et al., Citation2020). Lastly, a prospective French cohort of newly HIV-diagnosed patients even found that starting treatment within 9 days was negatively associated with care engagement after 1 year of follow-up (Cuzin et al., Citation2019).
To improve our knowledge about the impact of accelerated ART initiation in real-life practice in a high-income country like Italy, we aimed to investigate rates and predictors of virological response among newly diagnosed HIV patients according to the timing to ART initiation in a large HIV clinic in Brescia, Lombardy (Northern Italy).
Methods
A retrospective, observational, single-centre study was conducted in the Infectious and Tropical Diseases Department of the Azienda Socio Sanitaria Territoriale (ASST) Spedali Civili Hospital in Brescia, which represents the only tertiary referral center for HIV care of the entire Brescia Province (about 1,200,000 inhabitants). The Infectious and Tropical Diseases Department, following approximately 4,000 PLWH, is considered one of the largest outpatient clinics for HIV care in Italy.
We included all the newly HIV-diagnosed patients referred to our department from September 1, 2015, to July 31, 2019. We decided to start our study period in 2015 because it is recognized as the year of introduction of the WHO’s “treat all” recommendation and the publication of the START Trial. As we decided to conduct this study in late 2019, we chose to finish the study period in that year. We considered all patients receiving a new HIV-positive test result without setting any temporal criteria about the test execution, both as inpatients in our Infectious Diseases (ID) ward at the time of diagnosis, and as outpatients, normally after receiving a positive HIV test in one of the community-based checkpoints or private labs. We considered any clinical stage of HIV infection, including acute or early HIV infection (AEHI) defined according to Fiebig criteria (Fiebig et al., Citation2003) and AIDS presenters.
We retrospectively extracted data of patients from an electronic database (NetCare, Healthware Technology SpA, Salerno, Italy), generally used in our department in our daily clinical activity. Thus, patients’ baseline characteristics at the moment of linkage to our Clinic were collected in an anonymized database, including demographics (sex, age and nationality), the date and the modality of the first HIV care access (as outpatient or inpatient), risk factor for HIV acquisition (heterosexual, males who have sex with male [MSM], bisexual, intravenous drug user [IVDU]), co-infections (HCV and HBV active infections), virologic and immunological status (CD4+ T-cell count, CD4+/CD8+ T-cell ratio and HIV viral load), clinical stage of infection (primary HIV infection or AIDS), and time period occurred between the first HIV positive test and the first referral to our Clinic.
For patients starting ART, we recorded drugs (reverse transcriptase inhibitors [NRTIs], non-nucleoside reverse transcriptase inhibitors [NNRTIs], integrase strand transfer inhibitors [INSTIs], boosted protease inhibitors [PIs]) as triple or dual regimens and the date of ART initiation. Normally, the assessment of virologic/immunological profile (HIV viral load and CD4 T cell count) and execution of genotypic resistance test and HLA-B5701 precede ART initiation, which is selected by the physician based on the national and international HIV guidelines and his own clinical experience. Nevertheless, the readiness of the genotypic resistance test and HLA B5701 results is not mandatory to begin the initial ART regimen.
All patients were followed up for 400 days from their very first referral at our Clinic. We recorded the status of retention in care in the sixth and twelveth months (actively followed, lost to follow-up [LTFU], transferred to another Centre, deceased) and the rate of viral suppression (HIV RNA < 50 copies/ml) at the end of follow-up period. We defined LTFU as any patient missing follow-up visit and viro-immunological determination (HIV viral load and CD4+ T cell count) at the sixth and/or twelfth month from their first access without re-engagement in care.
Therefore, all new HIV out- and inpatients accepting to receive ART were divided into three groups according to the time of ART initiation from the first HIV care access (the rapid start group [≤ 7 days], the intermediate start group [between 8 and 30 days] and the late start group [>30 days]) and followed up over time, assessing for each group rate of viral suppression in the first, third, sixth and twelfth months. Inpatients initiating ART during their hospital stay were followed up after their discharge as outpatients.
Statistical analysis
The baseline characteristics of all new HIV-diagnosed patients were summarized in a descriptive analysis. Median, first and third quartile were used for continuous variables, while counts and percentages were used for categorical variables.
For the survival analyses, we excluded patients not starting ART. Hazard ratios (HR) of viral load suppression among continuous and categorical variables (gender, age, the timing of ART initiation, antiretroviral pharmacological class, HIV viral load at baseline and CD4+ T cell count) were estimated through a Cox proportional hazard model and 95% confidence intervals (CI) were calculated. The difference in the effect of ART initiation and viral suppression between in- and outpatients was tested through an interaction term between the two variables. The prediction obtained by the Cox model of the proportion of viral suppression depending on the time of follow-up among three different groups of patients according to the time of ART initiation was represented.
Results
Baseline characteristics of the study population
Three hundred twenty new HIV-diagnosed patients, including 206 outpatients and 114 inpatients, were initially selected for our study. All patients’ baseline characteristics are summarized in .
Table 1. Patients’ baseline characteristics.
Overall, the median age was 41.8 years (interquartile range [IQR] 32.0–51.5), most participants were male (n = 243, 75.9%) and Italian (n = 215, 67.2%), while among foreign patients the majority were from Africa (n = 48, 15.0%), followed by patients from Eastern Europe (n = 25, 7.8%), South/Central America (n = 16, 5.0%), Asia (n = 12, 3.8%), North America and Western Europe (n = 2, 0.6%, each). Overall, the main risk factor for HIV acquisition was heterosexual exposure (n = 201, 62.8%).
Regarding the baseline virological and immunological profile, CD4+ T cell absolute and percentage mean counts were 341.5 cells/mmc and 19%, respectively, and in patients with HIV RNA load <100.000 copies/ml were the majority (n = 192, 61.0%). Compared to outpatients, the inpatients’ cohort showed a significantly worse status, having a lower CD4+ T cell count (mean absolute count 250.3 cells/mmc, mean percentage 16.4%) and HIV RNA viral load >100.000 copies/ml in more than one-half of cases (n = 63, 56.2%).
Regarding the duration of infection at the time of HIV diagnosis, overall, 25.6% of patients were found at the AIDS stage, of whom just 19 were outpatients, while the great majority (n = 63) were inpatients, accounting for more than one-half of all inpatients (55.3%). A minority of patients (n = 37, 11.6%) were diagnosed with AEHI.
We observed a median time of 15 days (IQR 8.0-28.0) from the first positive HIV test to the first access to our Clinic.
ART initiation and regimens
Nine patients (all outpatients) never initiated ART. Characteristics of ART timing initiation and prescription are summarized in .
Table 2. Characteristics of ART timing initiation and prescription.
Overall, among patients receiving ART (n = 311) the median duration from the first Clinic access to ART prescription was 24 (IQR: 2.5-41.0) days, with a significantly lower time interval (4 days, IQR: 0.2-17.8) registered in inpatients (34 days, IQR: 9.0-44.0). Therefore, we distinguished three groups of patients according to the time of ART initiation from their first access to our clinic: 117 patients (37.6%), 46 outpatients (23.4%) and 71 inpatients (62.3%), started ART within 7 days (rapid start group). Sixty-four (20.6%) patients, 40 outpatients (20.3%) and 24 inpatients (21.1%) began ART between 8 and 30 days (intermediate start group). Lastly, 130 patients (41.8%), 111 outpatients (56.3%) and 19 inpatients (16.7%), started ART after 30 days (the late start group).
Regarding ART prescription attitude, a three-drug ART regimen was prescribed in all cases but two. The preferred 2 NRTIs back-bones were emtricitabine/tenofovir alafenamide (FTC/TAF) (37.2%), abacavir/lamivudine (ABC/3TC) (31.7%) and emtricitabine/tenofovir disoproxil (FTC/TDF) (29.8%). Among pharmacological classes used as the third drug, INSTIs were used in 243 cases (78.1%), with dolutegravir (DTG) as a preferred choice in 207 (66.6%) patients, boosted PIs (mostly darunavir) in 46 patients (14.7%) and NNRTI (only rilpivirine) in 28 cases (9.0%). A dual therapy containing DTG + 3TC was prescribed in just 2 cases.
Rates of retention in care and virological response during follow-up
As shown in , the median time to viral suppression was 84 days (IQR: 35.0-161.0). At the sixth month of follow-up the great majority of patients (n = 302, 94.4%) were still linked to care, while 4.7% were LTFU and just 3 patients had been transferred to another ID centre. By the twelfth month 87.5% of patients were actively followed, 9.4% were LTFU and 10 subjects were transferred (n = 5, 1.6%) or deceased (n = 5, 1.6%).
Table 3. Median time to viral suppression and rates of retention in care during follow-up.
and show the predictions of the proportion of patients achieving HIV RNA below 50 copies/ml during a 400 d of follow-up obtained by the Cox model setting initiation ART within 1 week, between the eighth and thirtieth day and after 4 weeks (rapid, intermediate and late start, respectively). At 3 months of follow-up the percentage of patients with virological success was 69.4%, 67.6% and 64.1% for the rapid start, the intermediate start and the late start group, respectively. After 6 months these proportions exceeded 90% in all groups, while at 12 months 99% of the patients in each group showed viral load suppression.
Figure 1. Time from ART starts to virological response (HIV RNA < 50 copies/ml) according to different timings of initiation (rapid, intermediate and late).
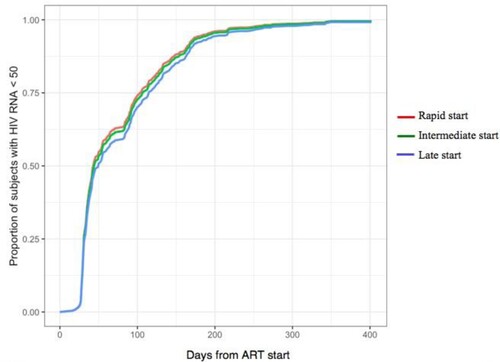
Table 4. Rates of viral suppression according to the ART initiation.
The Cox proportional hazard model was performed to investigate the association between ART start and virological response (HIV RNA < 50 copies/ml) adjusting for gender, age, being in- or outpatient, antiretroviral pharmacological class, HIV viral load at baseline and CD4+ T cell count ().
Table 5. Multivariable Cox Proportional Hazard model for Predictors of viral suppression.
Because of missing values among covariates, 293 subjects were included in the model. For each week awaiting initiation of therapy there was 4% less probability to achieve HIV RNA < 50 copies/ml (HR 0.96, 95% CI 0.93-0.99, p 0.016). Starting ART with boosted PI or NNRTI-containing regimens was less likely associated with virological response overtime than INSTI-containing initial regimen (PI vs INSTI HR 0.52, 95% CI 0.36-0.77 p 0.001; NNRTI vs INSTI HR 0.63, 95% CI 0.41-0.97 p 0.036). Baseline viro-immunological status was a significant predictor of virological response: higher viral load showed a statistically significant effect in reducing the probability of viral suppression (HR 0.85, 95% CI 0.80-0.90 p < 0.001), while a high CD4+ T cell count was associated with a higher probability to achieve viral suppression (HR 1.18, 95% CI 1.04-1.34 p 0.008). We observed no difference in the role of time to ART start on viral suppression between in- and outpatients (HR 1.03, 95% CI 0.97–1.10, p 0.280).
Discussion
In our cohort, the rapid ART approach has seemed to be useful to accelerate time to viral suppression which tends to be great over time regardless of the timing of ART initiation. This result is important considering that assessment of the long-term efficacy and acceptability of rapid ART in real-life practice remains challenging, especially in developed countries.
The proportion of patients receiving rapid ART, within 7 days from the first HIV medical referral, was higher in the inpatients’ cohort (62.3%) rather than the outpatients’ one (23.4%). This observation might be due to the greater probability of inpatients being diagnosed with HIV in an advanced stage of infection, as demonstrated by the high percentage of AIDS-defining events in the inpatients’ group (55.3%). These clinical conditions were already recognized by major national and international guidelines to require an immediate start of adequate therapy (EACS, Citation2020; Saag et al., Citation2018; SIMIT, Citation2017; Thompson et al., Citation2020). Recently, small cohorts from high-income settings also provided increasing evidence of the safety and acceptability of rapid ART initiation in the very early stage of infection out of clinical trials. For example, one cohort from London showed rapid viral suppression and high ART uptake by 24 weeks (99%) among those who initiated ART at the first medical appointment (Girometti et al., Citation2017). Similarly, another cohort from San Diego demonstrated increased ART uptake and a higher likelihood of viral suppression (91%) by 24 weeks for participants initiating ART within 7 days (Martin et al., Citation2021).
Nonetheless, clear evidence that certain timing of ART initiation impacts not on long-term care engagement and virologic suppression still lacks and previous studies carried out in high-income settings sometimes highlighted discordant results. Researchers from ICONA Foundation Study Cohort did not observe any clear benefit of rapid ART start in terms of virological success after one year of follow-up (D'Arminio Monforte et al., Citation2020). Differently, one cohort study from France even showed that a long time between the first medical visit and the first ART prescription was associated with better 1-year retention in care (Cuzin et al., Citation2019). By contrast, a real-life world retrospective study in Taiwan demonstrated a higher rate of attrition from care together with a lower rate of LTFU at 12 months in a population receiving ART within 7 days (Huang et al., Citation2019).
Our Clinic follows around 4000 HIV-infected patients from the Province of Brescia (Lombardy), which counts one of the highest incidences of HIV/AIDS diagnosis and the fourth largest migrant population in Italy (Caritas e Migrantes, Citation2019; ISS, Citation2020). Another study carried out in our HIV Clinic between 2012 and 2018 estimated a rate of 86.7% of outpatients retained in care with a rate of viral suppression (HIV RNA < 37 copies/ml) of more than 94% (Comelli et al., Citation2019). Although the current study corroborates our previous findings, with <10% rate of LTFU and almost 90% of patients still linked to care after 1 year of follow-up, we did not find a connection between the time of ART start and the likelihood of durable viral suppression. We observed a great rate of viral suppression (up to 99%) among people who remained in care regardless of the timing of ART initiation (rapid, intermediate and late).
Shortening the time to ART initiation leads to faster viral load suppression with incontrovertible individual and collective benefits (Cuzin et al., Citation2019; Hoenigl et al., Citation2016; OARAC, Citation2021; Pilcher et al., Citation2017; Radix & Shalev, Citation2021). Indeed, although the timing of ART initiation does not seem to be a successful guarantee of long-term retention in care (Ford et al., Citation2018; Rosen et al., Citation2016), one of the greatest goals of rapid ART start remains faster control of “community viremia”. In our population each week delaying the initiation of therapy brought to 4% less probability to reach an undetectable HIV viral load (see ). Most patients (41.8%) still began ART after one month after their first medical encounter and almost 3 months pass from ART prescription to the first undetectable HIV RNA, clearly more than what was observed by other cohorts (Girometti et al., Citation2017; Huang et al., Citation2019; Martin et al., Citation2021). This finding might be concerning from a public health point of view and there is room for improvement.
Obviously, fast initiation of treatment requires first removal of any cultural, financial and structural barriers which might prevent patients from diagnosis and care engagement. As well, optimizing strategies able to guarantee easy prompt access to HIV care remains a crucial topic (Montaner et al., Citation2014). Compared with published data, in our Clinic time from HIV diagnosis to linkage to care is still too long (around two weeks), as well as the interval from the first HIV referral to ART initiation is even wider, especially for outpatients who might have to wait for ART initiation up to 1 month (Cuzin et al., Citation2019; D'Arminio Monforte et al., Citation2020; Girometti et al., Citation2017; Martin et al., Citation2021). As previously underlined by d’Arminio Monforte and colleagues, this delay could be due not only to the prescription policy of the Centre but also to extrahospital settings where HIV diagnosis often occurs (i.e., Private laboratories, community-based checkpoints) and the patient’s referral process might be difficult (D'Arminio Monforte et al., Citation2020).
It is broadly recognized that predictors of virologic success include low baseline viremia, highly potent ART regimen, drug tolerability, the convenience of the regimen and appropriate adherence to the treatment (OARAC, Citation2021). Thus, as shown already by other cohorts, in our study the achievement of viral suppression was more likely associated with patients’ lower HIV RNA viral load and initial INSTI-containing regimens, the latter proven safe, non-inferior and less toxic in dual therapy for naïve HIV patients by GEMINI trials (Cahn et al., Citation2019; D'Arminio Monforte et al., Citation2020; Girometti et al., Citation2017; Hoenigl et al., Citation2016; Huang et al., Citation2019; Martin et al., Citation2021). In our cohort, anyway, even though DTG turned out to be largely prescribed, its use in dual therapy continues to lack, resulting in paltry compared with its proportion in a three-drug regimen.
Our study has several limitations. Its single-centre retrospective, observational design might present bias related to retrospective data collecting and poor generalizability of findings to other settings. Secondly, these data may suffer from the prescription habits of physicians from the same medical centre and HIV care experience. Thirdly, we did not characterize initial AIDS-defining events, including eventual TB co-infections, which have probably influenced differently the choice of ART start timing. Therefore, we cannot draw precise conclusions regarding the reasons behind clinicians’ decisions regarding ART timing initiation in such cases. Also, since September 2015, several aspects of our HIV continuum of care have been changing. On one hand, bureaucratic procedures have been simplified, on the other national and international guidelines have progressed and our clinical practice in HIV care and ART prescription attitudes too, going beyond the latest version of HIV care Italian guidelines from 2017, which recommended clinicians offering rapid ART initiation primarily to those highly motivated patients (SIMIT, Citation2017). However, DTG-based dual therapy in naïve patients has been implemented only in the last 24 months, due to the availability of a single tablet regimen and according to the results of newer randomized clinical trials on this topic. Nowadays, the main guidelines in HIV care strongly recommend offering rapid strategy in all but very few exceptions. Moreover, the results are not controlled for important risk factors of virologic failure such as adherence. However, in clinical practice we did not evaluate the adherence using standardized methods, but we looked at the therapy refill as a surrogate. We assumed that the maintenance in follow-up and the virological success might be the best proxy for the patients’ adherence. Finally, we must consider immortal-time bias and exposure misclassification since our ID Department is not a screening Centre. Therefore, there is an accounted latency time between the HIV test positivity and the first visit to the clinic.
The strength of our study is mainly due to its perspective of “real -life” setting over a 4-year period in HIV care still deeply unexplored. Moreover, differently from most published studies, we included both in- and outpatients’ cohorts reflecting our clinical practice even in severely ill patients.
Conclusions
Ensuring the best HIV linkage to care and adequate therapy adherence remains a priority in HIV continuum of care, to successfully pursue the “third 90” goal advocated by United Nations Program UNAIDS (Joint United Nations Programme on HIV/AIDS [UNAIDS], Citation2014). In a real-life setting of a high-income country like Italy, where a public national health system guarantees equal good-quality care and personalized health care is promoted, we may give priority to each patient’s clinical and social conditions as guidance to establish the best-individualized timing for antiretroviral therapy initiation.
Authors’ contributions
EQR, II and EF contributed to study design, analysis and data interpretation. SR and SC determined statistical methods and performed the statistical analysis. NG and GF contributed to data collection. NG and EF first drafted the article. EQR, EF and FC contributed to the critical reviewing of the article. All authors reviewed the article, read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was conducted in accordance with the rules of good clinical practice (GCP-ICH) and according to the Declaration of Helsinki. The study was approved by the Ethical Board of the Brescia Province on October 1 2020 and all efforts were made to obtain the patient’s written consent.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank the participants who contributed to this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Boyd, M. A., Boffito, M., Castagna, A., & Estrada, V. (2019). Rapid initiation of antiretroviral therapy at HIV diagnosis: Definition, process, knowledge gaps. HIV Medicine, 20(1), 3–11. https://doi.org/10.1111/hiv.12708
- Cahn, P., Madero, J. S., Arribas, J. R., Antinori, A., Ortiz, R., Clarke, A. E., Hung, C.-C., Rockstroh, J. K., Girard, P.-M., Sievers, J., Man, C., Currie, A., Underwood, M., Tenorio, A. R., Pappa, K., Wynne, B., Fettiplace, A., Gartland, M., Aboud, M., … Perea, R. T. (2019). Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. The Lancet, 393(10167), 143–155. https://doi.org/10.1016/S0140-6736(18)32462-0
- Caritas e Migrantes. (2019). XXVIII Rapporto Immigrazione 2018-2019. https://www.migrantes.it/wp-content/uploads/sites/50/2019/09/20190927-XXVIII-RICM-Presentazione.pdf
- Coffey, S., Bacchetti, P., Sachdev, D., Bacon, O., Jones, D., Ospina-Norvell, C., Torres, S., Lynch, E., Camp, C., Mercer-Slomoff, R., Lee, S., Christopoulos, K., Pilcher, C., Hsu, L., Jin, C., Scheer, S., Havlir, D., & Gandhi, M. (2019). RAPID antiretroviral therapy: High virologic suppression rates with immediate antiretroviral therapy initiation in a vulnerable urban clinic population. AIDS (London, England), 33(5), 825–832. https://doi.org/10.1097/QAD.0000000000002124
- Cohen, M. S., Chen, Y. Q., McCauley, M., Gamble, T., Hosseinipour, M. C., Kumarasamy, N., Hakim, J. G., Kumwenda, J., Grinsztejn, B., Pilotto, J. H., Godbole, S. V., Chariyalertsak, S., Santos, B. R., Mayer, K. H., Hoffman, I. F., Eshleman, S. H., Piwowar-Manning, E., Cottle, L., Zhang, X. C., Makhema, J., … HPTN 052 Study Team (2016). Antiretroviral therapy for the prevention of HIV-1 transmission. The New England Journal of Medicine, 375(9), 830–839. https://doi.org/10.1056/NEJMoa1600693
- Cohen, M. S., Chen, Y. Q., McCauley, M., Gamble, T., Hosseinipour, M. C., Kumarasamy, N., Hakim, J. G., Kumwenda, J., Grinsztejn, B., Pilotto, J. H. S., Godbole, S. V., Mehendale, S., Chariyalertsak, S., Santos, B. R., Mayer, K. H., Hoffman, I. F., Eshleman, S. H., Piwowar-Manning, E., Wang, L., … Khptn 052 Study Team (2011). Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine, 365(6), 493–505. https://doi.org/10.1056/NEJMoa1105243
- Comelli, A., Izzo, I., Donato, F., Celotti, A., Focà, E., Pezzoli, C., Castelli, F., & Quiros-Roldan, E. (2019). Disengagement and reengagement of HIV continuum of care in a single center cohort in northern Italy. HIV Research & Clinical Practice, 20(1), 1–11. https://doi.org/10.1080/15284336.2019.1595887
- Cuzin, L., Cotte, L., Delpierre, C., Allavena, C., Valantin, M. A., Rey, D., Delobel, P., Pugliese, P., Raffi, F., Cabié, A., & Dat’AIDS Study group (2019). Too fast to stay on track? Shorter time to first anti-retroviral regimen is not associated with better retention in care in the French Dat'AIDS cohort. PloS one, 14(9), e0222067. https://doi.org/10.1371/journal.pone.0222067
- D'Arminio Monforte, A., Tavelli, A., Cozzi-Lepri, A., Castagna, A., Passerini, S., Francisci, D., Saracino, A., Maggiolo, F., Lapadula, G., Girardi, E., Perno, C. F., Antinori, A., & Icona Foundation Study Group (2020). Virological response and retention in care according to time of starting ART in Italy: Data from the icona foundation study cohort. Journal of Antimicrobial Chemotherapy, 75(3), 681–689. https://doi.org/10.1093/jac/dkz512
- EACS. (2020). EACS guidelines version 10.1. https://www.eacsociety.org/files/guidelines-10.1_finaljan2021_1.pdf
- Fiebig, E. W., Wright, D. J., Rawal, B. D., Garrett, P., Schumacher, R., Peddada, L., Heldebrant, C., Smith, R., Conrad, A., Kleinman, S., & Busch, M. (2003). Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. AIDS (London, England), 17(13), 1871–1879. https://doi.org/10.1097/00002030-200309050-00005
- Ford, N., Migone, C., Calmy, A., Kerschberger, B., Kanters, S., Nsanzimana, S., Mills, E. J., Meintjes, G., Vitoria, M., Doherty, M., & Shubber, Z. (2018). Benefits and risks of rapid initiation of antiretroviral therapy. AIDS (London, England), 32(1), 17–23. https://doi.org/10.1097/QAD.0000000000001671
- Girometti, N., Nwokolo, N., McOwan, A., & Whitlock, G. (2017). Outcomes of acutely HIV-1-infected individuals following rapid antiretroviral therapy initiation. Antiviral Therapy, 22(1), 77–80. https://doi.org/10.3851/IMP3080
- Hoenigl, M., Chaillon, A., Moore, D. J., Morris, S. R., Mehta, S. R., Gianella, S., Amico, K. R., & Little, S. J. (2016). Rapid HIV viral load suppression in those initiating antiretroviral therapy at first visit after HIV diagnosis. Scientific Reports, 6(1), 32947. https://doi.org/10.1038/srep32947
- Huang, Y. C., Sun, H. Y., Chuang, Y. C., Huang, Y. S., Lin, K. Y., Huang, S. H., Chen, G. J., Luo, Y. Z., Wu, P. Y., Liu, W. C., Hung, C. C., & Chang, S. C. (2019). Short-term outcomes of rapid initiation of antiretroviral therapy among HIV-positive patients: Real-world experience from a single-centre retrospective cohort in Taiwan. BMJ Open, 9(9), e033246. https://doi.org/10.1136/bmjopen-2019-033246
- INSIGHT START Study Group, Lundgren, J. D., Babiker, A. G., Gordin, F., Emery, S., Grund, B., Sharma, S., Avihingsanon, A., Cooper, D. A., Fätkenheuer, G., Llibre, J. M., Molina, J. M., Munderi, P., Schechter, M., Wood, R., Klingman, K. L., Collins, S., Lane, H. C., Phillips, A. N., & Neaton, J. D. (2015). Initiation of antiretroviral therapy in early asymptomatic HIV infection. The New England Journal of Medicine, 373(9), 795–807. https://doi.org/10.1056/NEJMoa1506816
- Istituto Superiore di Sanità. (2020, November). Notiziario dell’Istituto Superiore di Sanità, 33(11). http://www.salute.gov.it/imgs/C_17_pubblicazioni_2979_allegato.pdf
- Joint United Nations Programme on HIV/AIDS (UNAIDS). (2014). 90-90-90: An ambitious treatment target to help end the AIDS epidemic. https://www.unaids.org/en/resources/documents/2017/90-90-90
- Koenig, S. P., Dorvil, N., Dévieux, J. G., Hedt-Gauthier, B. L., Riviere, C., Faustin, M., Lavoile, K., Perodin, C., Apollon, A., Duverger, L., McNairy, M. L., Hennessey, K. A., Souroutzidis, A., Cremieux, P. Y., Severe, P., & Pape, J. W. (2017). Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomized unblinded trial. PLoS Medicine, 14(7), e1002357. https://doi.org/10.1371/journal.pmed.1002357
- Labhardt, N. D., Ringera, I., Lejone, T. I., Klimkait, T., Muhairwe, J., Amstutz, A., & Glass, T. R. (2018). Effect of offering same-day ART vs usual health facility referral during home-based HIV testing on linkage to care and viral suppression Among adults With HIV in Lesotho: The CASCADE randomized clinical trial. JAMA, 319(11), 1103–1112. https://doi.org/10.1001/jama.2018.1818
- Lapadula, G., Chatenoud, L., Gori, A., Castelli, F., Di Giambenedetto, S., Fabbiani, M., Maggiolo, F., Focà, E., Ladisa, N., Sighinolfi, L., Di Pietro, M., Pan, A., Torti, C., & Italian MASTER Cohort (2015). Risk of severe non AIDS events Is increased among patients unable to increase their CD4+ T-cell counts >200+/μl despite effective HAART. PloS one, 10(5), e0124741. https://doi.org/10.1371/journal.pone.0124741
- Martin, T. C. S., Abrams, M., Anderson, C., & Little, S. J. (2021). Rapid antiretroviral therapy Among individuals With acute and early HIV. Clinical Infectious Diseases, 73(1), 130–133. https://doi.org/10.1093/cid/ciaa1174
- Mateo-Urdiales, A., Johnson, S., Smith, R., Nachega, J. B., & Eshun-Wilson, I. (2019). Rapid initiation of antiretroviral therapy for people living with HIV. Cochrane Database of Systematic Reviews, 6(6), 18–19. https://doi.org/10.1002/14651858.CD012962.pub2
- Montaner, J. S., Lima, V. D., Harrigan, P. R., Lourenço, L., Yip, B., Nosyk, B., Wood, E., Kerr, T., Shannon, K., Moore, D., Hogg, R. S., Barrios, R., Gilbert, M., Krajden, M., Gustafson, R., Daly, P., & Kendall, P. (2014). Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: The “HIV treatment as prevention” experience in a Canadian setting. PloS one, 9(2), e87872. https://doi.org/10.1371/journal.pone.0087872
- Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council (OARAC). (2021). Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. U.S. Department of Health & Human Services. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
- Pilcher, C. D., Ospina-Norvell, C., Dasgupta, A., Jones, D., Hartogensis, W., Torres, S., Calderon, F., Demicco, E., Geng, E., Gandhi, M., Havlir, D. V., & Hatano, H. (2017). The effect of same-day observed initiation of antiretroviral therapy on HIV viral load and treatment outcomes in a US public health setting. Journal of Acquired Immune Deficiency Syndromes (1999), 74(1), 44–51. https://doi.org/10.1097/QAI.0000000000001134
- Radix, A., & Shalev, N. (2021). When to Initiate Antiretroviral Therapy, With Protocol for Rapid Initiation. Johns Hopkins University. https://www.ncbi.nlm.nih.gov/books/NBK557123/
- Rosen, S., Maskew, M., Fox, M. P., Nyoni, C., Mongwenyana, C., Malete, G., Sanne, I., Bokaba, D., Sauls, C., Rohr, J., & Long, L. (2016). Initiating antiretroviral therapy for HIV at a patient's first clinic visit: The RapIT randomized controlled trial. PLoS Medicine, 13(5), e1002015. https://doi.org/10.1371/journal.pmed.1002015
- Saag, M. S., Benson, C. A., Gandhi, R. T., Hoy, J. F., Landovitz, R. J., Mugavero, M. J., Sax, P. E., Smith, D. M., Thompson, M. A., Buchbinder, S. P., Del Rio, C., Eron, J. J. J., Fätkenheuer, G., Günthard, H. F., Molina, J. M., Jacobsen, D. M., & Volberding, P. A. (2018). Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the international antiviral society-USA panel. JAMA, 320(4), 379–396. https://doi.org/10.1001/jama.2018.8431
- SIMIT. (2017). Linee guida Italiane sull’utilizzo della Terapia Antiretrovirale e la gestione diagnostico-clinica delle persone con infezione da HIV-1. https://www.salute.gov.it/imgs/C_17_pubblicazioni_2696_allegato.pdf
- Thompson, M. A., Horberg, M. A., Agwu, A. L., Colasanti, J. A., Jain, M. A., Short, W. R., Singh, T., & Aberg, J. A. (2020). Primary care guidance for persons With human immunodeficiency virus: 2020 update by the HIV medicine association of the infectious diseases society of America. Clinical Infectious Diseases, 73(11), e3572–e3605. https://doi.org/10.1093/cid/ciaa1391
- World Health Organization. (2017). Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. https://www.who.int/hiv/pub/guidelines/advanced-HIV-disease/en/
- World Health Organization. (2021). Updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring. https://www.who.int/publications/i/item/9789240022232
