ABSTRACT
We estimate the effectiveness of antiretroviral therapy (ART) among individuals receiving HIV care in Rio de Janeiro, Brazil. Adults (18y+) initiating ART between Jan/2008 and Dec/2018 (follow-up through Dec/2020) were included. First-line ART (two nucleoside reverse transcriptase inhibitors plus one antiretroviral from another class) was categorized into four categories: non-nucleoside reverse transcriptase inhibitor/NNRTI-based, protease inhibitor/PI-based, integrase strand transfer inhibitor/INSTI-based, and single-tablet regimen (STR, Tenofovir 300mg + Lamivudine 300mg + Efavirenz 600mg). Effectiveness (viral load ≤50 copies/µL) was evaluated at 6(3–9) and 12(9–15) months from ART initiation. Bayesian logistic regression models were used to quantify the association between exposure and outcomes while accounting for missing data. Overall, 1863(57%), 652(19.9%), 412(12.6%), and 342(10.5%) individuals used, respectively, NNRTI-based, PI-based, INSTI-based regimens, and STR. Compared to NNRTIs, the odds of viral suppression with INSTI-based regimens was 76% higher (adjusted OR:1.76, 95%CI:1.23–2.51) at six months but no higher at 12 months. Older age, higher education, CD4 count ≥500 cells/mm3 and viral load <100,000 copies/µL at ART initiation increased the odds of viral suppression. Viral suppression at six months was the strongest predictor of viral suppression at 12 months. These results highlight population groups that could benefit from close monitoring during the first year of ART.
Background
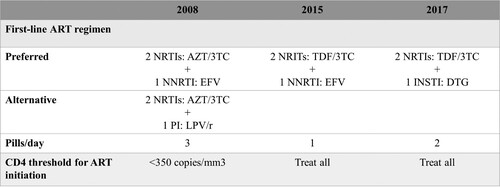
In Brazil, a national program of universal access to antiretroviral therapy (ART) has been in place since 1996. ART is offered within the Unified Health System (SUS, Sistema Único de Saúde), a government-run, publicly funded health care system that is entirely free of any cost at the point of service for any person. Guidelines for when, how and for whom ART should be started are elaborated by the national ART program and regularly updated with new antiretrovirals as well as revised recommendations for first-line and salvage regimens (the most recent as well as previous guidelines can be found at MS, Citation2018). Over the years, several therapeutic modifications have been recommended reflecting advancements in HIV treatment.
Of relevance to this study (), in 2008, guidelines for first-line ART included a preferred regimen that had non-nucleoside reverse transcriptase inhibitors (NNRTIs) as “core” drugs and an alternative regimen that had protease inhibitors (PIs) as “core” drugs, both were to be associated with 2 nucleoside reverse transcriptase inhibitors (NRTIs). At the time, immunologic criteria for treatment initiation was at 350 cells/mL CD4 cells (MS, Citation2008). In 2015, immunologic criteria had already been removed such that ART was to be offered to all people living with HIV (PWHIV) and recommended first-line ART was a single tablet regimen (STR) of Tenofovir 300 mg, Lamivudine 300 mg, and Efavirenz 600 mg. Later, in late 2017, guidelines were updated to incorporate WHO’s recommended first-line regimens that had dolutegravir, an integrase strand transfer inhibitor (INSTI), as the “core” drug (MS, Citation2018).
Figure 1. Temporal evolution first-line regimens and immunologic criteria for treatment initiation based on Brazilian ART guidelines, 2008–2017.
A cohort study conducted in Rio de Janeiro from 2000 to 2010 was one of the first to report on the effectiveness of first-line NNRTI-based regimens when compared to PI-based regimens: 79% of participants achieved viral suppression (viral load ≤ 400 copies/mL) at six months with NNRTIs compared to 71% with PIs (Cardoso et al., Citation2014a). Later, a study conducted among PWHIV initiating ART in Belo Horizonte between 2014 and 2015 reported 81% effectiveness at six months with STRs, where adherence was found to be a significant predictor of viral suppression (Costa et al., Citation2018b), as systematic reviews with meta-analysis have also evidenced (Clay et al., Citation2015, Citation2018). In a national study among over 100,000 PWHIV who initiated first-line ART between January 2014 and June 2017, viral suppression (viral load < 50 copies/mL) was achieved by 84 and 90% of those using NNRTI-based regimens (tenofovir/TDF + lamivudine/3TC + efavirenz/EFV used by 72% of the study population used) and INSTI-based regimens (TDF + 3TC + dolutegravir/DTG used by 10% of the study population), respectively (Meireles et al., Citation2019). In the present study, we investigated whether the incorporation of INSTI for first-line regimens has resulted in increased real-world effectiveness in a single tertiary health care service in Rio de Janeiro, Brazil.
Methods
Study population
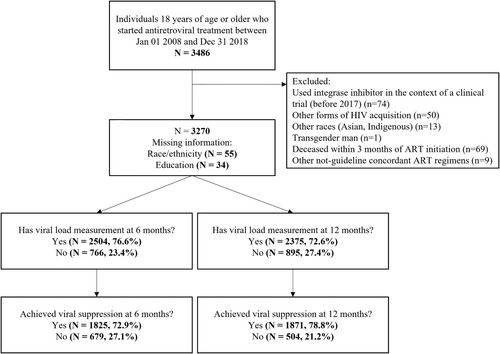
HIV care and treatment are provided at Instituto Nacional de Infectologia Evandro Chagas/Fundação Oswaldo Cruz (INI/FIOCRUZ) since 1986. Data from the longitudinal observational clinical cohort of PWHIV receiving care at INI is updated regularly and has been used in several studies (Coelho et al., Citation2016; Grinsztejn et al., Citation2013; Teixeira da Silva et al., Citation2017). For the present study, the population included adults (≥18 years) living with HIV who started ART between 1 January 2008, and 31 December 2018, with follow-up information through 31 December 2020. As ART regimen was our primary exposure of interest, only adults initiating ART regimens concordant with Brazilian guidelines (as per ) were included. ART was defined as two nucleoside reverse transcriptase inhibitors (NRTI) in combination with an “core” drug that was either a non-nucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI), or integrase strand transfer inhibitor (INSTI). Exclusion criteria included: individuals who used INSTI-based first-line regimens before 2017, subpopulations not adequately represented in the sample, individuals using non-guideline concordant ART regimens, and individuals who died immediately after ART start, defined as with <3 months of follow-up.
Study definitions
Exposure
For the primary exposure of interest, the first-line ART regimen, we used information on the prescribed antiretrovirals to define four mutually exclusive categories: NNRTI-based regimens (composed of at least 2 NRTIs plus 1 NNRTI), PI-based regimens (composed of at least 2 NRTIs plus 1 ritonavir boosted PI), INSTI-based regimens (composed of at least 2 NRTIs plus 1 INSTI), and a single-tablet regimen (STR, composed of 2 NRTIs [Tenofovir 300 mg + Lamivudine 300 mg] plus 1 NNRTI [Efavirenz 600 mg] as a single pill).
Outcomes
ART effectiveness was defined as having an HIV-1 viral load ≤ 50 copies/µL. We evaluated effectiveness at six and 12 months from ART initiation with grace periods defined for each time point as 3–9 months as 9–15 months, respectively. The viral load measurement occurring closest to the target time point (either before or after) was chosen within each window. Additionally, we report the CD4 cell counts at six and 12 months from ART initiation, with grace periods defined above. The CD4 cell count occurring closest to the target time point (either before or after) was chosen within each window.
Covariates
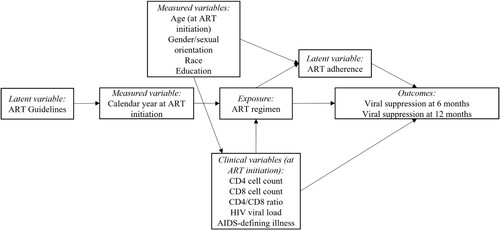
An operational model was constructed to represent the hypothesized relationship between first-line ART regimen and viral suppression, as well as other covariates (). Based on the authors’ conceptualization of the problem and published literature, we represented both objectively measured and latent variables as needed to portray the relationship among covariates. Objectively measured variables were age, gender/sexual orientation, skin color/race, level of education, and calendar year at ART initiation. The gender/sexual orientation variable combines information of a person’s gender identity and sexual orientation to create the following categories: cisgender women, cisgender men who have sex with men, heterosexual cisgender men, and other cisgender men for whom the mode of HIV acquisition is unknown, and transgender women. In addition, we categorized education according to UNESCO’s International Standard Classification of Education into the following strata: (1) incomplete basic education comprising illiteracy and incomplete early childhood education, (2) basic education from sixth to ninth grade, (3) secondary level education that includes individuals who have completed high school and (4) tertiary level education comprising any level of education beyond high school, including undergraduate and graduate degree (Socio-economic et al., Citation2014). ART adherence, a latent variable, was unfortunately not measured. The clinical variables measured at ART initiation included CD4 and CD8 cell counts (window of 6 months prior to 1 month after), CD4/CD8 ratio, HIV viral load (window of 6 months prior to 7 days after), and presence of an AIDS-defining illness (window of 6 months prior to 1 month after).
Statistical analysis
Median and interquartile range (IQR) and absolute number and proportions were used to describe the characteristics of the study population according to the exposure of interest (Hayes-Larson et al., Citation2019). Statistical differences were assessed with the Kruskal–Wallis test for continuous asymmetric variables and with the chi-squared test for categorical variables. We used the chi-squared test for trends to assess differences in the proportion over the years.
Though only a few participants had missing socio-demographic information (, race [n = 55, 1.7%], education [n = 34, 1%]), a significant fraction did not have laboratory measurements at ART initiation: CD4 cell count (n = 438, 13.4%), CD8 cell count (n = 861, 26.3%), CD4/CD8 ratio (863, 26.3%), or an HIV viral load (n = 720, 22.0%). We use Gamma-distribution regression models to impute the missing CD4 and CD8 data and Normal-distribution regression models to impute the log of the HIV viral load with a Bayesian approach (Migon et al., Citation2015). In this framework, the missing values are estimated directly from the model, as predicted values. The following variables were used as independent variables in both the Gamma- and Normal-distribution regression models: age, race/ethnicity, gender/sexual orientation, education, and presence of an AIDS-defining illness at ART initiation. For each dependent variable (CD4 cell count, CD8 cell count, and log of the HIV viral load), the initial model contained the five potential independent variables. Other models were then explored where one or a subset of the variables were removed from this initial model; the final model was determined based on Watanabe-Akaike information criterion (WAIC) (Watanabe, Citation2010). We present models’ results as Supplementary Material.
Figure 3. Flowchart of the study population, INI Clinical Cohort, Rio de Janeiro, Brazil, 2008–2018.
After modeling the missing data for the three variables (CD4 cell count, CD8 cell count, and log of the HIV viral load), the missing values were replaced with the posterior means from the predictive distributions to proceed with the Bayesian logistic regression models of the two outcomes of interest, viral suppression at six and 12 months (Little & Rubin, Citation2002). From the Bayesian point of view, the model's specification is complete when prior distributions are assigned to the hyperparameters. We used a normal distribution with mean zero and variance equal to 1000 as the priors for the regression model coefficients. The parametric inference was approximated using Integrated Nested Laplace Approximations (INLA) (Rue et al., Citation2009). The following variables were used as potential independent variables of the two outcomes of interest: age, race/ethnicity, gender/sexual orientation, and education, as well as the clinical variables measured at ART initiation: CD4 and CD8 cell counts, CD4/CD8 ratio, HIV viral load, and presence of an AIDS-defining illness. After including the primary exposure of interest (first-line ART regimen), other variables were sequentially included starting with the proximal variables (i.e., clinical variables) and later including the socio-demographic variables. The regression model for viral suppression at 12 months also included viral suppression at six months as a potential independent variable, missing values (n = 766, 23.4%) were replaced with the posterior means from the predictive distribution. As for the previous models, the final model was determined based on Watanabe-Akaike information criterion (WAIC).
All analyzes were performed in R version 4.04 (R Core Team, Citation2020) using the R-INLA package (Project, Citation2021).
Results
From 1 January 2008, to 31 December 2018, 3486 individuals 18 years of age or older initiated ART at INI/FIOCRUZ (). From these, 217 were excluded. We excluded participants who started INSTI-based first-line regimens before 2017 as these represent the use of such drugs within the context of clinical trials (n = 74). Additionally, given the small sample size and therefore our inability to accurately speak of certain subpopulations, we excluded participants that had acquired HIV through mother-to-child transmission (n = 9), occupational exposure (n = 6), blood transfusion (n = 15), and injection drug use (n = 20), as well as participants of Asian or Indigenous race/ethnicity (n = 13) and one transgender man. Moreover, those who died within 90 days of starting ART were excluded as they no longer contributed to the risk set (n = 69). Finally, 9 participants started ART regimens that were non-concordant with Brazilian guidelines and were therefore excluded.
The final study population included 3270 individuals. Overall, 1862 (56.9%), 652 (19.9%), 412 (12.6%), and 344 (10.5%) individuals used NNRTI-based, PI-based, INSTI-based regimens, and the STR, respectively. As expected, given the changes in Brazilian guidelines, the proportional use ART regimens changed significantly over time with a predominance of NNRTI-based regimens in 2008–2014, STR in 2015–2016, and INSTI-based regimens in 2017–2018 ().
Figure 4. Percentage of individuals initiating first-line antiretroviral therapy (ART) by type of ART regimen (NNRTI-based regimens [at least 2 NRTIs plus 1 NNRTI], PI-based regimens [at least 2 NRTIs plus 1 PI], INSTI-based regimens [at least 2 NRTIs plus 1 INSTI], and single-tablet regimen [STR, Tenofovir 300 mg, Lamivudine 300 mg plus Efavirenz 600 mg as a single pill]) and year, INI Clinical Cohort, Rio de Janeiro, Brazil, 2008–2018.
![Figure 4. Percentage of individuals initiating first-line antiretroviral therapy (ART) by type of ART regimen (NNRTI-based regimens [at least 2 NRTIs plus 1 NNRTI], PI-based regimens [at least 2 NRTIs plus 1 PI], INSTI-based regimens [at least 2 NRTIs plus 1 INSTI], and single-tablet regimen [STR, Tenofovir 300 mg, Lamivudine 300 mg plus Efavirenz 600 mg as a single pill]) and year, INI Clinical Cohort, Rio de Janeiro, Brazil, 2008–2018.](/cms/asset/dd905572-46ea-4ebd-bc4d-208d91c0f7ee/caic_a_2190954_f0004_ob.jpg)
Overall, the median age at ART initiation was 33.9 years, 25% were cis-women, and 39.8% were cis-men who have sex with men. Most participants were non-white (22.7% Black and 37.3% mixed/Pardo, ). Clinically, at ART initiation, median CD4 and CD8 cell counts were 292 and 900 cells/µL, respectively, and around a third of participants had >100,000 HIV-1 viral load copies/mL or an opportunistic illness. There were significant differences when comparing sociodemographic and clinic characteristics among participants initiating each type of ART regimen. Compared to those initiating an NNRTI- or PI-based regimens, those initiating INSTI-based regimens or STR (i.e., those initiating ART from 2015 onwards) were more likely to be younger, non-white, cis-men who have sex with men, and have tertiary education. Clinically, these individuals had higher CD4 and CD8 cell counts, and a higher percentage had a CD4/CD8 ratio greater than 0.20.
Table 1. Socio-demographic and clinical characteristics at first-line antiretroviral therapy (ART) initiation, overall, and by first-line ART regimen, INI Clinical Cohort, 2008-2018, Rio de Janeiro, Brazil.
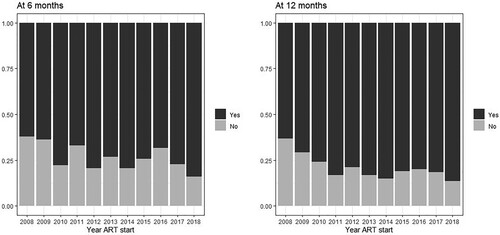
Across all years, viral suppression was observed for 1825 (72.9%) at six months and 1871 (78.8%) at 12 months (). Overall, 766 (23.4%) and 895 (27.4%) individuals did not have HIV viral load measurements within the established time frames for the 6- and 12-months outcomes, respectively (). shows how viral load measurements availability have decreased over time (Chi-square test p < 0.001) to a maximum of approximately 40% in 2018. This pattern is contrasted by viral suppression results, which have increased over time (, Chi-square test p < 0.001) to a maximum of approximately 85%. When stratified by ART regimen, across all years, viral suppression at six months was observed for 72%, 69%, 83%, and 75% of the individuals starting, respectively, NNRTI-based, PI-based, INSTI-based regimens, and STR. The corresponding proportions at 12-months were 79%, 74%, 87%, and 80%. The “core” antiretrovirals most frequently used in each of the regimens were: for NNRTI-based regimens: 98% used efavirenz (1.7% nevirapine); for PI-based regimens: 45% used boosted atazanavir (37% boosted lopinavir); and for INSTI-based regimens: 90% used dolutegravir (10% raltegravir).
Figure 5. Viral load availability: percentage of individuals with viral load measurements at 6- (left) and 12-months (right) of initiating ART by year of ART initiation, INI Clinical Cohort, Rio de Janeiro, Brazil, 2008–2018.
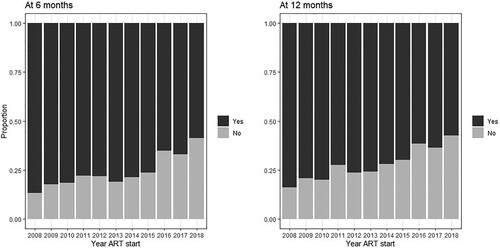
Figure 6. Viral suppression: percentage of individuals with viral suppresion at 6- (left) and 12-months (right) of initiating ART by year of ART initiation, INI Clinical Cohort, Rio de Janeiro, Brazil, 2008–2018.
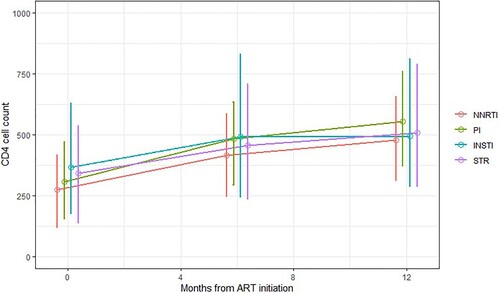
shows how the median CD4 cell count (and interquartile ranged) varied from baseline to 6 and 12 months. Median CD4 cell count was highest for INSTI-based regimens at baseline and 6-months (491, IQR 245-829) and lowest for NNRTI-based regimens (at 6 months: 415 [IQR 248-584]). Though still within the ∼500 cells range, median CD4 cell count at 12-months were higher than the 6-months values for all ART regimens with the highest observed values for PI-based regimens.
Figure 7. Immune response: CD4 cell counts (median and interquartile range) at ART initiation and at 6- and 12-months after initiating ART by ART regimen, INI Clinical Cohort, Rio de Janeiro, Brazil, 2008–2018.
The first-line ART regimen was significantly associated with viral suppression at six and 12 months in unadjusted models but remained statistically significant only in the adjusted model for viral suppression at six months ( and ). Compared to the effectiveness of NNRTI-based regimens, the odds of achieving viral suppression at six months with INSTI-based regimens was 89% higher (OR 1.89, 95% credibility interval [95%CI] 1.34-2.67) in unadjusted and 76% higher (aOR 1.76, 95%CI 1.23-2.51) in adjusted models (). Other factors significantly associated with viral suppression at six months included age (increased odds of viral suppression with increasing age), gender/sexual orientation (with cisgender men who have sex with men having higher odds of viral suppression compared to cisgender women). Education was associated with the outcome in a dose–response manner such that the higher the educational level, the greater the odds of viral suppression. Additionally, having a CD4 cell count of ≥500 cells/mm3 or a viral load <100,000 copies/mL at ART initiation both increased the odds of viral suppression.
Table 2. Unadjusted and adjusted odds ratio (95% credibility interval) for factors associated with viral suppression at six months, INI Clinical Cohort, 2008-2018, Rio de Janeiro, Brazil.
Table 3. Unadjusted and adjusted odds ratio (95% credibility interval) for factors associated with viral suppression at 12 months, INI Clinical Cohort, 2008-2018, Rio de Janeiro, Brazil.
Compared to the effectiveness of NNRTI-based regimens, the odds of achieving viral suppression at 12 months with INSTI-based regimens were 80% higher in unadjusted and 22% (non-significant) higher in adjusted models. Like the model for viral suppression at six months, age (increased odds of viral suppression with increasing age), gender/sexual orientation (with cisgender men who have sex with men having higher odds of viral suppression compared to cisgender women), and education (the higher the educational level, the greater the odds of viral suppression) were associated with the outcome at 12 months. The strongest predictor of viral suppression at 12 months was having achieved viral suppression at six months (aOR 8.70, 95%CI 6.91-10.94).
Discussion
Our findings show the superior effectiveness of the INSTI-based first-line ART regimen among PLWH followed at a tertiary health service in Rio de Janeiro, Brazil. Among individuals with viral load measurements within the established windows, a higher proportion achieved viral suppression at six and 12 months with INSTI-based regimens (83 and 87%). Moreover, when evaluating the association between ART regimen and viral suppression with Bayesian logistic regression models, results showed that INSTI-based regimens were statistically superior at 6 months of follow-up. Demographic (age, gender/sexual orientation and education) and clinical variables indicative of advanced disease at the time of ART initiation were also associated with the studied outcomes. Additionally, having achieved viral suppression at six months was strongly associated with viral suppression at 12 months.
Across the study period (2008–2018) and irrespective of the ART regimen, viral suppression was observed for 73% of participants at six months and 78% at 12 months. In a prior analysis of our cohort that included 1311 PWHIV enrolled from 2000 to 2010, similar results were reported, with 77% and 76% of participants achieving viral suppression at 6- and 12-months (Cardoso et al., Citation2014a). Given the study period of the prior study, only NNRTI-based and PI-based regimens were included, with results showing higher effectiveness for NNRIT-based regimens. Results for the STR were reported from a clinic-based cohort study from Belo Horizonte (Costa et al., Citation2018b), and effectiveness results were similar to those reported here: 81% at 6-months and 83% at 12 months. A national study reporting on the effectiveness of dolutegravir-based regimens estimated it at 90% at 12-months (Meireles et al., Citation2019) which is slightly higher than our 87%.
Our results align with recent systematic reviews and meta-analyzes that showed the superiority of INSTI-based regimens compared to other regimens (Balayan et al., Citation2017; Jiang et al., Citation2016). Of note is the almost exclusive representation of experimental studies in the published systematic reviews. Our results thus extend these findings to an observational study conducted in a middle-income country with free-of-charge access to ART. One of the reasons why INSTI-based regimens yields greater effectiveness may be due to greater tolerability and less side effects. A recent analysis of the ECOART cohort followed in Minas Gerais, Brazil, showed that participants who started a dolutegravir-based regimen had improved adherence (measured with an 8-item self-report instrument) compared to participants who used other multiple-dose regimens or a STR (Cardoso et al., Citation2019). That is, despite the intake of more than one pill when using an INSTI-based regimen, results showed no impact on adherence compared to STR. Another study from Brazil that used national data of over 100,000 ART-naïve PLWH who started treatment between 2014 and 2017 also showed greater effectiveness at 12-months of dolutegravir-based regimens (Meireles et al., Citation2019). In the same study, a sub-analysis restricted to individuals who did not change any antiretrovirals during the 12 months and maintained good treatment adherence suggested that dolutegravir’s superiority was not due solely to lower discontinuation of drugs or improved tolerability (Meireles et al., Citation2019).
In the present study, we could not assess the role of adherence on viral suppression because adherence data were not routinely collected. However, as presented in our operational model, we hypothesized that sociodemographic factors would be associated with the studied outcomes, as these factors have been shown to impact adherence in our setting (Costa et al., Citation2018a). Our results show an association of age, gender/sexual orientation, and education with viral suppression. These results are in accordance with prior analyzes of our cohort that evaluated the effectiveness of first-line regimens (Cardoso et al., Citation2014a) as well as outcomes of second-line ART regimens (Cardoso et al., Citation2014b). We found that for each 10-years increase in age, the odds of viral suppression increased 15% at six months and 28% at 12 months. These findings might result from an improved understanding of the importance of treatment and a greater commitment to oneself, both of which correlate with older age (Barclay et al., Citation2007; Sayegh et al., Citation2016). In addition, we found that MSM, when compared to cisgender women, had 32% and 83% higher odds of viral suppression at six and 12 months, respectively. Similar results were observed among participants of a clinical cohort in Belo Horizonte (Costa et al., Citation2018b), and also in a national analysis with MSM having the highest observed viral suppression of all groups (groups were defined by gender and probable mode of HIV acquisition) (Meireles et al., Citation2018). These findings may be the result of higher adherence among MSM, a hypothesis supported by a qualitative review of factors associated with adherence in Latin America, which found that most studies showed poorer adherence among women compared with men or heterosexuals in comparison to MSM (Costa et al., Citation2018a). Our results also show that those with higher education had increased odds of viral suppression at 6 and 12 months. Multiple previous studies of this cohort have shown that educational attainment adversely affects several cascade stages (Cardoso et al., Citation2014a, Citation2014b). A recent analysis reported on a causal mechanism linking education level, a proxy for socioeconomic status, to late initiation of ART (Rodrigues et al., Citation2021). Other work has shown how education/socioeconomic status is associated with access to health services with higher education leading to early HIV diagnosis and ART initiation (Girardi et al., Citation2004; Sobrino-Vegas et al., Citation2012; Socio-economic et al., Citation2014). Lastly, we found that having achieved viral suppression at six months was the strongest predictor of viral suppression at 12 months, a finding also reported in other studies from Brazil: odds of viral suppression at 12 months was eightfold higher among those achieving viral suppression at six months (Costa et al., Citation2018b).
Our results also highlight the effect of clinical variables on viral suppression. We found that compared to initiating ART with a CD4 cell count <200 cells/mm3, a CD4 cell count ≥500 cells/mm3 was associated with increased odds of viral suppression at six months. Similarly, initiating ART with a low HIV viral load burden (<100,000 copies/mL) led to a significant increase in the odds of viral suppression at six months. Although the threshold for initiation of ART that maximizes the benefit of therapy was debated for years, studies conducted over the past decade have reached a consensus that ART should be started as soon as possible (Group et al., Citation2015). Late ART initiation, i.e., at low CD4 cell counts, is associated with poorer prognosis and increased mortality (Kitahata et al., Citation2009). In this regard, it is disturbing to recognize that late ART initiation is widespread, as observed for our cohort (Rodrigues et al., Citation2021), for our region (Belaunzaran-Zamudio et al., Citation2020), and globally (IeDea et al., Citation2014). Our results highlight one of the consequences of late initiation of ART: poorer viral suppression.
Our study has limitations that should be acknowledged. First, the significant fraction of missing viral load measurements, at baseline, 6 and 12 months. Compared to prior studies from this cohort, and as the data here presented suggests, the percent of missing data has increased over time. To address this limitation, we used Bayesian logistic regressions for model estimation using a two-step approach that included modeling the baseline CD4, CD8 and HIV viral load data and then, subsequently, the outcome. Secondly, our analysis assumed an “intention to treat” approach, where changes to antiretrovirals over follow-up were not considered. This limitation may be particularly relevant for the year following a guideline update (in 2015 and 2017, respectively, when guidelines were updated to STR and INSTI-based regimens). Switches from previously recommended regimens to the new recommendations likely happened for a fraction of the participants. If the fraction of switches of antiretrovirals is large, then misclassification of exposure would be present. Thirdly, we assumed that individuals who died within 90 days of ART start were not at risk of experiencing the outcome of interest and were therefore excluded. An alternative assumption would have been to classify these individuals as having virologic failure. We believe the impact of our assumption on the results is likely minimal given that only 69 individuals were excluded for this reason (0.2%) and because the fraction of individuals using each ART regimen resembles that of the included participants (41 (59.4%), 22 (31.9%), 3 (4.3%), and 4 (4.3%) individuals used NNRTI-based, PI-based, INSTI-based regimens, and the STR, respectively). Lastly, it is essential to recognize that the population receiving care at our center constitutes a convenience sample of participants who reside in Rio de Janeiro city and its metropolitan region (Hovhannisyan et al., Citation2022). Moreover, our results cannot be extrapolated to individuals who acquired HIV from injection drug use or other non-sexual routes who were excluded from the present analysis due to their low prevalence in our setting (representing only 1.4% of the study population).
In conclusion, we used real-world clinic-based data from a middle-income country to provide evidence on the effectiveness of currently recommended first-line ART regimens. We found that initiating ART with INSTI-based regimens was associated with higher odds of viral suppression at 6 and 12 months of follow-up. These findings support Brazilian guidelines for HIV treatment which, in late 2017, switch its recommendations for first-line ART from NNRTI-based to INSTI-based regimens. Similarly, it supports WHO’s recommendation for national programs of lower/middle and high-income countries to transition to dolutegravir-based regimens. Beyond the ART regimen, age, gender/sexual orientation, education, and clinical markers of advanced HIV disease were also associated with the odds of viral suppression. These findings highlight population groups at increased odds of poor outcomes, younger PWHIV with low education, and advanced HIV disease, that could benefit from more closely monitoring of response upon ART initiation.
Ethical approval and consent to participate
This study was approved by the Ethics Committee of the Evandro Chagas Clinical Research Institute of the Oswaldo Cruz Foundation (INI/FIOCRUZ, CAAE 0032.0.009.000-10). It was conducted according to the principles expressed in the Declaration of Helsinki. All patient records/information were anonymized prior to analysis.
Supplemental Material
Download MS Word (158 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The final dataset used for the current study is available from the corresponding author on reasonable request.
Additional information
Funding
References
- Balayan, T., Horvath, H., & Rutherford, G. W. (2017). Ritonavir-Boosted darunavir plus two nucleoside reverse transcriptase inhibitors versus other regimens for initial antiretroviral therapy for people with HIV infection: A systematic review. AIDS Research and Treatment, 2017, 9. Article ID 2345617. https://doi.org/10.1155/2017/2345617
- Barclay, T. R., Hinkin, C. H., Castellon, S. A., Mason, K. I., Reinhard, M. J., Marion, S. D., Levine, A. J., & Durvasula, R. S. (2007). Age-associated predictors of medication adherence in HIV-positive adults: Health beliefs, self-efficacy, and neurocognitive status. Health Psychology, 26(1), 40–49. https://doi.org/10.1037/0278-6133.26.1.40
- Belaunzaran-Zamudio, P. F., Caro-Vega, Y. N., Shepherd, B. E., Rebeiro, P. F., Crabtree-Ramirez, B. E., Cortes, C. P., Grinsztejn, B., Gotuzzo, E., Mejia, F., Padgett, D., Pape, J. W., Rouzier, V., Veloso, V., Cardoso, S. W., McGowan, C. C., Sierra-Madero, J. G., Caribbean, C., & South America network for, H. I. V. e. (2020). The population impact of late presentation with advanced HIV disease and delayed antiretroviral therapy in adults receiving HIV care in Latin America. American Journal of Epidemiology, 189(6), 564–572. https://doi.org/10.1093/aje/kwz252
- Cardoso, S. W., Luz, P. M., Velasque, L., Torres, T., Coelho, L., Freedberg, K. A., Veloso, V. G., Walensky, R. P., & Grinsztejn, B. (2014a). Effectiveness of first-line antiretroviral therapy in the IPEC cohort, Rio de Janeiro, Brazil. AIDS Research and Therapy, 11(1), 29. https://doi.org/10.1186/1742-6405-11-29
- Cardoso, S. W., Luz, P. M., Velasque, L., Torres, T. S., Tavares, I. C., Ribeiro, S. R., Moreira, R. I., Veloso, V. G., Moore, R. D., & Grinsztejn, B. (2014b). Outcomes of second-line combination antiretroviral therapy for HIV-infected patients: A cohort study from Rio de Janeiro, Brazil. BMC Infectious Diseases, 14(1), 699. https://doi.org/10.1186/s12879-014-0699-5
- Cardoso, T. S., Costa, J. O., Reis, E. A., Silveira, M. R., Bonolo, P. F., Santos, S. F. D., & Ceccato, M. (2019). Which antiretroviral regimen is associated with higher adherence in Brazil? A comparison of single, multi, and dolutegravir-based regimens. Cadernos de Saúde Pública, 35(9), e00115518. https://doi.org/10.1590/0102-311x00115518
- Clay, P. G., Nag, S., Graham, C. M., & Narayanan, S. (2015). Meta-analysis of studies comparing single and multi-tablet fixed dose combination HIV treatment regimens. Medicine (Baltimore), 94(42), e1677. https://doi.org/10.1097/MD.0000000000001677
- Clay, P. G., Yuet, W. C., Moecklinghoff, C. H., Duchesne, I., Tronczynski, K. L., Shah, S., & Shao, D. (2018). A meta-analysis comparing 48-week treatment outcomes of single and multi-tablet antiretroviral regimens for the treatment of people living with HIV. AIDS Research and Therapy, 15(1), 17. https://doi.org/10.1186/s12981-018-0204-0
- Coelho, L. E., Cardoso, S. W., Amancio, R. T., Moreira, R. I., Ribeiro, S. R., Coelho, A. B., Campos, D. P., Veloso, V. G., Grinsztejn, B., & Luz, P. M. (2016). Predictors of opportunistic illnesses incidence in post combination antiretroviral therapy era in an urban cohort from Rio de Janeiro, Brazil. BMC Infectious Diseases, 16(1), 134. https://doi.org/10.1186/s12879-016-1462-x
- Costa, J. M., Torres, T. S., Coelho, L. E., & Luz, P. M. (2018a). Adherence to antiretroviral therapy for HIV/AIDS in Latin America and the Caribbean: Systematic review and meta-analysis. Journal of the International AIDS Society, 21(1), 1. https://doi.org/10.1002/jia2.25066
- Costa, J. O., Ceccato, M., Silveira, M. R., Bonolo, P. F., Reis, E. A., & Acurcio, F. A. (2018b). Effectiveness of antiretroviral therapy in the single-tablet regimen era. Revista de Saúde Pública, 52, 87. https://doi.org/10.11606/S1518-8787.2018052000399.
- Girardi, E., Aloisi, M. S., Arici, C., Pezzotti, P., Serraino, D., Balzano, R., Vigevani, G., Alberici, F., Ursitti, M., D'Alessandro, M., d'Arminio Monforte, A., Ippolito, G., & Group, I. C. B. E. S. (2004). Delayed presentation and late testing for HIV: Demographic and behavioral risk factors in a multicenter study in Italy. JAIDS Journal of Acquired Immune Deficiency Syndromes, 36(4), 951–959. https://doi.org/10.1097/00126334-200408010-00009
- Grinsztejn, B., Luz, P. M., Pacheco, A. G., Santos, D. V., Velasque, L., Moreira, R. I., Guimaraes, M. R., Nunes, E. P., Lemos, A. S., Ribeiro, S. R., Campos, D. P., Vitoria, M. A., & Veloso, V. G. (2013). Changing mortality profile among HIV-infected patients in Rio de Janeiro, Brazil: Shifting from AIDS to non-AIDS related conditions in the HAART era. PLoS One, 8(4), e59768. https://doi.org/10.1371/journal.pone.0059768
- Group, I. S. S., Lundgren, J. D., Babiker, A. G., Gordin, F., Emery, S., Grund, B., Sharma, S., Avihingsanon, A., Cooper, D. A., Fatkenheuer, G., Llibre, J. M., Molina, J. M., Munderi, P., Schechter, M., Wood, R., Klingman, K. L., Collins, S., Lane, H. C., Phillips, A. N., & Neaton, J. D. (2015). Initiation of antiretroviral therapy in early asymptomatic HIV infection. New England Journal of Medicine, 373(9), 795–807. https://doi.org/10.1056/NEJMoa1506816
- Hayes-Larson, E., Kezios, K. L., Mooney, S. J., & Lovasi, G. (2019). Who is in this study, anyway? Guidelines for a useful table 1. Journal of Clinical Epidemiology, 114, 125–132. https://doi.org/10.1016/j.jclinepi.2019.06.011
- Hovhannisyan, L., Coelho, L. E., Velasque, L., De Boni, R. B., Clark, J., Cardoso, S. W., Lake, J., Veloso, V. G., Grinsztejn, B., & Luz, P. M. (2022). Multilevel analysis of individual and neighborhood characteristics associated with viral suppression among adults with HIV in Rio de Janeiro, Brazil. AIDS and Behavior, 26(3), 947–962. https://doi.org/10.1007/s10461-021-03450-2
- IeDea, C. A. R. T. C., Avila, D., Althoff, K. N., Mugglin, C., Wools-Kaloustian, K., Koller, M., Dabis, F., Nash, D., Gsponer, T., Sungkanuparph, S., McGowan, C., May, M., Cooper, D., Chimbetete, C., Wolff, M., Collier, A., McManus, H., Davies, M. A., Costagliola, D., … & Egger, M. (2014). Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. JAIDS Journal of Acquired Immune Deficiency Syndromes, 65(1), e8–16. https://doi.org/10.1097/QAI.0b013e3182a39979
- Jiang, J., Xu, X., Guo, W., Su, J., Huang, J., Liang, B., Chen, H., Zang, N., Liao, Y., Ye, L., & Liang, H. (2016). Dolutegravir(DTG, S/GSK1349572) combined with other ARTs is superior to RAL- or EFV-based regimens for treatment of HIV-1 infection: A meta-analysis of randomized controlled trials. AIDS Research and Therapy, 13(1), 30. https://doi.org/10.1186/s12981-016-0115-x
- Kitahata, M. M., Gange, S. J., Abraham, A. G., Merriman, B., Saag, M. S., Justice, A. C., Hogg, R. S., Deeks, S. G., Eron, J. J., Brooks, J. T., Rourke, S. B., Gill, M. J., Bosch, R. J., Martin, J. N., Klein, M. B., Jacobson, L. P., Rodriguez, B., Sterling, T. R., Kirk, G. D., … Investigators, N.-A. (2009). Effect of early versus deferred antiretroviral therapy for HIV on survival. New England Journal of Medicine, 360(18), 1815–1826. https://doi.org/10.1056/NEJMoa0807252
- Little, R. J. A., & Rubin, D. B. (2002). Statistical analysis with missing data (2nd ed.). Wiley.
- Socio-economic inequalities and HIV Writing Group, Lodi, S., Dray-Spira, R., Touloumi, G., Braun, D., Teira, R., D'Arminio Monforte, A., Gallois, A., Zangerle, R., Spire, B., Dabis, F., Stahelin, C., Termote, M., Kirk, O., Chene, G., Egger, M., & del Amo, J. (2014). Delayed HIV diagnosis and initiation of antiretroviral therapy: Inequalities by educational level, COHERE in EuroCoord. Aids (london, England), 28(15), 2297–2306. https://doi.org/10.1097/QAD.0000000000000410
- Meireles, M. V., Pascom, A. R. P., & Duarte, E. C. (2018). Factors associated with early virological response in HIV-infected individuals starting antiretroviral therapy in Brazil (2014-2015): Results from a large HIV surveillance cohort. JAIDS Journal of Acquired Immune Deficiency Syndromes, 78(4), e19–e27. https://doi.org/10.1097/QAI.0000000000001684
- Meireles, M. V., Pascom, A. R. P., Duarte, E. C., & McFarland, W. (2019). Comparative effectiveness of first-line antiretroviral therapy: Results from a large real-world cohort after the implementation of dolutegravir. Aids (London, England), 33(10), 1663–1668. https://doi.org/10.1097/QAD.0000000000002254
- Migon, H. d. S., Gamerman, D., & Louzada-Neto, F. (2015). Statistical inference: An integrated approach (2nd ed.). CRC Press, Taylor & Francis Group.
- MS. (2008). Protocolo Clínico e Diretrizes Terapêuticas para Manejo da Infecção pelo HIV em Adultos. Accessed on October 25, 2021, from http://www.aids.gov.br/sites/default/files/pub/2016/59204/consensoadulto005c_2008montado.pdf
- MS. (2018). Protocolo clínico e diretrizes terapêuticas para manejo da infecção pelo HIV em adultos. Accessed on October 25, 2021, from http://www.aids.gov.br/pt-br/pub/2013/protocolo-clinico-e-diretrizes-terapeuticas-para-manejo-da-infeccao-pelo-hiv-em-adultos
- Project, T. R.-I. (2021). http://www.r-inla.org/
- R Core Team. (2020). R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/
- Rodrigues, A., Struchiner, C. J., Coelho, L. E., Veloso, V. G., Grinsztejn, B., & Luz, P. M. (2021). Late initiation of antiretroviral therapy: Inequalities by educational level despite universal access to care and treatment. BMC Public Health, 21(1), 389. https://doi.org/10.1186/s12889-021-10421-8
- Rue, H., Martino, S., & Chopin, N. (2009). Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 71(2), 319–392. https://doi.org/10.1111/j.1467-9868.2008.00700.x
- Sayegh, P., Thaler, N. S., Arentoft, A., Kuhn, T. P., Schonfeld, D., Castellon, S. A., Durvasula, R. S., Myers, H. F., & Hinkin, C. H. (2016). Medication adherence in HIV-positive African Americans: The roles of Age, health beliefs, and sensation seeking. Cogent Psychology, 3(1), 1137207. https://doi.org/10.1080/23311908.2015.1137207
- Sobrino-Vegas, P., Rodriguez-Urrego, J., Berenguer, J., Caro-Murillo, A. M., Blanco, J. R., Viciana, P., Moreno, S., Bernardino, I., del Amo, J., & CoRis. (2012). Educational gradient in HIV diagnosis delay, mortality, antiretroviral treatment initiation and response in a country with universal health care. Antiviral Therapy, 17(1), 1–8. https://doi.org/10.3851/IMP1939
- Teixeira da Silva, D. S., Luz, P. M., Lake, J. E., Cardoso, S. W., Ribeiro, S., Moreira, R. I., Clark, J. L., Veloso, V. G., Grinsztejn, B., & De Boni, R. B. (2017). Poor retention in early care increases risk of mortality in a Brazilian HIV-infected clinical cohort. AIDS Care, 29(2), 263–267. https://doi.org/10.1080/09540121.2016.1211610
- Watanabe, S. (2010). Asymptotic equivalence of Bayes cross validation and widely applicable information criterion in singular learning theory. Journal of Machine Learning Research, 11(116), 3571–3594.