ABSTRACT
The medicine burden of people living with HIV (PLWH) is unknown. Between 2018 and 2020, participants completed a survey comprising outcome measures for medicine burden (LMQ-3) and stigma experiences (SSCI-8). Participants were HIV+ adults (≥18 years), using antiretrovirals (ARV) with or without non-ARV medicines, recruited via two outpatient clinics in southeast England and online via HIV charities across the UK. Spearman’s correlations between medicine burden levels and stigma scores were calculated. Participants were mostly males (72%, 101/141) of mean (SD) age 48.6 (±12.31) years. Total number of medicines ranged from 1-20. High medicine burden was self-reported by 21.3% (30) and was associated with polypharmacy (≥ 5 medicines) (101.52 Vs 85.08, p = 0.006); multiple doses versus once daily regimes (109.31 Vs 85.65, p = 0.001); unemployment (98.23 Vs 84.46, p = 0.004); and ethnicity (97 Vs 86.85, p = 0.041 for non-White versus White participants). A correlation between medicine burden and stigma was observed (r = 0.576, p < 0.001). The LMQ-3 demonstrated adequate construct validity and reliability (domain loadings ranging 0.617-0.933 and Cronbach’s α of 0.714-0.932). Assessment of medicine burden and psychosocial stigma in PLWH could enable identification of those needing additional support in future research and practice.
Introduction
The UK surpassed the United Nations’ 90:90:90 goals, with 98% of people living with HIV (PLWH) on long-term antiretrovirals (ARVs) to manage this chronic condition (Kirby, Citation2018). For the 105200 PLWH in the UK, engagement with care is critical for optimal health outcomes and prevention of HIV transmission (Genberg et al., Citation2016). Due to advances in treatments and successful early initiation of ARVs, by 2030, 75% of PLWH will be aged 50 years or older, and up to 28% will have at least three comorbidities (Smit et al., Citation2015). HIV increases individuals’ risk of co-morbidities (e.g., cardiovascular-, mental health-, bone- and renal- conditions) and is associated with polypharmacy (using ≥ 5 medicines).
Managing ARVs and non-ARV medicines, which now account for more than 50% of all medicines used by PLWH, can be challenging. Up to 65% of PLWH in the UK use at least one non-ARV medicine while 17% experience non-ARV polypharmacy, thereby juggling the demands of concomitant medicines use (Okoli et al., Citation2020). Polypharmacy is associated with increased incidence of adverse drug reactions, drug-interactions, and medicine burden (Back & Marzolini, Citation2020; Carter, Citation2018; Edelman et al., Citation2020; Guaraldi et al., Citation2017). Prescribing cascades, aiming to manage side effects of ARVs, may further complicate medicine use and contribute to problematic polypharmacy (Marzolini & Livio, Citation2019).
Medicine burden is “a patient’s subjective experience in response to physical, psychosocial and financial impacts of medicines to maintain their daily lives, health and well-being [and involves] adapting to challenges of living with a medicine” (Mohammed et al., Citation2016). Medicine burden, including but not limited to side effects and interruptions to daily routines, impacts adherence to treatments and contributes to disengagement with care and treatment discontinuation (Claborn et al., Citation2017).
Various systematic reviews and meta-analyses have shown that lower adherence to ARVs is associated with more complex regimens, leading to higher risk of treatment failure and risk of antiretroviral drug resistance (Altice et al., Citation2019; Bhatta et al., Citation2017; Shah et al., Citation2019; Shubber et al., Citation2016; Taiwo et al., Citation2023). Few studies have attempted to determine extent to which HIV-related treatment burden affects PLWH generally (Schreiner et al., Citation2019; Tran et al., Citation2019). We are not aware of studies that have explored HIV-related medicine burden in the UK.
Despite HIV services and ARVs being offered free within the National Health Service (NHS), some PLWH in England may face challenges with making co-payments for their non-ARV prescriptions. Organising and attending different healthcare appointments, and difficulties accessing some or all their medicines can be challenging (Tran et al., Citation2019). Moreover, stigma and secrecy related to HIV (and medicine use) may further exacerbate actual or perceived medicine burden (Katz et al., Citation2013; Pound et al., Citation2005; Shubber et al., Citation2016). Higher levels of HIV-related stigma and not sharing information about HIV status are also associated with nonadherence to ARVs (Katz et al., Citation2013). Research is needed to understand medicine burden in PLWH and to ascertain the extent to which it correlates to stigma.
Person-centred care, particularly monitoring health outcomes from the patient’s perspective, is increasingly accepted, despite predominant biomedical approaches to HIV care (Bristowe et al., Citation2019). Patient-reported outcome measures (PROMs) play a role in supporting person-centred care and they are continuing to emerge as relevant tools for identifying challenging aspects of HIV care (Bristowe et al., Citation2018). We measured the extent of medicine burden as perceived by PLWH using a valid and reliable instrument. The Living with Medicines Questionnaire (LMQ-3) is a 41-item, 8-domain, generic, PROM for medicine burden which measures a broad range of medicine-related issues which affect everyday lives of people using medicines long-term (Katusiime et al., Citation2018; Krska et al., Citation2018). It has been used internationally to assess medicine burden in the general population (Tordoff et al., Citation2019) and in older Chinese over 50 years living with HIV (Zheng et al., Citation2022) but not in other PLWH. This study also investigated factors correlated with medicine burden and stigma.
Materials and methods
Design and sample
Between 2018 and 2020, a cross-sectional survey was conducted to gather views of eligible HIV + adults, aged 18 years or older, using ARVs for at least 6 months. Participants who were not using ARVs long-term or on pre-exposure prophylaxis were excluded. We also excluded those unable to understand English to a level necessary for providing consent to study procedures; logistical challenges prevented the use of translation services.
Data collection and setting
Participants were recruited through two outpatient HIV clinics in southeast England. Screening to identify eligible patients was done by the care team. Eligible participants were informed about the study, provided an information pack, and gave their written consent. The anonymous survey could be completed in the clinic or at home. A printed paper version or electronic survey was available with the latter accessed via a scannable QR code, or online link. A prepaid envelope was provided for paper surveys. Survey links were also promoted via social media and websites of eligible patient organisations providing HIV-related services/support across the UK. Informed consent was recorded for all survey participants via Qualtrics™ platform.
Measures
The LMQ-3 assessed overall medicine burden, which was defined as a sum of all participants’ collated ratings on the questionnaire, in eight domains: practical difficulties (7 items), general concerns (7 items), interferences to day-to-day life (6 items), perceived effectiveness (6 items), patient–doctor relationships/communication (5 items), side effects (4 items), cost-related burden (3 items), and autonomy/control over medicine use (3 items). Items were rated on a 5-point scale (strongly agree to strongly disagree), and reverse scoring used as appropriate, with higher scores depicting higher medicine burden. Overall medicine burden was then categorised using previously published cut-off values for levels of burden (low, moderate, high as 41-87, 88-110, and > 110 respectively). The LMQ-3 also includes a section for obtaining free-text qualitative data and participant characteristics (Katusiime et al., Citation2018).
The 8-item Stigma Scale for Chronic Illnesses (SSCI-8) is a generic measure of stigma related to living with a long-term condition. It was used, with permission, to assess forms of stigma: internalised or self-stigma correlated with awareness of negative stereotypes around an individual’s illness (2 items) and enacted/actual experiences of stigma and discriminatory behaviour (5 items) or internalised and enacted stigma (1 item). Items were rated on a 5-point scale (never, rarely, sometimes, often, always) and total scores calculated (range 8-40) with higher scores indicating greater stigma (Molina et al., Citation2013; Rao et al., Citation2009). We hypothesised a priori that stigma would be positively correlated with medicine burden.
Data analysis
Validity and reliability of the LMQ-3 were assessed by conducting a confirmatory factor analysis (CFA) and measuring Cronbach’s alpha for the eight domains respectively. To investigate the correlations between medicine burden and stigma, Spearman’s correlations were calculated using scores obtained by the previously described outcome measures with p-values < 0.05 considered statistically significant.
Ethics
Ethics approval was obtained from the Northeast Newcastle and North Tyneside Research Ethics Committee for recruitment via outpatient NHS clinics (REF 18/NE/0321) and Medway School of Pharmacy Research Ethics Committee (SREC) for the UK-wide on-line survey (REF001018).
Results
Participant characteristics
A total of 217 participants accessed the survey. There were variations in response rates by the source of data and recruitment methods used: relatively more questionnaires (66.8%, 145) were returned from participants using social media/online platforms of HIV-related patient organisations/charities or support groups compared to sample data collected from two outpatient clinics in southeast England (33.2%, 72). Non-responders were mostly those who viewed the electronic survey- no demographic data were provided by all non-responders. The first and last LMQ-3 items were completed by 175 and 154 participants, respectively. Two thirds (141, 65%) completed all LMQ-3 items. Seventy-two percent were male, of mean age in years 48.6 (SD ± 12.31); age range 18–79 ().
Table 1. Demographic characteristics of participants.
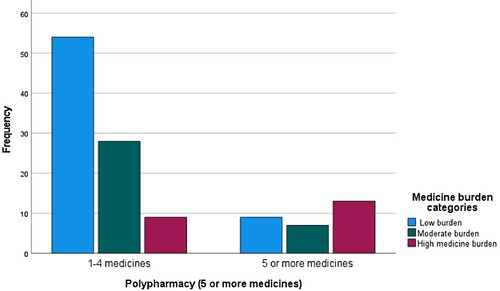
Overall, participants used up to 20 medicines (median, 3) (). Polypharmacy (≥ 5 medicines) was experienced by 23.7% (32/135). Compared to 18–49-year-olds (12.9%, 4/31), polypharmacy was more frequently experienced among participants aged ≥ 50 years (87.1%, 27/31) (p < 0.001). Concomitant medicines commonly reported included statins/cholesterol lowering medicines, diuretics, asthma inhalers, and vitamin supplements.
Table 2. Medicine characteristics of participants.
Experiences of medicine burden
High- and moderate- levels of medicine burden were experienced by 21.3% (30/141) and 29.1% (41/141) respectively. The median LMQ-3 total score was 88 suggesting moderate medicine burden for this sample population (range 57-170). shows results from LMQ-3 statements. Notably, over a third (36.7%, 62/169) were concerned that they may forget to use their medicines, while 11.4% (18/159) felt it was neither easy to keep their medicines routine nor were they comfortable with the timing of their regular doses (5.7%, 10/174). Within the domain “interference to daily life, a third (32.9%, 51/155) agreed/strongly agreed that their medicines interfered with their sexual life while 23.6% (37/157) had interferences to their social relationships. Quotes taken from participants’ written comments illustrate aspects of medicine burden in the eight LMQ-3 domain areas ().
Table 3. Participants’ responses to the statements in the LMQ-3 survey.
Table 4. Qualitative analysis of free-text comments in the eight medicine burden domains.
Factors correlated with medicine burden
Polypharmacy was significantly correlated with medicine burden (). Mean LMQ-3 burden scores were higher among participants using 5 or more medicines, those using medicines more frequently than once daily (). Data showed statistically significant differences in medicine burden scores with respect to employment status. Domain analyses showed cost burden scores were higher in the unemployed group (mean ± SD 6.44 ± 3.31) compared to those employed (5.04 ± 2.38) (p = 0.018) and comparable results were obtained with Mann–Whitney tests (mean rank, 80.27 Vs 62.46 respectively, p = 0.012). Non-White participants had higher medicine burden scores (97.00 ± 24.12) than White participants (86.85 ± 20.33, p = 0.041); domain analyses revealed statistically significant differences in the following areas: practical difficulties (16 Vs 13, p = 0.006), cost-burden related to non-ARV prescriptions (7.0 Vs 5.2, p = 0.005), general concerns (21.3 Vs 18.3, p = 0.017), and lack of autonomy (10.5 Vs 11.9, p = 0.021).
Table 5. Factors associated with medicine burden among people living with HIV.
Correlations between medicine burden and stigma
A third (32.8%) of the participants often or always felt embarrassed about living with HIV (and taking medicines) while 22.8% often or always felt blamed for their HIV diagnosis (). A 49-year-old male illustrated the burden of stigma: “some medicines are arguably difficult to take in front of people as this may lead to being stigmatised”. Some participants were concerned about the size of tablets, like this 58-year-old female “I don’t like the size of some of them as it makes it difficult to be discreet when taking them in public”.
Table 6. Responses to the eight stigma items in the stigma questionnaire.
Medicine burden scores were strongly positively correlated with total stigma scores (0.576, p < 0.001 respectively). The correlation between total LMQ-3 scores and internalised/perceived stigma (0.543) was stronger than total LMQ-3 and enacted/actual stigma (0.441). Of the eight LMQ-3 domains, “general concerns’ had the strongest positive correlation with total stigma scores (rho = 0.565, p < 0.001) ().
Table 7. Correlations between medicine burden and stigma.
LMQ-3 validity and reliability
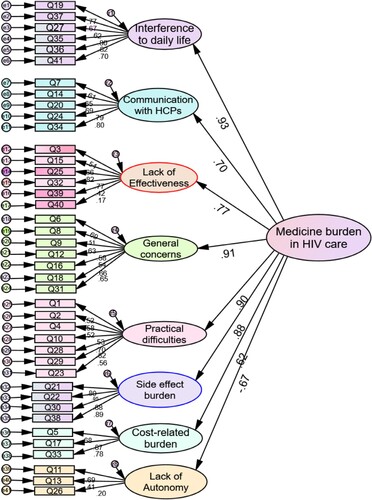
Factor loadings for the eight medicine burden domains ranged from 0.617-0.933 (p < 0.001), being strongest for “interferences (0.93), general concerns (0.91), practical difficulties (0.90), and side effects (0.88) (). Seven of eight LMQ-3 subscales had excellent internal consistency (Cronbach’s alpha range, 0.714–0.932).
Figure 2. Confirmatory factor analysis of the LMQ-3 when completed by people living with HIV (N = 141). Notes: Standardised regression coefficients for all 41 LMQ-3 items are shown in brackets: Interferences to daily life- Q19(0.77), Q37(0.67), Q27(0.62), Q35(0.80), Q36(0.82), Q41(0.70);Communication with HCPs - Q7(0.61), Q14(0.65), Q20(0.69), Q24(0.79), Q34(0.80); Lack of effectiveness -Q3(0.54), Q15(0.66), Q25(0.82), Q32(0.77) and Q39(0.42), Q40 (0.17);General concerns – Q6(0.69), Q8(0.51), Q9(0.63), Q12(0.58); Q16 (0.54), Q18(0.66), Q31(0.65); Practical difficulties -Q1(0.52), Q2(0.58), Q4(0.52), Q10(0.53), Q28(0.70), Q29(0.82), Q23 (0.56); Side effect burden - Q21(0.80), Q22(0.95), Q30(0.88),Q38(0.89); Cost-related burden- Q5 (0.68), Q17 (0.87), Q33 (0.78); and Lack of autonomy- Q11 (0.69), Q13 (0.41) and Q26 (0.20).
Discussion
To the best of our knowledge, this is the first study investigating the extent to which PLWH experience medicine burden and correlations with stigma. High medicine burden was experienced by 1 in 5 participants (21%), slightly less than that found in the UK’s general adult population (23.5%) (Krska et al., Citation2018). All eight domains of medicine burden were experienced by some individuals to varying magnitudes. For instance, “medicine-related interference” affected up to a third of all participants’ social relationships and sexual life, a bigger impact than that seen in the general population. Side effects affected around 25% and generated most of the free-text comments compared to around 19% in the general population; General concerns about possible long-term effects of medicines were more common in PLWH than in the general population (62.9% versus 54.4%); PLWH may experience significant medicine burden from either ARVs or non-ARV medicine, although likely to report less burden due to placing more emphasis on medicine need and gratitude for lifesaving ARVs. Stronger beliefs around necessity, effectiveness, and longevity may explain the lower prevalence of medicine burden among PLWH (Fall et al., Citation2014; Horne et al., Citation1999). More PLWH are living longer due to advances in antiretroviral medicines, and this may influence risk-benefit assessments in this population (Pound et al., Citation2005). Further qualitative research is needed to fully investigate perceptions of burden in this population.
Factors significantly correlated with increasing medicine burden included polypharmacy (5 or more medicines), multiple daily dosing regimens, and needing a carer to managing medicines use. Like the general population, higher medicine burden increased with the total number of medicines and more frequent daily use (Katusiime et al., Citation2018; Krska et al., Citation2018). Polypharmacy among PLWH is increasing (Edelman et al., Citation2020; Halloran et al. Citation2019). Nearly half (47%, 2195/4630) of PLWH in the UK use two or more non-ARVs plus their usual ARV regime; the prevalence of polypharmacy is even higher in among PLWH over 50 years of age (61%) (Okoli et al., Citation2020). Polypharmacy contributes to drug–drug interactions, adverse drug reactions, nonadherence and poor health outcomes (Carter, Citation2018; Edelman et al., Citation2020; Guaraldi et al., Citation2017; Okoli et al., Citation2020). More frequent use of ARVs and non-ARV medicines (> more than once daily) was associated with higher medicine burden. Practical difficulties, particularly concerns about adherence and timing of doses were also more common in PLWH than in the general population (36.7% compared to 25.2%). Lack of autonomy to vary the timing of doses appeared to have a greater influence on experiences of medicine burden for PLWH (55.7%) compared to the general population (45.8%) (Krska et al., Citation2018). Factor analyses of our sample data showed a stronger correlation between “lack of autonomy” and medicine burden (0.674, p < 0.001) compared to the general population coefficient (0.1, p = 0.224) (Katusiime et al., Citation2018), suggesting that autonomy is a crucial factor to consider in medicine-related consultations for PLWH. Not surprisingly, fewer PLWH reported being able to vary the dose and/or timing of their medicines due to a greater emphasis on adherence to achieve undetectable viral loads (Altice et al., Citation2019). Pound et al. (Citation2005) hinted on the potential burden of strict adherence to HIV medicines, which can be very demanding and disruptive to life (including mealtimes, sleep, social life) leading to lack of control and regimen fatigue. Patient autonomy with regards to timing of ARVs, where clinically appropriate, may reduce burden for PLWH and achieve person-centred care. Interventions to reduce regimen complexity are key to preventing non-adherence (Zhou et al., Citation2014). Long acting antiretrovirals such as once-a-month cabotegravir/rilpivirine injections are promising (Voelker, Citation2021), yet to become adopted routinely within practice.
Increasing stigma was correlated with increasing medicine burden. PLWH may experience stigma associated with the condition itself, using medicines, or older age.
Higher stigma may exacerbate fatigue and contribute to cognitive decline (Rao et al., Citation2012) impacting medicines use and adherence behaviour. Our study showed higher medicine burden levels in non-White participants. Rao et al. (Citation2016) who used a similar measure of stigma found higher internalised stigma among African Americans living with HIV. Medicine burden interventions need to be inclusive for all PLWH, in addition to providing more support to ethnic minorities. PLWH needing a carer to support medicine use perceived a higher medicine burden than those managing independently, like the general population (Krska et al., Citation2018). Decreasing treatment burden is correlated with increasing social support (e.g., from close friends and family) (r = −0.2, p = 0.03) (Schreiner et al., Citation2019). However, perceptions or experiences of stigma may deter PLWH from seeking social support and those requiring formal carers may feel burdened by issues around unintended disclosure of HIV status.
Implications for practice and policy
To the best of our knowledge, this is the first study to assess medicine burden among PLWH with a validated generic measure. The LMQ-3 could help to identify those needing additional support given the increasing multimorbidity and polypharmacy in this population. Medicine reviews should be prioritised for PLWH experiencing polypharmacy including both ARVs and non-ARV medicines. We know that the number of medicines alone is not a sufficient indicator of medicine burden (Krska et al., Citation2018), therefore other factors, particularly the timing of doses and how this affects everyday life should be considered by clinicians, as well as experiences of stigma. Additional support for ethnic minorities needs to be considered when prioritising patients for intervention. As HIV treatment is lifelong, clinicians and policymakers should consider integration of medicine burden measures (such as the LMQ-3) in future HIV care models to achieve person-centred care. Future healthcare funding policies in England should include HIV on the list of long-term conditions exempted from the NHS prescription charge like exemptions provided to people with certain life-long conditions (e.g., diabetes, hypothyroidism, epilepsy) especially for those on low household income. Other studies involving larger samples of PLWH are needed to study how medicine burden impacts adherence and clinical outcomes (e.g., viral load).
Strengths and limitations
Online recruitment methods, via social media and websites of HIV-related patient organisations and within selected HIV clinics, were used to widen inclusion of participants across the UK, contributing to a relatively small but adequate sample size. We do not anticipate that the sample population was unusual, especially that increasingly more PLWH seek support from online platforms. This may have increased during the data collection period which partially encompassed the COVID-19 pandemic. A survey led by one of the UK’s leading women’s HIV charities (n = 75) found that nearly half (45%) of participants had problems accessing HIV care during the lockdown period, with a quarter (25%) highlighted their main concern as “lack of access to their HIV clinic”. (National AIDS Trust, Citation2020). It is possible that these recruitment methods may have biased the sample to younger persons or those with access to the internet.
Validated instruments (the LMQ-3 and SSCI-8) were used, allowing measurement of medicine burden and correlations with stigma. This is the first application of a validated PROM of medicine burden in PLWH within the UK, and confirmatory factor analyses demonstrated adequate construct validity and reliability. All eight domains of the LMQ-3 loaded strongly on the overarching construct of medicine burden, suggesting reliability of the instrument. Nevertheless, missing data may be due to potential survey fatigue from the questionnaire length. In addition, no demographic data were collected for non-responders to the electronic or paper-based survey. Regardless, a shorter, tailored, version of the LMQ-3 may benefit future studies. Although demographic characteristics of our sample comprised mostly White males, this was representative of the HIV population in the UK. The sample population, however, may not fully represent the views or experiences of all PLWH.
Conclusion
One in five PLWH experience high medicine burden. Our data showed that higher medicine burden is correlated with increasing stigma, polypharmacy (5 or more medicines), multiple daily dosing (more than twice daily), as well as needing support with medicines use (e.g., from a carer). Of the eight aspects of medicine burden, lack of autonomy to vary the timing of medicines appeared to be the highest contributor to burden for PLWH. The LMQ-3 is a valid and reliable outcome measure in this population, but further work is needed to harness a larger sample. This study confirms that perceptions and experiences of medicine burden are related to stigma, both of which can affect an individual's adherence to treatments for HIV and co-morbidities. Highlighting this interrelationship has the potential to shape the development of future interventions aimed at reducing the burden to improve overall health and quality of life for PLWH.
Acknowledgements
The authors thank all study participants and the various patient organisations and HIV charities which promoted the study. We also thank the HIV-specialist pharmacists, pharmacy technicians, nurses, consultants, and the research teams who supported recruitment from the outpatient clinics involved in this study – we are more than grateful for their involvement in this project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Altice, F., Evuarherhe, O., Shina, S., Carter, G., & Beaubrun, A. C. (2019). Adherence to HIV treatment regimens: Systematic literature review and meta-analysis. Patient Preference and Adherence, 13, 475–490. https://doi.org/10.2147/PPA.S192735
- Back, D., & Marzolini, C. (2020). The challenge of HIV treatment in an era of polypharmacy. Journal of the International AIDS Society, 23(2), e25449. https://doi.org/10.1002/jia2.25449
- Bhatta, D. N., Liabsuetrakul, T., & McNeil, E. B. (2017). Social and behavioral interventions for improving quality of life of HIV infected people receiving antiretroviral therapy: A systematic review and meta-analysis. Health and Quality of Life Outcomes, 15(1), 80. https://doi.org/10.1186/s12955-017-0662-4
- Bristowe, K. (2018). A novel patient reported outcome measure for people living with HIV: Development, face and content validity and stakeholder views on implementation. In Fourth joint conference of the British HIV Association with the British Associaton for Sexual Health and HIV (pp. 1–5). Edinburgh International Conference Centre. https://www.bhiva.org/file/xRtasJbTvYFkh/KatherineBristowe.pdf
- Bristowe, K., Clift, P., James, R., Josh, J., Platt, M., Whetham, J., Nixon, E., Post, F. A., McQuillan, K., Ní Cheallaigh, C., Murtagh, F. E. M., Anderson, J., Sullivan, A. K., & Harding, R. (2019). Towards person-centred care for people living with HIV: What core outcomes matter, and how might we assess them? A cross-national multi-centre qualitative study with key stakeholders. HIV Medicine, 20(8), 542–554. https://doi.org/10.1111/hiv.v20.8
- Carter, M. (2018). Taking large numbers of non-ART drugs associated with increased risk of hospitalisation and death for HIV-positive people. Aidsmap.Com. https://www.aidsmap.com/news/may-2018/taking-large-numbers-non-art-drugs-associated-increased-risk-hospitalisation-and.
- Claborn, K., Miller, M. B., Meier, E., & Carbone, S. (2017). Development of a conceptual etiological model of treatment regimen fatigue Among patients engaged in HIV care: A qualitative study. The Journal of the Correlation of Nurses in AIDS Care: JANAC, 28(4), 479–490. https://doi.org/10.1016/j.jana.2017.02.008
- Edelman, E. J., Rentsch, C. T., & Justice, A. C. (2020). Polypharmacy in HIV: Recent insights and future directions. Current Opinion in HIV and AIDS, 15(2), 126–133. https://doi.org/10.1097/COH.0000000000000608
- Fall, E., Gauchet, A., Izaute, M., Horne, R., & Chakroun, N. (2014). Validation of the French version of the beliefs about Medicines Questionnaire (BMQ) among diabetes and HIV patients. European Review of Applied Psychology-Revue Europeenne De Psychologie Appliquee, 64(6), 335–343. https://doi.org/10.1016/j.erap.2014.08.005
- Genberg, B. L., Shangani, S., Sabatino, K., Rachlis, B., Wachira, J., Braitstein, P., & Operario, D. (2016). Improving engagement in the HIV care cascade: A systematic review of interventions involving people living with HIV/AIDS as peers. AIDS and Behavior, 20(10), 2452–2463. https://doi.org/10.1007/s10461-016-1307-z
- Guaraldi, G., Menozzi, M., Zona, S., Calcagno, A., Silva, A. R., Santoro, A., Malagoli, A., Dolci, G., Mussi, C., Mussini, C., Cesari, M., & Khoo, S. H. (2017). Impact of polypharmacy on antiretroviral prescription in people living with HIV. Journal of Antimicrobial Chemotherapy, 72(2), 511–514. https://doi.org/10.1093/jac/dkw437
- Halloran, M. O., Boyle, C., Kehoe, B., Bagkeris, E., Mallon, P., Post, F. A., Vera, J., Williams, I., Anderson, J., Winston, A., Sachikonye, M., Sabin, C., & Boffito, M. (2019). Polypharmacy and drug-drug interactions in older and younger people living with HIV: The POPPY study. Antiviral Therapy, 24(3), 193–201. https://doi.org/10.3851/IMP3293
- Horne, R., Weinman, J., & Hankins, M. (1999). The Beliefs about Medicines Questionnaire: The development and evaluation of a new method for assessing the cognitive representation of medication. Psychology & Health, 14(1), 1–24. https://doi.org/10.1080/08870449908407311
- Katusiime, B., Corlett, S. A., & Krska, J. (2018). Development and validation of a revised instrument to measure burden of long-term medicines use: The Living with Medicines Questionnaire version 3. Patient Related Outcome Measures, 9, 155–168. https://doi.org/10.2147/PROM.S151143
- Katz, I. T., Ryu, A. E., Onuegbu, A. G., Psaros, C., Weiser, S. D., Bangsberg, D. R., & Tsai, A. C. (2013). Impact of HIV-related stigma on treatment adherence: Systematic review and meta-synthesis. Journal of the International AIDS Society, 16(3 Suppl 2), 18640. https://doi.org/10.7448/IAS.16.3.18640
- Kirby, T. (2018). The UK reaches UNAIDS 90-90-90 targets. The Lancet, 392(10163), 2427. https://doi.org/10.1016/S0140-6736(18)33117-9
- Krska, J., Katusiime, B., & Corlett, S. A. (2018). Patient experiences of the burden of using medicines for long-term conditions and factors affecting burden: A cross-sectional survey. Health & Social Care in the Community, 26(6), 946–959. https://doi.org/10.1111/hsc.12624
- Marzolini, C., & Livio, F. (2019). Prescribing issues in elderly individuals living with HIV. Expert Review of Clinical Pharmacology, 12(7), 643–659. https://doi.org/10.1080/17512433.2019.1627200
- Mohammed, M. A., Moles, R. J., & Chen, T. F. (2016). Medication-related burden and patients’ lived experience with medicine: A systematic review and metasynthesis of qualitative studies. BMJ Open, 6(2), 1–16. https://doi.org/10.1136/bmjopen-2015-010035
- Molina, Y., Choi, S. W., Cella, D., & Rao, D. (2013). The stigma scale for chronic illnesses 8-item version (SSCI-8): Development, validation and use across neurological conditions. International Journal of Behavioral Medicine, 20(3), 450–460. https://doi.org/10.1007/s12529-012-9243-4
- National AIDS Trust. (2020). HIV COVID-19 Network Briefing Six- a fortnightly update from the voluntary sector. https://www.nat.org.uk/sites/default/files/publications/Network%20Briefing%20Six%20-%2026%20June%202020.pdf.
- Okoli, C., Schwenk, A., Radford, M., Myland, M., Taylor, S., Darley, A., Barnes, J., Fox, A., Grimson, F., Reeves, I., Munshi, S., Croucher, A., Boxall, N., Benn, P., Paice, A., Wyk, J. v., & Khoo, S. (2020). Polypharmacy and potential drug–drug interactions for people with HIV in the UK from the Climate-HIV database. HIV Medicine, 21(8), 471–480. https://doi.org/10.1111/hiv.12879
- Pound, P., Britten, N., Morgan, M., Yardley, L., Pope, C., Daker-White, G., & Campbell, R. (2005). Resisting medicines: A synthesis of qualitative studies of medicine taking. Social Science & Medicine, 61(1), 133–155. https://doi.org/10.1016/j.socscimed.2004.11.063
- Rao, D., Choi, S. W., Victorson, D., Bode, R., Peterman, A., Heinemann, A., & Cella, D. (2009). Measuring stigma across neurological conditions: The development of the stigma scale for chronic illness (SSCI). Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 18(5), 585–595. https://doi.org/10.1007/s11136-009-9475-1
- Rao, D., Feldman, B. J., Fredericksen, R. J., Crane, P. K., Simoni, J. M., Kitahata, M. M., & Crane, H. M. (2012). A structural equation model of HIV-related stigma, depressive symptoms, and medication adherence. AIDS and Behavior, 16(3), 711–716. https://doi.org/10.1007/s10461-011-9915-0
- Rao, D., Molina, Y., Lambert, N., & Cohn, S. E. (2016). Assessing stigma among African Americans living with HIV. Stigma and Health, 1(3), 146–155. https://doi.org/10.1037/sah0000027
- Schreiner, N., Perazzo, J., Currie, J., Daly, B., & Webel, A. (2019). A descriptive, cross-sectional study examining treatment burden in people living with HIV. Applied Nursing Research: ANR, 46, 31–36. https://doi.org/10.1016/j.apnr.2019.02.009
- Shah, R., Watson, J., & Free, C. (2019). A systematic review and meta-analysis in the effectiveness of mobile phone interventions used to improve adherence to antiretroviral therapy in HIV infection. BMC Public Health, 19(1), 915. https://doi.org/10.1186/s12889-019-6899-6
- Shubber, Z., Mills, E. J., Nachega, J. B., Vreeman, R., Freitas, M., Bock, P., Nsanzimana, S., Penazzato, M., Appolo, T., Doherty, M., & Ford, N. (2016). Patient-Reported barriers to adherence to antiretroviral therapy: A systematic review and meta-analysis. PLOS Medicine, 13(11), e1002183. https://doi.org/10.1371/journal.pmed.1002183
- Smit, M., Brinkman, K., Geerlings, S., Smit, C., Thyagarajan, K., Sighem, A. v., de Wolf, F., & Hallett, T. B. (2015). Future challenges for clinical care of an ageing population infected with HIV: A modelling study. The Lancet. Infectious Diseases, 15(7), 810–818. https://doi.org/10.1016/S1473-3099(15)00056-0
- Taiwo, B. O., Romdhani, H., Lafeuille, M.-H., Bhojwani, R., Milbers, K., & Donga, P. (2023). Treatment and comorbidity burden among people living with HIV: A review of systematic literature reviews. Journal of Drug Assessment, 12(1), 1–11. https://doi.org/10.1080/21556660.2022.2149963
- Tordoff, J. M., Brenkley, C., Krska, J., & Smith, A. (2019). Exploring medicines burden among adults in New Zealand: A cross-sectional survey. Patient Preference and Adherence, 13, 2171–2184. https://doi.org/10.2147/PPA.S231202
- Tran, V.-T., Messou, E., Mama Djima, M., Ravaud, P., & Ekouevi, D. K. (2019). Patients’ perspectives on how to decrease the burden of treatment: A qualitative study of HIV care in sub-Saharan Africa. BMJ Quality & Safety, 28(4), 266–275. https://doi.org/10.1136/bmjqs-2017-007564
- Voelker, R. (2021). Monthly injection is approved for patients With HIV. JAMA, 325(9), 816–816. https://doi.org/10.1001/jama.2021.1932
- Zheng, C., Meng, J., Xiao, X., Xie, Y., Zhao, D., & Wang, H. (2022). Polypharmacy, medication-related burden and antiretroviral therapy adherence in people living with HIV aged 50 and above: A cross-sectional study in Hunan, China. Patient Preference and Adherence, 16, 41–49. https://doi.org/10.2147/PPA.S340621. PMID: 35027822; PMCID: PMC8752076.
- Zhou, S., Martin, K., Corbett, A., Napravnik, S., Eron, J., Zhu, Y., Casciere, B., Boulton, C., Loy, B., Smith, S., Woods, A., Murray, M., Ramsdell, L., & Wohl, D. A. (2014). Total daily pill burden in HIV-infected patients in the southern United States. AIDS Patient Care and STDs, 28(6), 311–317. https://doi.org/10.1089/apc.2014.0010