Abstract
Elderly patients are at increased risk for persistent complaints after mild traumatic brain injury (MTBI). This study aimed to investigate the role of post-concussive symptoms, mood, post-traumatic stress, and coping on functional outcome in elderly with MTBI. Information on mood, post-concussive symptoms, post-traumatic stress, and coping was collected 2 weeks post-injury. Six months post-injury functional outcome was assessed with the Glasgow Outcome Scale Extended. One hundred and sixty-two patients aged ≥ 60 years were included, 55% male, mean age = 71 (±6.2) years. The most frequent cause of injury was falls from standing height (75%). Two weeks post-injury anxiety, depression, and post-traumatic stress were present in 15%, 12%, and 38% of patients, respectively, with 73% reporting post-concussive symptoms. Avoidant coping was the most frequently used coping style. Six months post-injury, 44% showed incomplete recovery. Higher depression scores (OR = 0.87, p = 0.005) and number of post-concussive symptoms (OR = 0.91, p = 0.03) were associated with incomplete recovery. Half of the elderly showed incomplete recovery 6 months after MTBI, with early depression or post-concussive symptoms as important factors. Coping style was not related to outcome. These results underline the need for a different approach in elderly patients, focusing on other predicting factors and fall prevention strategies.
Introduction
Traumatic brain injury (TBI), with mild traumatic brain injury (MTBI) accounting for the vast majority of all TBIs, is a major worldwide problem. TBI is associated with high utilization of medical care and substantial medical costs (Fu, Jing, Fu, & Cusimano, Citation2016; McMillan, Weir, & Wainman-Lefley, Citation2014; Mosenthal et al., Citation2004; Scholten, Haagsma, Panneman, Van Beeck, & Polinder, Citation2014; Thurman, Alverson, Dunn, Guerrero, & Sniezek, Citation1999). The elderly population is generally the largest consumer of health services and critical care resources. By 2050, 40% of all trauma patients will consist of adults aged 65 years or older (Tillou et al., Citation2014). Both the bimodal distribution of TBI, with a higher risk of TBI in both younger (younger than 15 years) and older subjects (older than 65 years) (Taylor, Bell, Breiding, & Xu, Citation2017), and the increasing life expectancy of people are believed to lead to higher mortality and morbidity rates after sustaining a TBI. Elderly not only have an increased risk of sustaining a TBI, but advanced age is also a risk factor for higher mortality and morbidity rates after sustaining a TBI (Mosenthal, Lavery, & Addis, Citation2002; Pennings, Bachulis, Simons, & Slazinski, Citation1993; Rozzelle, Wofford, & Branch, Citation1995; Zonfrillo, Kim, & Arbogast, Citation2015). Although the majority of elderly patients return to their homes after sustaining a TBI (Rapoport & Feinstein, Citation2000; Røe, Skandsen, Manskow, Ader, & Anke, Citation2015), elderly patients are reported to experience more complications (Papa, Mendes, & Braga, Citation2012), and a greater dependence as compared to their younger counterparts (Mosenthal et al., Citation2002).
Despite the increase in elderly patients who sustain a TBI, the majority of studies focus on outcomes of working-age individuals as opposed to older adults (LeBlanc, de Guise, Gosselin, & Feyz, Citation2006; Livingston et al., Citation2005; Mosenthal et al., Citation2004; Susman et al., Citation2002; Weber et al., Citation2016; Zonfrillo et al., Citation2015). This underlines the need for prognostic models in this patient group. Previous studies have shown that older age is a main prognostic factor for outcome, whereas elderly patients also often suffer from a high burden of chronic health conditions. These chronic health conditions contribute to falls, which subsequently increases the risk of TBI. Mosenthal et al. (Citation2004) reported that 73% of elderly patients with TBI had one or more medical conditions prior to injury. In addition, Selassie, McCarthy, Ferguson, Tian, and Langlois (Citation2005) have demonstrated that older TBI patients with three or more pre-existing comorbid diseases showed mortality rates that were four times higher than subjects without any pre-existing disease.
Mood (i.e. anxiety and depression), post-concussive symptoms, and post-traumatic stress are important factors that are associated with outcome after TBI (Silverberg et al., Citation2015; Wäljas et al., Citation2015). For instance, many studies have shown that the occurrence of a TBI increases the likelihood of developing depression (Fedoroff et al., Citation1992; Jorge et al., Citation2004; Kreutzer, Seel, & Gourley, Citation2001). Rapoport, McCullagh, Streiner, and Feinstein (Citation2003) reported that major depression was seen in 15% of patients with MTBI in a cohort with ages ranging from 15–91 years (Rapoport et al., Citation2003). It was also found that individuals with depression showed poorer outcome than subjects without depression (Rapoport et al., Citation2003). Post-concussive symptoms are also associated with the presence of anxiety, depression, and stress (Haagsma et al., Citation2015; Lagarde et al., Citation2014). However, specific information on the relation between emotional distress and post-concussive symptoms in elderly is lacking.
In addition, coping, which refers to ‘the thoughts and acts people use to manage the demands of stressful situations’ is also believed to play an important role in the recovery process after TBI (Chen, Peng, Xu, & O’Brien, Citation2018). The most commonly used coping styles comprise the active, passive, and avoidant coping style, of which passive coping is associated with incomplete recovery and lower quality-of-life (Maestas et al., Citation2014; Scheenen et al., Citation2017; Snell, Siegert, Hay-Smith, & Surgenor, Citation2011; van der Naalt et al., Citation2017). Although studies on coping styles in elderly patients with MTBI are very limited, one study in older adults reported that an avoidant coping style was associated with more emotional distress (Couture, Larivière, & Lefrançois, Citation2005). Resilience is also assumed to contribute to adverse outcome following MTBI. For instance, Losoi et al. (Citation2015) reported that higher levels of resilience were associated with fewer post-concussive symptoms and a better quality-of-life (Losoi et al., Citation2015). Moreover, resilience was found to be associated with lower use of an avoidant coping style in veterans who sustained a TBI (Elliott et al., Citation2015).
Elderly also experience an age-associated loss of cognitive and physical resources that can make it difficult to adjust to life following a TBI. For instance, frailty (i.e. defined as ‘the attribute of aged people who are at an increased risk of adverse health outcomes as a consequence of a diminished ability to respond to stress’). Rockwood, Song, and MacKnight (Citation2005) has emerged as an independent predictor of risk of adverse outcome in older people (Abdulle et al., Citation2018). Therefore, we postulate that the ability to adapt and cope with TBI is a determining factor in satisfactory outcome in elderly patients. The current study aims to investigate the role of post-concussive symptoms, mood, post-traumatic stress and coping (assessed at 2 weeks post-injury) on functional outcome measured 6 months after sustaining an MTBI in elderly patients.
Method
Participants
This study is part of a larger observational cohort study on outcome following MTBI (UPFRONT-study) (van der Naalt et al., Citation2017). All patients were recruited from 2013 to 2015 at the emergency department (ED) of three Dutch hospitals designated as Level-1 trauma centres. MTBI was defined by a Glasgow Coma Score (GCS) 13–15, loss of consciousness (LOC) ≤ 30 min, and/or duration of post-traumatic amnesia (PTA) < 24 h (ACRM criteria). In the current study, only data from patients aged ≥ 60 years were used for the analyses. No one was suffering from psychiatric disorders at the time of the MTBI, and none of the patients were clinically diagnosed with dementia. The study was approved by the Medical Ethical Committee of the UMCG, and all patients gave written informed consent.
Clinical measures
Data were collected anonymously and comprised information on general characteristics (e.g. gender and age), injury-related characteristics (e.g. mechanism of trauma, CT abnormalities, Injury Severity Scale (ISS), hospital admission, and discharge destination). In addition, the presence of pre-injury mental health problems and pre-injury physical complaints were assessed based on two specific questions (‘Did you experience physical complaints prior to your accident?’, ‘Did you experience mental health problems prior to your accident?’). Data were obtained at several intervals post-injury, but for the current study data from the questionnaires assessed at 2 weeks injury were used unless stated otherwise. These questionnaires included:
Coping: The Utrecht Coping List (UCL) is a 47-item questionnaire to investigate coping style (Curran, Ponsford, & Crowe, Citation2000). The UCL has seven sub-scales representing different coping styles including problem-solving, avoidance, self-distraction, social support, withdrawal, expression of emotions, and positive reframing. For the current study, we investigated three commonly used coping styles (i.e. active, passive, and avoidant coping style) (Maestas et al., Citation2014). The scores range from low (1) to very high (5), and higher scores on the scales indicate a greater tendency to adopt that particular coping style. For the current study, values were dichotomized in high (range = 4–5) and low (<4) use of the coping style (Scheenen et al., Citation2017; van der Naalt et al., Citation2017). The UCL is frequently applied in Dutch clinical practice and has been used in several earlier studies among people with other types of brain injury or stroke (Aben, Busschbach, Ponds, & Ribbers, Citation2008; Wolters, Stapert, Brands, & Van Heugten, Citation2010).
Post-concussive symptoms: Commonly occurring post-concussive symptoms were assessed with the Head Injury Symptom Checklist (HISC) (de Koning et al., Citation2016; van der Naalt, van Zomeren A, Sluiter, & Minderhoud, Citation1999). The HISC contains 21 items with scores ranging from 0 to 42 (0 = never, 1 = sometimes and 2 = often) corrected for pre-injury complaints. The presence of post-concussive symptoms was defined as reporting one or more symptoms, and dichotomized into 0 (no complaints) and 1 (one or more complaints).
Mood: The Hospital Anxiety and Depression Scale (HADS) was used to measure anxiety and depression (Snaith & Zigmond, Citation1983). This instrument contains 14 items with seven items on depression and seven items on anxiety, with scores ranging from 0–21. HADS scores were dichotomized, with a score ≥8 indicating the presence of clinical anxiety and/or depression.
Post-traumatic stress: The Impact of Event Scale (IES) was used to assess post-traumatic stress. This 15-item questionnaire has a 5-point Likert scale (sum scores 0–75), and a cut-off score of 19 was indicative of post-traumatic stress disorder (Horowitz, Wilner, & Alvarez, Citation1979).
Outcome: Functional outcome, assessed with the Glasgow Outcome Scale Extended (GOSE), was measured 6 months after the MTBI (Wilson, Pettigrew, & Teasdale, Citation1998). The GOSE questionnaire has an 8-point scale, ranging from death (1) to complete recovery (8). The GOSE scores were dichotomized, with a score of 0–7 indicating incomplete functional recovery and a score of 8 indicating complete functional recovery.
Statistical analysis
Statistic calculations were performed with the Statistical Package for the Social Sciences version 23.0 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0; Armonk, NY). Descriptive statistics were used to describe patient characteristics. Parametric tests (one-way ANOVA) were used to assess group differences for normally distributed variables. Non-normally distributed data were assessed using non-parametric tests (e.g. Kruskal Wallis, Chi-square test). Correlations between variables were calculated using binary logistic regression, with the dichotomized GOSE scores as the dependent variable. A p-value below 0.05 was considered significant.
Results
Patients characteristics
A total of 162 patients were included. Patient characteristics are displayed in . The majority of patients were males (55%) and had a mean age of 71 (± 6.2) years. At 6 months post-injury, 56% of the patients had a complete recovery. The majority (73%) of patients were hospitalized and were eventually discharged to their homes. No significant differences in patient characteristics were present when comparing patients with complete recovery to those with incomplete recovery at 6 months post-injury.
Table 1. Patients characteristics of the total group, patients with an incomplete recovery, and patients with a complete recovery.
Table 2. Univariate analysis (variables assessed at baseline or 2 weeks post-injury), with the dichotomized GOSE scores as dependent variable.
Mood, post-traumatic stress, and post-concussive symptoms
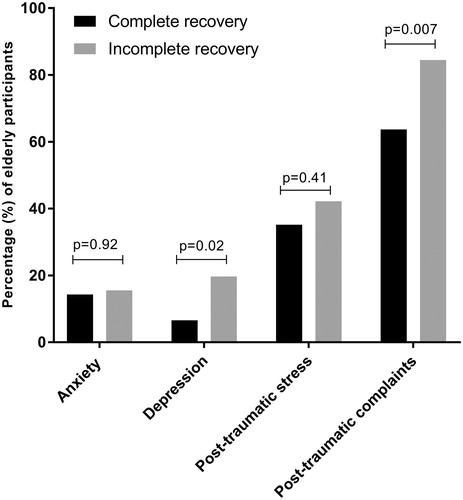
At 2 weeks post-injury, 15% of patients reported to experience anxiety (14% of patients with complete recovery and 16% of patients with an incomplete recovery, p = 0.92, ). Regarding depression, 12% of the patients reported to experience depression-related complaints. When comparing patients with a complete and incomplete recovery, a significantly higher prevalence of depression was found in patients with an incomplete recovery (20%), as compared to patients with complete recovery (7%, p = 0.02). Moreover, 73% of patients reported at least one post-concussive symptom. The most frequent prevailing post-concussive symptoms for both groups were dizziness, fatigue, and headache. When comparing the two groups, we found that patients with an incomplete recovery more frequently experienced at least one post-concussive symptom (85%), compared to patients with a complete recovery (64%, p = 0.007). In addition, 38% of the total group reported to experience post-traumatic stress. However, no significant difference between the groups was present (p = 0.41).
Coping style
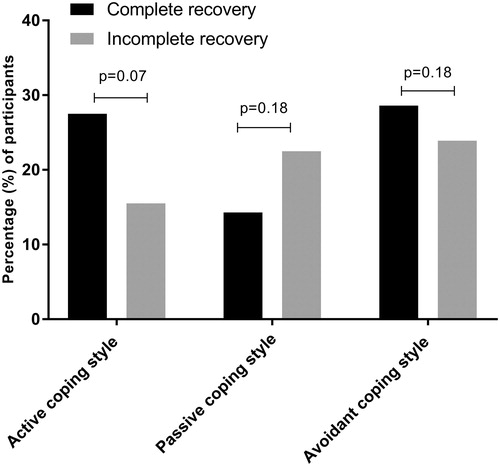
In the total group, 27% of the patients showed high use of the avoidant coping style, 22% showed high use of the active coping style, and 18% showed high use of the passive coping style. The differences in distinct coping styles between subjects with a complete and incomplete recovery are displayed in . Although we observed a higher use of the active and avoidant coping style in patients with complete recovery as compared to patients with an incomplete recovery, no significant differences were present (active coping style, p = 0.07; passive coping style, p = 0.18; avoidant coping style, p = 0.18).
Role of mood, post-concussive symptoms, post-traumatic stress, and coping on outcome
In the univariate analysis (), higher depression scores and higher HISC scores were significantly associated with a decreased odds of complete recovery (HADS depression scores: OR = 0.87 [0.78–0.96], p = 0.005; HISC scores: OR = 0.91 [0.84–0.99], p = 0.03). Higher use of an active coping style was found to be borderline associated with an increased odds of complete recovery (OR = 1.42 [0.96–2.10], p = 0.08). The other variables (i.e. age, GCS score, ISS score, gender, hospital admission, CT-abnormalities, pre-injury physical and psychological complaints, post-injury anxiety, post-traumatic stress, and post-concussive symptoms) were not found to be associated with outcome assessed at 6 months post-injury.
Discussion
The current study aimed to investigate the role of mood, post-concussive symptoms, post-traumatic stress and coping on outcome in elderly patients after sustaining an MTBI. The main finding of our study was that almost half (44%) of the elderly showed incomplete recovery 6 months post-injury. In addition, elderly patients who experienced depression-related complaints and post-concussive symptoms early after MTBI (i.e. 2 weeks post-injury) were more likely to show an incomplete recovery at 6 months post-injury. In addition, coping style was not found to be significantly associated with outcome.
A total of 162 elderly patients were included in the current study, and 44% of these elderly patients showed an incomplete recovery 6 months after sustaining an MTBI. This finding is in accordance with Rothweiler, Temkin, and Dikmen (Citation1998), who reported that with increasing age the proportion of patients who recover completely decreases. They also reported that only 20% of the subjects aged 60 years or older achieved complete recovery (Rothweiler et al., Citation1998). This high number of incomplete recoveries might partly be explained by mood problems. For instance, the current study found that 12% of the elderly patients experienced early depression, and the presence of depression was associated with a higher likelihood of incomplete recovery 6 months post-injury. Conversely, anxiety was not found to be associated with outcome in our elderly cohort. In line with our findings, it was previously reported that patients frequently develop depression following TBI and also show poorer recovery (Fann, Katon, Uomoto, & Esselman, Citation1995; Menzel, Citation2008). In addition, Goldstein, Levin, Goldman, Clark, & Kenehan Altonen (Citation2001) found that elderly MTBI patients, although having a relatively good cognitive recovery, showed significant depression and anxiety.
In addition to mood, post-concussive symptoms might also affect the outcome of these elderly patients. Our study demonstrated that the majority of patients experience at least one post-concussive symptom 2 weeks after injury. We have also shown that higher numbers of post-concussive symptoms are significantly associated with a decreased likelihood of complete recovery. In accordance with previous studies, the most frequently reported complaints in our cohort were dizziness, fatigue, and headache (van der Naalt et al., Citation1999; Wylie & Flashman, Citation2017). Collectively, these findings further underline the importance of early post-concussive symptoms and depression as determining factors for outcome, and suggest that elderly patients might benefit from early rehabilitation.
Pre-injury physical status, as well as pre-existing mental health problems, should always be taken into account when assessing elderly MTBI patients. The importance of early physical complaints is underlined by the postulation that elderly have reduced physical reserves (LeBlanc et al., Citation2006; Elliott et al., Citation2015). In addition, it was previously reported that pre-existing mental health problems are associated with an increased risk of a worse outcome (Fedoroff et al., Citation1992; Curran et al., Citation2000). However, in our elderly cohort the prevalence of pre-injury mental health problems was rather low. Although not being statistically significant, we did observe a higher prevalence of pre-injury physical complaints in subjects with an incomplete recovery 6 months post-injury.
Fall accidents are the leading cause of MTBI in elderly patients, and in our cohort fall accidents from standing height was the mechanism of injury in the majority of patients. Given the fact that falls were the most prevailing cause of injury, elderly patients might potentially benefit from primary fall prevention strategies. In addition, falls have been associated with post-traumatic stress (Rockwood et al., Citation2005). In our cohort, one in three of the elderly patients reported to experience early post-traumatic stress. O’Donnell et al. (Citation2012) have previously shown that psychological interventions, early after sustaining an injury, could have a positive impact on outcome. Therefore, the initiation of such strategies should be encouraged.
We further investigated the use of the three most frequently used coping styles (i.e. active, passive, and avoidant). Contrary to what was expected, we found that high use of these coping styles was not frequently present in elderly patients. The avoidant coping style was the most used coping style. In addition, the active coping style was borderline significantly associated with a decreased risk of incomplete recovery. In earlier studies, the passive coping style was associated with incomplete recovery and a reduced HRQoL (Aben et al., Citation2008). Studies on coping styles in elderly patients who sustained an MTBI are very limited, and interpretation of our current results might be speculative. For instance, the high use of the avoidant coping style might be the best choice in these elderly patients in order to deal with limitations in their daily life. By using the avoidant coping style instead of the active coping style, elderly would be less likely to get frustrated by their complaints. Conversely, it could be reasoned that this would lead to depression, which is also more present in our cohort. Although we have not investigated social engagement, previous studies have shown that social participation can constitute a means of coping with health changes (Wolters et al., Citation2010). Subsequently, social relationships might aid in the recovery following an MTBI. Therefore, future studies in elderly patients should also investigate the role of social participation in coping with outcome following an MTBI.
Despite the very interesting results, this present study also has some limitations. The first limitation of this study is the lack of a control group (one that consists of elderly who have not sustained a brain injury) to disentangle the effect of age and the effect of the MTBI. Furthermore, it could be argued that the GOSE is not an applicable instrument to measure outcome in these elderly patients, as the focus is on functional outcome. Nevertheless, the GOSE is a valid scale and is applied worldwide to monitor functional recovery, even several years after injury. The GOSE has been used in all age-categories and enables us to compare our results with other studies. Another limitation is that the current study did not measure cognitive impairments, which made it difficult to determine whether patients were suffering from age-associated mild cognitive impairment. However, none of the patients in this study were clinically diagnosed with dementia. In addition, the diagnosis of MTBI in these elderly patients is often considered difficult, due to the fact that elderly patients might experience mild cognitive impairment, use medication, and suffer from multiple comorbidities which might affect their brain function (Page, Linnebur, Bryant, & Ruscin, Citation2010; Papa et al., Citation2012). Therefore, the number of elderly sustaining an MTBI might even be under-estimated due to these known difficulties (de Koning et al., Citation2016; Horowitz et al., Citation1979; Snaith & Zigmond, Citation1983).
Conclusion
Our study showed that almost half of the elderly had an incomplete recovery 6 months after sustaining an MTBI. Elderly patients who experienced depression and post-concussive symptoms early after sustaining an MTBI were more likely to have an incomplete recovery. Collectively, our results underline the need for a different approach in these elderly patients. Therefore, other strategies are needed to support these elderly patients with incomplete recovery, focusing more on pre-injury status, cognitive reserve, and social participation. Since the most common cause of injury in our elderly cohort was fall accidents from standing height, more awareness of fall prevention is warranted.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Abdulle, A. E., de Koning, M. E., van der Horn, H. J., Scheenen, M. E., Roks, G., Hageman, G., … van der Naalt, J. (2018). Early predictors for long-term functional outcome after mild traumatic brain injury in frail elderly patients. Journal of Head Trauma Rehabilitation, 33, E59–E67. doi:10.1097/HTR.0000000000000368
- Aben, L., Busschbach, J. J. V., Ponds, R., & Ribbers, G. M. (2008). Memory self-efficacy and psychosocial factors in stroke. Journal of Rehabilitation Medicine, 40(8), 681–683. doi:10.2340/16501977-0227
- Chen, Y., Peng, Y., Xu, H., & O’Brien, W. H. (2018). Age differences in stress and coping: Problem-focused strategies mediate the relationship between age and positive affect. The International Journal of Aging and Human Development, 86(4), 347–363. doi:10.1177/0091415017720890
- Couture, M., Larivière, N., & Lefrançois, R. (2005). Psychological distress in older adults with low functional independence: A multidimensional perspective. Archives of Gerontology and Geriatrics, 41(1), 101–111. doi:10.1016/j.archger.2004.12.004
- Curran, C. A., Ponsford, J. L., & Crowe, S. (2000). Coping strategies and emotional outcome following traumatic brain injury: A comparison with orthopedic patients. Journal of Head Trauma Rehabilitation, 15(6), 1256–1274. doi:10.1097/00001199-200012000-00006
- de Koning, M. E., Gareb, B., El Moumni, M., Scheenen, M. E., van der Horn, H. J., Timmerman, M. E., … van der Naalt, J. (2016). Subacute posttraumatic complaints and psychological distress in trauma patients with or without mild traumatic brain injury. Injury, 47(9), 2041–2047. doi:10.1016/j.injury.2016.04.036
- Elliott, T. R., Hsiao, Y.-Y., Kimbrel, N. A., Meyer, E. C., DeBeer, B. B., Gulliver, S. B., … Morissette, S. B. (2015). Resilience, traumatic brain injury, depression, and posttraumatic stress among Iraq/Afghanistan war veterans. Rehabilitation Psychology, 60(3), 263–276. doi:10.1037/rep0000050
- Fann, J. R., Katon, W. J., Uomoto, J. M., & Esselman, P. C. (1995). Psychiatric disorders and functional disability in outpatients with traumatic brain injuries. The American Journal of Psychiatry, 152(10), 1493–1499. doi:10.1176/ajp.152.10.1493
- Fedoroff, J. P., Starkstein, S. E., Forrester, A. W., Geisler, F. H., Jorge, R. E., Arndt, S. V., & Robinson, R. G. (1992). Depression in patients with acute traumatic brain injury. The American Journal of Psychiatry, 149(7), 918–923. doi:10.1176/ajp.149.7.918
- Fu, T. S., Jing, R., Fu, W. W., & Cusimano, M. D. (2016). Epidemiological trends of traumatic brain injury identified in the emergency department in a publicly-insured population, 2002–2010. PLoS One, 11(1), 2002–2010. doi:10.1371/journal.pone.0145469
- Goldstein, F. C., Levin, H. S., Goldman, W.P., Clark, A. N., & Kenehan Altonen, T. (2001). Cognitive and neurobehavioral functioning after mild versus moderate traumatic brain injury in older adults. Journal of the International Neuropsychological Society, 7(3), 373–383. doi:10.1017/S1355617701733115
- Haagsma, J. A., Scholten, A. C., Andriessen, T., Vos, P. E., Van Beeck, E. F., & Polinder, S. (2015). Impact of depression and post-traumatic stress disorder on functional outcome and health-related quality of life of patients with mild traumatic brain injury. Journal of Neurotrauma, 32(11), 853–862. doi:10.1089/neu.2013.3283
- Horowitz, M., Wilner, N., & Alvarez, W. (1979). Impact of event scale: A measure of subjective stress. Psychosomatic Medicine, 41(3), 209–218. doi:10.1097/00006842-197905000-00004
- Jorge, R. E., Robinson, R. G., Moser, D., Tateno, A., Crespo-Facorro, B., & Arndt, S. (2004). Major depression following traumatic brain injury. Archives of General Psychiatry, 61(1), 42–50. doi:10.1001/archpsyc.61.1.42
- Kreutzer, J. S., Seel, R. T., & Gourley, E. (2001). The prevalence and symptom rates of depression after traumatic brain injury: A comprehensive examination. Brain Injury, 15(7), 563–576. doi:10.1080/02699050010009108
- Lagarde, E., Salmi, L.-R., Holm, L. W., Contrand, B., Masson, F., Ribéreau-Gayon, R., … Cassidy, J. D. (2014). Association of symptoms following mild traumatic brain injury with posttraumatic stress disorder vs postconcussion syndrome. JAMA Psychiatry, 71(9), 1032–1040. doi:10.1001/jamapsychiatry.2014.666
- LeBlanc, J., de Guise, E., Gosselin, N., & Feyz, M. (2006). Comparison of functional outcome following acute care in young, middle-aged and elderly patients with traumatic brain injury. Brain Injury, 20(8), 779–790. doi:10.1080/02699050600831835
- Livingston, D. H., Lavery, R. F., Mosenthal, A. C., Knudson, M. M., Lee, S., Morabito, D., … Coimbra, R. (2005). Recovery at one year following isolated traumatic brain injury: A Western Trauma Association prospective multicenter trial. The Journal of Trauma, 59(6), 1298–1304. doi:10.1097/01.ta.0000196002.03681.18
- Losoi, H., Silverberg, N. D., Wäljas, M., Turunen, S., Rosti-Otajärvi, E., Helminen, M., … Iverson, G. L. (2015). Resilience is associated with outcome from mild traumatic brain injury. Journal of Neurotrauma, 32(13), 942–949. doi:10.1089/neu.2014.3799
- Maestas, K. L., Sander, A. M., Clark, A. N., van Veldhoven, L. M., Struchen, M. A., Sherer, M., & Hannay, H. J. (2014). Preinjury coping, emotional functioning, and quality of life following uncomplicated and complicated mild traumatic brain injury. Journal of Head Trauma Rehabilitation, 29(5), 407–417. doi:10.1097/HTR.0b013e31828654b4
- McMillan, T. M., Weir, C. J., & Wainman-Lefley, J. (2014). Mortality and morbidity 15 years after hospital admission with mild head injury: A prospective case-controlled population study. Journal of Neurology, Neurosurgery, and Psychiatry, 85(11):1214–1220. doi:10.1136/jnnp-2013-307279
- Menzel, J. C. (2008). Depression in the elderly after traumatic brain injury: A systematic review. Brain Injury, 22(5), 375–380. doi:10.1080/02699050802001492
- Mosenthal, A. C., Lavery, R. F., & Addis, M. (2002). Isolated traumatic brain injury; age is an independent predictor of mortality and early outcome. The Journal of Trauma, 52, 907–911. doi:10.1097/00005373-200205000-00015
- Mosenthal, A. C., Livingston, D. H., Lavery, R. F., Knudson, M. M., Lee, S., Morabito, D., … Coimbra, R. (2004). The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. The Journal of Trauma, 56(5), 1042–1048. doi:10.1097/01.TA.0000127767.83267.33
- O'Donnell, M. L., Lau, W., Tipping, S., Holmes, A. C. N., Ellen, S., Judson, R., ... Forbes, D. (2012). Stepped early psychological intervention for posttraumatic stress disorder, other anxiety disorders, and depression following serious injury. Journal of Traumatic Stress, 25(2), 125–133. doi: 10.1002/jts.21677.
- Page, R. L., Linnebur, S. A., Bryant, L. L., & Ruscin, J. M. (2010). Inappropriate prescribing in the hospitalized elderly patient: Defining the problem, evaluation tools, and possible solutions. Clinical Interventions in Aging, 5(5), 75–87.
- Papa, L., Mendes, M. E., & Braga, C. F. (2012). Mild traumatic brain injury among the geriatric population. Current Translational Geriatrics and Experimental Gerontology Reports, 1(3), 135–142. doi:10.1007/s13670-012-0019-0
- Pennings, J. L., Bachulis, B. L., Simons, C. T., & Slazinski, T. (1993). Survival after severe brain injury in the aged. Archives of Surgery, 128(7), 787–793. doi:10.1001/archsurg.1993.01420190083011
- Rapoport, M. J., & Feinstein, A. (2000). Outcome following traumatic brain injury in the elderly: A critical review. Brain Injury, 14(8), 749–761. doi:10.1080/026990500413777
- Rapoport, M. J., McCullagh, S., Streiner, D., & Feinstein, A. (2003). The clinical significance of major depression following mild traumatic brain injury. Psychosomatics, 44(1), 31–37. doi:10.1176/appi.psy.44.1.31
- Rockwood, K., Song, X., & MacKnight, C. (2005). A global clinical measure of fitness and frailty in elderly people. Canadian Medical Association Journal, 173(5), 489–495. doi:10.1503/cmaj.050051
- Røe, C., Skandsen, T., Manskow, U., Ader, T., & Anke, A. (2015). Mortality and one-year functional outcome in elderly and very old patients with severe traumatic brain injuries: Observed and predicted. Behavioural Neurology, 2015, 1. doi:10.1155/2015/845491
- Rothweiler, B., Temkin, N. R., & Dikmen, S. S. (1998). Aging effect on psychosocial outcome in traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 79(8), 881–887. doi:10.1016/S0003-9993(98)90082-X
- Rozzelle, C. J., Wofford, J. L., & Branch, C. L. (1995). Predictors of hospital mortality in older patients with subdural hematoma. Journal of the American Geriatrics Society, 43(3), 240–244. doi:10.1111/j.1532-5415.1995.tb07329.x
- Scheenen, M. E., Spikman, J. M., de Koning, M. E., van der Horn, H. J., Roks, G., Hageman, G., & van der Naalt, J. (2017). Patients ‘“at risk”’ of suffering from persistent complaints after mild traumatic brain injury: The role of coping, mood disorders, and post-traumatic stress. Journal of Neurotrauma, 34(1), 31–37. doi:10.1089/neu.2015.4381
- Scholten, A. C., Haagsma, J. A., Panneman, M. J. M., Van Beeck, E. F., & Polinder, S. (2014). Traumatic brain injury in the netherlands: Incidence, costs and disability-adjusted life years. PLoS One, 9(10), e110905. doi:10.1371/journal.pone.0110905
- Selassie, A. W., McCarthy, M. L., Ferguson, P. L., Tian, J., & Langlois, J. A. (2005). Risk of posthospitalization mortality among persons with traumatic brain injury, South Carolina 1999–2001. Journal of Head Trauma Rehabilitation, 20(3), 257–269. doi:10.1097/00001199-200505000-00008
- Silverberg, N. D., Gardner, A. J., Brubacher, J. R., Panenka, W. J., Li, J. J., & Iverson, G. L. (2015). Systematic review of multivariable prognostic models for mild traumatic brain injury. Journal of Neurotrauma, 32(8), 517–526. doi:10.1089/neu.2014.3600
- Snaith, R. P., & Zigmond, A. S. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. http://www.ncbi.nlm.nih.gov/pubmed/6880820%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1339318&tool=pmcentrez&rendertype=abstract.
- Snell, D. L., Siegert, R. J., Hay-Smith, E. J. C., & Surgenor, L. J. (2011). Associations between illness perceptions, coping styles and outcome after mild traumatic brain injury: Preliminary results from a cohort study. Brain Injury, 25(11), 1126–1138. doi:10.3109/02699052.2011.607786
- Susman, M., DiRusso, S. M., Sullivan, T., Risucci, D., Nealon, P., Cuff, S., … Benzil, D. (2002). Traumatic brain injury in the elderly: Increased mortality and worse functional outcome at discharge despite lower injury severity. The Journal of Trauma, 53(2), 219–224. doi:10.1097/01TA.0000024249.40070.BD
- Taylor, C. A., Bell, J. M., Breiding, M. J., & Xu, L. (2017). Traumatic brain injury–related emergency department visits, hospitalizations, and deaths — United States, 2007 and 2013. MMWR. Surveillance Summaries, 66(9), 1–16. doi:10.15585/mmwr.ss6609a1
- Thurman, D. J., Alverson, C., Dunn, K. A., Guerrero, J., & Sniezek, J. E. (1999). Traumatic brain injury in the United States: A public health perspective. Journal of Head Trauma Rehabilitation, 14(6), 602–615. doi:10.1097/00001199-199912000-00009
- Tillou, A., Kelley-Quon, L., Burruss, S., Morley, E., Cryer, H., Cohen, M., & Min, L. (2014). Long-term postinjury functional recovery: Outcomes of geriatric consultation. JAMA Surgery, 149(1), 83–89. doi:10.1001/jamasurg.2013.4244
- van der Naalt, J., Timmerman, M. E., de Koning, M. E., van der Horn, H. J., Scheenen, M. E., Jacobs, B., … Spikman, J. M. (2017). Early predictors of outcome after mild traumatic brain injury (UPFRONT): An observational cohort study. The Lancet Neurology, 16(7), 532–540. doi:10.1016/S1474-4422(17)30117-5
- van der Naalt, J., van Zomeren A, H., Sluiter, W. J., & Minderhoud, J. M. (1999). One year outcome in mild to moderate head injury: The predictive value of acute injury characteristics related to complaints and return to work. J Neurol Neurosurg Psychiatry, 66(2), 207–213. doi:10.1136/jnnp.66.2.207
- Wäljas, M., Iverson, G. L., Lange, R. T., Hakulinen, U., Dastidar, P., Huhtala, H., … Öhman, J. (2015). A prospective biopsychosocial study of the persistent post-concussion symptoms following mild traumatic brain injury. Journal of Neurotrauma, 32(8), 534–547. doi:10.1089/neu.2014.3339
- Weber, K. T., Guimarães, V. A., Pontes Neto, O. M., Leite, J. P., Takayanagui, O. M., & Santos-Pontelli, T. (2016). Predictors of quality of life after moderate to severe traumatic brain injury. Arquivos de Neuro-Psiquiatria, 74(5), 409–415. doi:10.1590/0004-282X20160053
- Wilson, J. T., Pettigrew, L. E., & Teasdale, G. M. (1998). Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. Journal of Neurotrauma, 15(8), 573–585. doi:10.1089/neu.1998.15.573
- Wolters, G., Stapert, S., Brands, I., & Van Heugten, C. (2010). Coping styles in relation to cognitive rehabilitation and quality of life after brain injury. Neuropsychological Rehabilitation, 20(4), 587–600. doi:10.1080/09602011003683836
- Wylie, G. R., & Flashman, L. A. (2017). Understanding the interplay between mild traumatic brain injury and cognitive fatigue: Models and treatments. Concussion, 2(4), CNC50. doi:10.2217/cnc-2017-0003
- Zonfrillo, M. R., Kim, K. H., & Arbogast, K. B. (2015). Emergency department visits and head computed tomography utilization for concussion patients from 2006 to 2011. Academic Emergency Medicine, 22(7), 872–877. doi:10.1111/acem.12696