Abstract
Physical inactivity in mid-life is a modifiable risk factor for dementia. Mild behavioral impairment (MBI) is a marker of potential neurodegenerative disease. We investigated the association between physical activity and MBI. Baseline data from the Canadian Platform for Research Online to Investigate Health, Quality of Life, Cognition, Behaviour, Function, and Caregiving in Aging (CAN-PROTECT) were used. Four categories of weekly physical activity (cardiovascular, mind-body, strength training, and physical labour) were derived from the Community Healthy Activities Model Program for Seniors questionnaire. MBI was measured using the MBI-Checklist. Multivariable negative binomial regressions modelled the association between the standardized physical activity duration and MBI severity, adjusted for age, sex, education, marital status, ethno cultural origin, occupation, hypertension, dyslipidemia, mobility, and body mass index. Every 1 SD increase in cardiovascular activity was associated with 8.42% lower MBI severity. In contrast, every 1 SD increase in physical labor duration was associated with 5.64% greater MBI severity. These associations were neither moderated by the frequency engaging in each physical activity nor by sex. Cardiovascular physical activity in older persons may reduce levels of non-cognitive dementia markers like MBI, comparable to effects seen in cognition, potentially modulating dementia risk.
Introduction
In less than 20 years, dementia is predicted to affect the lives of 1.1 million Canadians, with repercussions for the economy, healthcare system, long-term care facilities, and social and community services (Alzheimer Society of Canada, 2010). Dementia is a syndrome characterized by cognitive and behavioural changes severe enough to interfere with functional independence (Duong et al., Citation2017). Dementia is most often attributed to Alzheimer’s disease, although vascular contributions to cognitive impairment are increasingly more common with age.
While dementia typically begins in later life, many factors earlier in life contribute to dementia risk. According to the Lancet Commission on Dementia Prevention, Intervention, and Care, physical inactivity in mid-life is a modifiable risk factor for dementia (Livingston et al., Citation2020). From the Canadian Longitudinal Study on Aging, physical inactivity has the largest population-attributable fraction of all modifiable risk factors for dementia (Son et al., Citation2024). Thus, physical activity can play a great role in the prevention of dementia (Montero-Odasso et al., Citation2020). In light of this, several clinical trials have examined the efficacy of physical activity and shown promising results in managing cognitive impairment through exercise therapy (Hoffmann et al., Citation2016; Vidoni et al., Citation2019). Physical activity programs for those with dementia have shown numerous benefits, including improved cognition (Hokkanen et al., Citation2008), daily activities and independence (Arcoverde et al., Citation2008), functional ability, and mental health (Teri et al., Citation2008). Participating in physical activity in a group setting can also provide social benefits by increasing social networks and reducing feelings of loneliness and isolation, common issues for people living with dementia (Alzheimer’s Society, Citation2008). Evidence suggests that physical activity is important in both males and females; older males with walking rates less than 0.25 miles daily had a greater risk of dementia than those who walked more than two miles a day (Abbott et al., Citation2004), and females with high baseline physical activity levels experienced a lower risk of cognitive decline (Yaffe et al., Citation2001). However, not all types of physical activity may be beneficial (Holtermann et al., Citation2018).
While physical activity can benefit those with cognitive impairment and reduce the risk of incident dementia, there is less research on the impact of physical activity in at-risk populations with little cognitive impairment. As there are fewer barriers to administering physical activity to patients with little cognitive, behavioural, or functional impairment, secondary prevention programs, like physical activity, may be more beneficial at earlier stages of the disease. Therefore, it is important to investigate whether physical activity impacts pre-dementia risk markers to understand if individuals can benefit from physical activity prior to the onset of cognitive impairment.
Mild behavioral impairment (MBI) is a dementia risk marker. Characterized by later-life emergent and persistent neuropsychiatric symptoms (NPS), representing a change from longstanding behaviour and/or personality, MBI identifies a group at high risk for cognitive decline and incident dementia (Ismail et al., Citation2016). MBI symptoms are categorized into five domains, including decreased drive and motivation (apathy), affective dysregulation (mood and anxiety symptoms), impulse dyscontrol (agitation, impulsivity, abnormal reward salience), social inappropriateness (impaired social cognition), and abnormal perception or thought content (hallucinations and delusions, i.e. psychotic symptoms). In cohorts from several countries representing diverse populations, MBI has consistently demonstrated associations with cognitive decline and incident dementia (Creese et al., Citation2019; Ebrahim et al., Citation2023; Ismail et al., Citation2021, Citation2023; Kan et al., Citation2022; Matsuoka et al., Citation2019; Rouse et al., Citation2024; Wolfova et al., Citation2022; Yoon et al., Citation2022). Further, sex differences have been observed in prevalence of MBI and sex may be a moderator of the effect of physical activity on cognitive and neural outcomes, warranting further investigation (Barha et al., Citation2019; Wolfova et al., Citation2022).
We aimed to examine dementia-free older adults to determine the association between the duration of engagement in different types of physical activity and MBI severity. We hypothesized that longer duration of engagement in any type of physical activity would be associated with less severe MBI. We also aimed to explore whether the benefits of physical activity depended on frequency of engagement (i.e. longer but fewer sessions versus more distributed sessions). A secondary analysis investigated sex moderation for the association between physical activity duration and MBI.
Method
Study design
Data are from the Canadian Platform for Research Online to Investigate Health, Quality of Life, Cognition, Behavior, Function, and Caregiving in Aging (CAN-PROTECT) study. CAN-PROTECT is an online longitudinal observational cohort study of risk and resilience to brain aging in community-dwelling Canadian residents aged ≥18 years (Ismail et al., Citation2023). Participants are required to confirm they do not have dementia prior to enrolling in the study (Ismail et al., Citation2023). Participants with mild cognitive impairment (MCI) were identified through self-report. Annual questionnaires about the participant and their lifestyle are administered, as well as a cognitive test battery. Currently, only baseline participant data are available. Extensive descriptions of CAN-PROTECT recruitment and data collection procedures have been published elsewhere (Ismail et al., Citation2023). Participants provide informed consent as part of the registration process. The Conjoint Health Research Ethics Board at the University of Calgary approved the study (REB21-1065).
Participants
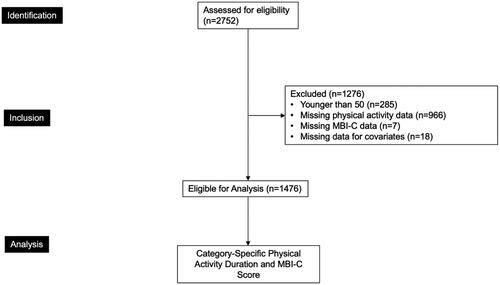
The overall sample assessed for eligibility consisted of 2752 participants. Participants were included in the present analyses if they were (1) older than 50 years of age, in accordance with the International Society to Advance Alzheimer’s Research and Treatment (ISTAART) MBI criteria (n = 2467); (2) had complete physical activity data (n = 1501); (3) had complete MBI Checklist (MBI-C) data (n = 1494); and (4) data for covariates including age, sex, years of education, marital status, ethnocultural origin, occupation type (manual labour, qualified manual labour, non-manual labour, professional, and leadership/managerial work), diagnosis of high blood pressure, and high cholesterol, issues with mobility, and body mass index (BMI) (n = 1476). The final sample size for the analyses was 1476 ().
Figure 1. Participant flow-diagram. Physical activity categories included cardiovascular activity, mind-body activity, strength training, and physical labour. Covariates included age, sex, years of education, marital status, ethnocultural origin, occupation type (manual labour, qualified manual labour, non-manual labour, professional, and leadership/managerial work), diagnosis of high blood pressure, high cholesterol, issues with mobility, and BMI. MBI-C: Mild Behavioral Impairment Checklist.
Measures
Physical activity type, duration, and frequency
Physical activity was measured using the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire (Stewart et al., Citation2001). Participants are first asked how many times per week they typically engage in physical activity (0 = did not engage, 1 = once per week, 2 = twice or more per week). If participants report engaging in physical activity, they are prompted to report the total weekly hours engaged in each type of activity endorsed. The total duration (hours) of weekly engagement in an activity is measured on a scale from 0 to 9, with 0 indicating less than 1 hour of physical activity and 9 indicating greater than 9 hours per week. To distinguish those engaging in no physical activity from those engaging in less than 1 hour of activity, only participants who reported no physical activity were scored as 0, while those endorsing the activity but for less than 1 hour were recoded as 0.5.
Four categories of physical activity (cardiovascular activities, mind-body activities, and strength training activities, physical labour) were derived based on the activities described in the CHAMPS questionnaire. Cardiovascular score comprised jogging, running, walking, hiking, skating, riding a bicycle or stationary cycle, playing sports (football, basketball, tennis, or other racquet sports), and other aerobic exercises (rowing or step machines, water exercises, swimming at various paces, aerobics, or aerobic dancing). Mind-body activity comprised stretching or flexibility exercises and yoga or tai-chi. Strength training activities comprised light, moderate, and heavy strength training and general conditioning exercises. Physical labour comprised engagement in heavy and light housework, gardening, and working on machinery.
Physical activity category duration scores (hours/week) were generated by summing the duration scores of all activities in the category. Cardiovascular activity duration could range from 0 to 135, mind-body activity from 0 to 18, strength training from 0 to 27, and physical labour from 0 to 45. Similarly, category-specific frequencies (times/week) were generated by summing the frequency scores of all activities within a category. Cardiovascular activity could range from 0 to 30, mind-body activity from 0 to 4, strength training from 0 to 6, and physical labour frequency from 0 to 10.
Mild behavioral impairment
MBI was measured by the self-reported MBI-C (Hu et al., Citation2023; Ismail et al., Citation2017; Kassam et al., Citation2023). The MBI-C comprises five domains assessed by 34 items: decreased motivation (6 items), emotional dysregulation (6 items), impulse dyscontrol (12 items), social inappropriateness (5 items), and abnormal perception or thought content (5 items). Each question assesses the presence and severity of symptoms. If a symptom has not been present for at least six months (continuously, or on and off) or is not a change from longstanding behavioral patterns, it is considered absent, and a score of 0 is assigned to that item. If symptoms are present, the severity can be rated on a scale from 1 to 3. A severity score of 1 indicates symptom severity that is mild (noticeable, but not a significant change), 2 indicates moderate (significant, but not a dramatic change), and 3 indicates severe (very marked or prominent, a dramatic change). The MBI total score, ranging from 0 to 102, was calculated by summing the scores for each item, with higher scores indicating more severe symptoms.
Statistical analysis
Participant demographics were summarized using descriptive statistics (means, standard deviations [SD], ranges, counts, and percentages), stratified by sex. Wilcoxon signed-rank tests and chi-squared tests were used to compare between groups.
As MBI-C scores resembled an over dispersed (variance > mean) Poisson distribution, multivariable negative binomial regression models were used in this study. Four regressions modelled the associations between standardized duration of engagement in individual physical activity categories (exposure) and MBI-C total score (outcome). For physical activity categories that were associated with MBI, moderation by frequency of engagement was tested by including the category-specific physical activity frequency as an interaction term to the subset of participants who reported engaging in that category. All models were adjusted for age, sex, years of education, marital status, ethnocultural origin, occupation type (manual labour, qualified manual labour, non-manual labour, professional, and leadership/managerial work), diagnosis of high blood pressure, and high cholesterol, issues with mobility, and BMI. Statistical significance was determined at the level of α = 0.05. All reported coefficients were exponentiated and then converted to percentages. R version 4.0.5 was used to conduct all statistical analyses (R Core Team, Citation2022).
Results
Participant characteristics
Participant characteristics for all analyses are summarized in . Of the total sample (n = 1476), mean participant age was 64.7 (SD = 7.6, range = 50–94) years, 1199 (81.2%) were female, had 15.8 (SD = 4.1, range = 6.0–26) years of education, 1142 (77.4%) were married, common law or cohabiting, 1244 (84.3%) were of European origins, 546 (37.0%) worked in occupations in a leadership/managerial capacity, 422 (28.6%) had high blood pressure, 351 (23.8%) had high cholesterol, 339 (23.0%) had mobility issues, and mean BMI was 28.2 (SD = 8.6, range = 18.8–59.2). For cognitive category, 1059 participants (71.7%) were cognitively normal, 404 (27.4%) had subjective cognitive decline, and 13 (0.9%) had MCI. Total cardiovascular duration was 8.1 hours/week (SD = 6.5, range = 0–43.0), mind-body duration was 1.2 hours/week (SD = 1.8, range = 0–14.0), strength training duration was 1.2 hours/week (SD = 1.8, range = 0–21.0), and physical labour duration was 6.0 hours/week (SD = 5.3, range = 0–37.0). Total cardiovascular frequency was 6.2 times/week (SD = 3.2, range = 0–19.0), mind-body frequency was 1.4 times/week (SD = 1.2, range = 0–4.0), strength training frequency was 1.7 times/week (SD = 1.7, range = 0–6.0), and physical labour frequency was 4.4 times/week (SD = 2.0, range = 0–10.0). Mean MBI-C score was 5.4 (SD = 7.4, range = 0–65.0).
Table 1. Participant characteristics.
Physical activity duration and MBI
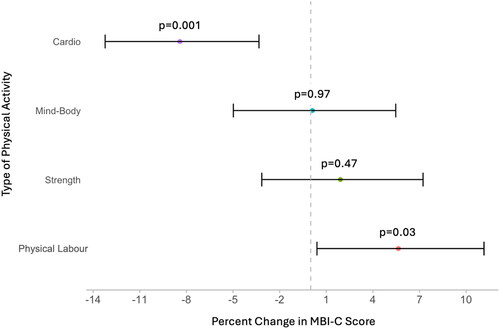
Distribution of the physical activity categories as a function of MBI-C score is shown in Supplementary Figure 1. As illustrated in , adjusting for all covariates, cardiovascular activity duration and physical labour duration were associated with differences in MBI severity. Every 1 SD increase in cardiovascular duration was associated with 8.42% (95%CI: −13.23 to −3.33, p = 0.001) lower MBI severity. In contrast, every 1 SD increase in physical labour duration was associated with 5.64% (95%CI: 0.40–11.15, p = 0.03) greater MBI severity. Neither mind-body activity duration (b = 0.11%, 95%CI: –4.98 to 5.47, p = 0.97) nor strength training duration (b = 1.91%, 95%CI: –3.15 to 7.23, p = 0.47) were associated with MBI severity. The associations between cardiovascular activity duration and physical labour duration and MBI were not moderated by the frequency of engagement in each type of physical activity (cardiovascular b = –0.06%, 95%CI: −0.27 to 0.16, p = 0.62; physical labour b = 0.30%, 95%CI: −0.17 to 0.76, p = 0.21). These associations were also not moderated by sex (cardiovascular b = 1.37%, 95%CI: −0.56 to 3.34, p = 0.16; physical labour b = –1.14%, 95%CI: −3.20 to 0.97, p = 0.29).
Figure 2. Forest plot of multivariable negative binomial regression model outputs. Percent change in Mild Behavioral Impairment Checklist (MBI-C) score for every 1 standard deviation increase in the duration of engagement in cardiovascular activities, mind-body activities, strength training activities, and physical labour, adjusting for age, sex, years of education, marital status, ethnocultural origin, occupation type (manual labour, qualified manual labour, non-manual labour, professional, and leadership/managerial work), diagnosis of high blood pressure, and high cholesterol, issues with mobility, body mass index. Error bars represent the 95% confidence interval (n = 1476).
Discussion
This study found that different forms of physical activity may not have the same effects for brain health. The direction of effect for cardiovascular activity duration was in association with lower MBI severity, whereas physical labour duration had an opposite direction of effect, associated with greater MBI severity.
Our findings are consistent with the physical activity paradox, a phenomenon where occupational physical activity (physical labour) may not have the same beneficial effects as leisure-time physical activity, possibly even contributing to detrimental health effects (Holtermann et al., Citation2018; Zotcheva et al., Citation2023). There are several potential explanations for this paradox. Physical labour activities may be too low in intensity or too long in duration for maintaining or improving cardiovascular health as compared to leisure-time cardiovascular activities. Additionally, long durations of engagement in physical labour activities tend to increase 24-hour heart rate and blood pressure, which are risk factors for cerebral small vessel disease (Holtermann et al., Citation2018) and may also contribute to increased MBI severity (Miao et al., Citation2021). Furthermore, excessive physical labour may be an indicator of low socioeconomic status, which has been indicated as a risk factor for dementia and dementia-related death (Cadar et al., Citation2018; Korhonen et al., Citation2020; Marden et al., Citation2017). Conversely, the ability to participate in leisure-time cardiovascular activity may also be an indicator of high socioeconomic status. In our study, physical activity was based primarily on voluntary activities rather than occupational obligation, but findings remained consistent with the physical activity paradox – a novel finding. Nonetheless, leisure-time cardiovascular activity may provide the most robust reductions in behavioural impairment preceding dementia, but this should be investigated in future clinical trials and potentially discussed at a public health level in terms of equal access to these activities.
These results extend the current literature. A systematic review and meta-analysis of randomized control trials on the effectiveness of physical activity in AD patients found that physical activity can improve cognition, non-cognitive symptoms such as NPS, and quality of life (Liang et al., Citation2022). For NPS specifically, several studies have found that in dementia patients, engagement in physical activity reduces NPS burden (Christofoletti et al., Citation2011; Veronese et al., Citation2019). Another study found that when older adults with AD were involved in a short-term exercise program in addition to treatment as usual, NPS severity was reduced (Fleiner et al., Citation2017). Of these studies, the most common physical activity intervention was aerobic physical activity, as captured by the cardiovascular activity category in this study.
While much of this research has been in older adults with dementia, few studies have explored the link between physical activity and NPS in dementia-free older adults. A report from the Mayo Clinic Study of Aging assessed several types of physical activity in a sample of participants with normal cognition and MCI. Greater late-life total physical activity was associated with lower odds of having apathy, appetite changes, nighttime disturbances, depression, irritability, clinical depression, and clinical anxiety (Krell-Roesch et al., Citation2023). Our findings are consistent but differ with respect to aims; we linked physical activity and dementia risk as measured by MBI. NPS that are later-life emergent and persistent, as per MBI criteria, better predict incident dementia than conventionally measured NPS (Ghahremani, Nathan, et al., Citation2023; Ismail et al., Citation2023; McGirr et al., Citation2022; Naude et al., Citation2024; Vellone et al., Citation2022). Thus, our findings may likely represent an association between cardiovascular physical activity and lower dementia risk.
Several mechanisms may help explain our findings. First, studies show that oxidative stress and a pro-inflammatory profile are associated with NPS in older adults (Büttiker et al., Citation2022; Esch et al., Citation2002; Esch & Stefano, Citation2002). However, aerobic physical activity, including cardiovascular physical activity, may provide neuroprotection by enhancing the body’s antioxidant defence mechanisms to resist oxidative stress (Tortosa-Martínez & Clow, Citation2012; Wang et al., Citation2023). Regular exercise also helps protect against visceral fat accumulation, a source of inflammatory cytokines (Pedersen, Citation2009). These mechanisms align with findings linking vitamin D deficiency and MBI (Rao et al., Citation2020), and with vitamin D supplementation being associated with lower incidence of AD (with greater effects in females than males) (Ghahremani, Smith, et al., Citation2023). As vitamin D protects against oxidative stress and may assist with amyloid-β clearance, these findings strengthen the evidence base that similar neurobiological mechanisms contribute to cognitive symptoms and behavioural symptoms. By extension, these symptoms could be amenable to non-pharmacological interventions such as exercise.
Second, cardiovascular physical activity shows protective effects against vascular pathology in the brain by inducing angiogenesis and increasing blood flow to the brain. For instance, a study showed that moderate-intensity exercise led to an immediate increase in blood flow to the brain. Thus, participants who exercised had higher levels of resting cerebral blood flow and brain metabolism, indicated by glucose uptake, compared to sedentary adults (Barnes, Citation2015; Chapman et al., Citation2013). In addition to cognition, this effect may help improve or mitigate the development of some non-cognitive symptoms like NPS, resulting from hypo perfusion or hypo metabolism in the brain. Relatedly, white matter hyper intensities have demonstrated associations with MBI, further supporting links between vascular disease and behavioural risk for dementia (Miao et al., Citation2021).
Lastly, several studies have shown that exercising can regulate the release of neurotransmitters like dopamine, norepinephrine, and serotonin, which can consequently reduce aggressive and depressive behaviour (Garcia et al., Citation2003; Zheng et al., Citation2018). The locus coeruleus is the primary site of human noradrenergic neurons. Reduced locus coeruleus integrity early in AD has been linked to MBI-impulse dyscontrol, providing a potential mechanism through which exercise may act (Cassidy et al., Citation2022). Additionally, norepinephrine and serotonin stimulation are important for enhanced brain-derived neurotrophic factor (BDNF) transcription after physical exercise (Ma, Citation2008). BDNF plays a crucial role in regulating neuronal survival, synaptic plasticity, and neurogenesis in the brain, particularly in the hippocampus, which is, to an extent, responsible for memory and behaviour (Cotman & Berchtold, Citation2002; Garza et al., Citation2004; Ma, Citation2008). Following exercise, BDNF is upregulated along with other growth factors in the hippocampus and improves mental performance in animal models (Cotman & Berchtold, Citation2002; García-Mesa et al., Citation2014; Garza et al., Citation2004; Ma, Citation2008). Norepinephrine and serotonin may further upregulate BDNF during exercise, thereby reducing NPS. Additionally, BDNF polymorphisms may also be associated with MBI (Matuskova et al., Citation2024; Ramezani et al., Citation2020). Finally, several studies have linked MBI and biomarkers for AD and other neurodegenerative diseases, associations that are not consistently found in groups with conventionally measured NPS (Creese et al., Citation2021; Creese & Ismail, Citation2022; Ghahremani, Nathan, et al., Citation2023; Ghahremani, Wang, et al., Citation2023; Ismail et al., Citation2023; Johansson et al., Citation2021; Lang et al., Citation2020; Lussier et al., Citation2020; Matsuoka et al., Citation2023; Matuskova et al., Citation2021; Miao et al., Citation2021; Citation2022; Naude et al., Citation2020; Ruthirakuhan et al., Citation2022; Showraki et al., Citation2019; Soto et al., Citation2024; Sun et al., Citation2021; Yoon et al., Citation2019; Citation2022).
Ultimately, this study adds to the current evidence indicating that secondary prevention programs like cardiovascular physical activity can be beneficial for individuals at risk for dementia. The fact that the benefits of cardiovascular physical activity can precede the onset of cognitive decline is welcome news. Healthcare practitioners can recommend increased duration and frequency of cardiovascular activity as early as possible to patients to minimize behavioural impairments and later-life risk for cognitive decline and dementia.
Despite these novel findings, this study is not without limitations. First, we were not able to explore socioeconomic status as a possible mediator, moderator, or confounder of the relationship, which should be explored in future studies; some data suggest that while higher recreational physical activity is associated with better cognition, higher work-related physical activity is associated with lower cognition (Zotcheva et al., Citation2023). Additionally, this sample consisted of primarily higher educated individuals of European origins, which limits the generalizability of these findings. The measure of physical activity is also a self-reported measure, which is subject to reporter bias. The CHAMPS questionnaire frequency scale combines frequencies of engagement ≥2 weekly into one category, somewhat limiting analysis. Lastly, this analysis was cross-sectional, and directionality cannot necessarily be implied. Longitudinal studies are needed to explore this further. Future studies should also investigate these relationships in terms of cognition to understand concurrently the impact of physical activity on later-life cognitive and behavioural changes.
Nonetheless, there are several strengths of this study. First, to our knowledge, this is the first study exploring the relationship between physical activity and MBI. Therefore, we have established a starting point for further investigations. Second, we focused on stratifying by the type, duration, and frequency of physical activity. This focus allowed us to explore relationships between durations and frequencies of physical activity in the same sample and establish clear associations between various types, durations, and frequencies of physical activities on MBI severity. This is particularly relevant as many previous studies tend to examine physical activity as a homogenous variable. Lastly, the large sample size of nearly 1500 participants allowed us to investigate multiple associations.
Conclusion
This study investigated the cross-sectional relationship between the type, weekly duration, and weekly frequency of various physical activities. It was found that longer durations cardiovascular activity were associated with less severe MBI, while longer durations of physical labour were associated with more severe MBI. These results can inform dementia risk reduction, where clinicians can include non-cognitive outcomes in addition to cognition when recommending cardiovascular physical exercise. Future studies should investigate this relationship further, particularly through a clinical trial with a longitudinal cohort, to assess whether the association between physical activity and MBI is causal, whether it is direct or indirect, and if there are associations with a decreased risk of developing dementia.
Consent and ethical publication statement
All participants provided electronic informed consent as part of the CAN-PROTECT online registration process. The research reported in this paper adhered to the ethical guidelines set forth by the Conjoint Health Research Ethics Board at the University of Calgary (REB21-1065).
Supplemental Material
Download MS Word (219.3 KB)Disclosure statement
EES reported consulting (unpaid) for Alnylam Pharmaceuticals and Eli Lilly, and an advisory board (unpaid) for Eisai. ZI has served as an advisor/consultant to CADTH, EISAI, Lilly, Lundbeck/Otsuka, Novo Nordisk, and Roche.
Additional information
Funding
References
- Abbott, R. D., White, L. R., Ross, G. W., Masaki, K. H., Curb, J. D., & Petrovitch, H. (2004). Walking and dementia in physically capable elderly men. JAMA, 292(12), 1447–1453. https://doi.org/10.1001/jama.292.12.1447
- Alzheimer Society of Canada. (2010). Rising tide: The impact of dementia on Canadian society. In Executive Summary (pp. 1–24). Alzheimer Society of Canada.
- Alzheimer’s Society. (2008). Dementia: Out of the shadows. Alzheimer’s Society London.
- Arcoverde, C., Deslandes, A., Rangel, A., Rangel, A., Pavão, R., Nigri, F., Engelhardt, E., & Laks, J. (2008). Role of physical activity on the maintenance of cognition and activities of daily living in elderly with Alzheimer’s disease. Arquivos de Neuro-Psiquiatria, 66(2B), 323–327. https://doi.org/10.1590/s0004-282x2008000300007
- Barha, C. K., Hsu, C.-L., Ten Brinke, L., & Liu-Ambrose, T. (2019). Biological sex: A potential moderator of physical activity efficacy on brain health. Frontiers in Aging Neuroscience, 11, 329. https://doi.org/10.3389/fnagi.2019.00329
- Barnes, J. N. (2015). Exercise, cognitive function, and aging. Advances in Physiology Education, 39(2), 55–62. https://doi.org/10.1152/advan.00101.2014
- Büttiker, P., Weissenberger, S., Esch, T., Anders, M., Raboch, J., Ptacek, R., Kream, R. M., & Stefano, G. B. (2022). Dysfunctional mitochondrial processes contribute to energy perturbations in the brain and neuropsychiatric symptoms. Frontiers in Pharmacology, 13, 1095923. https://doi.org/10.3389/fphar.2022.1095923
- Cadar, D., Lassale, C., Davies, H., Llewellyn, D. J., Batty, G. D., & Steptoe, A. (2018). Individual and area-based socioeconomic factors associated with dementia incidence in England: Evidence from a 12-year follow-up in the English longitudinal study of ageing. JAMA Psychiatry, 75(7), 723–732. https://doi.org/10.1001/jamapsychiatry.2018.1012
- Cassidy, C. M., Therriault, J., Pascoal, T. A., Cheung, V., Savard, M., Tuominen, L., Chamoun, M., McCall, A., Celebi, S., Lussier, F., Massarweh, G., Soucy, J.-P., Weinshenker, D., Tardif, C., Ismail, Z., Gauthier, S., & Rosa-Neto, P. (2022). Association of locus coeruleus integrity with Braak stage and neuropsychiatric symptom severity in Alzheimer’s disease. Neuropsychopharmacology, 47(5), 1128–1136. https://doi.org/10.1038/s41386-022-01293-6
- Chapman, S. B., Aslan, S., Spence, J. S., Defina, L. F., Keebler, M. W., Didehbani, N., & Lu, H. (2013). Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Frontiers in Aging Neuroscience, 5, 75. https://doi.org/10.3389/fnagi.2013.00075
- Christofoletti, G., Oliani, M. M., Bucken-Gobbi, L. T., Gobbi, S., Beinotti, F., & Stella, F. (2011). Physical activity attenuates neuropsychiatric disturbances and caregiver burden in patients with dementia. Clinics, 66(4), 613–618. https://doi.org/10.1590/S1807-59322011000400015
- Cotman, C. W., & Berchtold, N. C. (2002). Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences, 25(6), 295–301. https://doi.org/10.1016/s0166-2236(02)02143-4
- Creese, B., & Ismail, Z. (2022). Mild behavioral impairment: Measurement and clinical correlates of a novel marker of preclinical Alzheimer’s disease. Alzheimer’s Research & Therapy, 14(1), 2. https://doi.org/10.1186/s13195-021-00949-7
- Creese, B., Brooker, H., Ismail, Z., Wesnes, K. A., Hampshire, A., Khan, Z., Megalogeni, M., Corbett, A., Aarsland, D., & Ballard, C. (2019). Mild behavioral impairment as a marker of cognitive decline in cognitively normal older adults. The American Journal of Geriatric Psychiatry, 27(8), 823–834. https://doi.org/10.1016/j.jagp.2019.01.215
- Creese, B., Arathimos, R., Brooker, H., Aarsland, D., Corbett, A., Lewis, C., Ballard, C., Ismail, Z. (2021). Genetic risk for Alzheimer’s disease, cognition, and mild behavioral impairment in healthy older adults. Alzheimers Dementia, 13(1), e12164.
- Duong, S., Patel, T., & Chang, F. (2017). Dementia: What pharmacists need to know. Canadian Pharmacists Journal/Revue des Pharmaciens du Canada, 150(2), 118–129. https://doi.org/10.1177/1715163517690745
- Ebrahim, I. M., Ghahremani, M., Camicioli, R., Smith, E. E., & Ismail, Z. (2023). Effects of race, baseline cognition, and APOE on the association of affective dysregulation with incident dementia: A longitudinal study of dementia-free older adults. Journal of Affective Disorders, 332, 9–18. https://doi.org/10.1016/j.jad.2023.03.074
- Esch, T., & Stefano, G. (2002). Proinflammation: A common denominator or initiator of different pathophysiological disease processes. Signature, 8(5), 9.
- Esch, T., Stefano, GB., Fricchione, GL., Benson, H. (2002). The role of stress in neurodegenerative diseases and mental disorders. Neuroendocrinology Letters, 23(3), 199–208.
- Fleiner, T., Dauth, H., Gersie, M., Zijlstra, W., & Haussermann, P. (2017). Structured physical exercise improves neuropsychiatric symptoms in acute dementia care: A hospital-based RCT. Alzheimer’s Research & Therapy, 9(1), 68. https://doi.org/10.1186/s13195-017-0289-z
- Garcia, C., Chen, M. J., Garza, A. A., Cotman, C. W., & Russo-Neustadt, A. (2003). The influence of specific noradrenergic and serotonergic lesions on the expression of hippocampal brain-derived neurotrophic factor transcripts following voluntary physical activity. Neuroscience, 119(3), 721–732. https://doi.org/10.1016/s0306-4522(03)00192-1
- García-Mesa, Y., Pareja-Galeano, H., Bonet-Costa, V., Revilla, S., Gómez-Cabrera, M. C., Gambini, J., Giménez-Llort, L., Cristòfol, R., Viña, J., & Sanfeliu, C. (2014). Physical exercise neuroprotects ovariectomized 3xTg-AD mice through BDNF mechanisms. Psychoneuroendocrinology, 45, 154–166. https://doi.org/10.1016/j.psyneuen.2014.03.021
- Garza, A. A., Ha, T. G., Garcia, C., Chen, M. J., & Russo-Neustadt, A. A. (2004). Exercise, antidepressant treatment, and BDNF mRNA expression in the aging brain. Pharmacology Biochemistry and Behavior, 77(2), 209–220. https://doi.org/10.1016/j.pbb.2003.10.020
- Ghahremani, M., Nathan, S., Smith, E. E., McGirr, A., Goodyear, B., & Ismail, Z. (2023). Functional connectivity and mild behavioral impairment in dementia-free elderly. Alzheimer’s & Dementia, 9(1), e12371.
- Ghahremani, M., Smith, E. E., Chen, H., Creese, B., Goodarzi, Z., & Ismail, Z. (2023). Vitamin D supplementation and incident dementia: Effects of sex, APOE, and baseline cognitive status. Alzheimer’s & Dementia, 15(1), e12404. https://doi.org/10.1002/dad2.12404
- Ghahremani, M., Wang, M., Chen, H.-Y., Zetterberg, H., Smith, E., & Ismail, Z (2023). Plasma phosphorylated tau at threonine 181 and neuropsychiatric symptoms in preclinical and prodromal Alzheimer disease. Neurology, 100(7), e683–e693. https://doi.org/10.1212/WNL.0000000000201517
- Hoffmann, K., Sobol, N. A., Frederiksen, K. S., Beyer, N., Vogel, A., Vestergaard, K., Brændgaard, H., Gottrup, H., Lolk, A., Wermuth, L., Jacobsen, S., Laugesen, L. P., Gergelyffy, R. G., Høgh, P., Bjerregaard, E., Andersen, B. B., Siersma, V., Johannsen, P., Cotman, C. W., Waldemar, G., & Hasselbalch, S. G. (2016). Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: A randomized controlled trial. Journal of Alzheimer’s Disease, 50(2), 443–453. https://doi.org/10.3233/JAD-150817
- Hokkanen, L., Rantala, L., Remes, A. M., Härkönen, B., Viramo, P., & Winblad, I. (2008). Dance and movement therapeutic methods in management of dementia: A randomized, controlled study. Journal of the American Geriatrics Society, 56(4), 771–772. https://doi.org/10.1111/j.1532-5415.2008.01611.x
- Holtermann, A., Krause, N., van der Beek, AJ., Straker L. (2018). The physical activity paradox: Six reasons why occupational physical activity (OPA) does not confer the cardiovascular health benefits that leisure time physical activity does. British Journal of Sports Medicine, 52(3), 149.
- Hu, S., Patten, S., Charlton, A., Fischer, K., Fick, G., Smith, E. E., & Ismail, Z. (2023). Validating the mild behavioral impairment checklist in a cognitive clinic: Comparisons with the neuropsychiatric inventory questionnaire. Journal of Geriatric Psychiatry and Neurology, 36(2), 107–120. https://doi.org/10.1177/08919887221093353
- Ismail, Z., Agüera-Ortiz, L., Brodaty, H., Cieslak, A., Cummings, J., Fischer, C. E., Gauthier, S., Geda, Y. E., Herrmann, N., Kanji, J., Lanctôt, K. L., Miller, D. S., Mortby, M. E., Onyike, C. U., Rosenberg, P. B., Smith, E. E., Smith, G. S., Sultzer, D. L., Lyketsos, C., & NPS Professional Interest Area of the International Society of to Advance Alzheimer’s Research and Treatment (NPS-PIA of ISTAART). (2017). The Mild Behavioral Impairment Checklist (MBI-C): A rating scale for neuropsychiatric symptoms in pre-dementia populations. Journal of Alzheimer’s Disease, 56(3), 929–938. https://doi.org/10.3233/JAD-160979
- Ismail, Z., McGirr, A., Gill, S., Hu, S., Forkert, N. D., & Smith, E. E. (2021). Mild behavioral impairment and subjective cognitive decline predict cognitive and functional decline. Journal of Alzheimer’s Disease, 80(1), 459–469. https://doi.org/10.3233/JAD-201184
- Ismail, Z., Guan, D. X., Vellone, D., Ballard, C., Creese, B., Corbett, A., Pickering, E., Bloomfield, A., Hampshire, A., Sekhon, R., Roach, P., & Smith, E. E. (2023). The Canadian platform for research online to investigate health, quality of life, cognition, behaviour, function, and caregiving in aging (CAN-PROTECT): Study protocol, platform description, and preliminary analyses. medRxiv, p. 2023.12.16.23300094.
- Ismail, Z., Ghahremani, M., Amlish Munir, M., Fischer, C. E., Smith, E. E., & Creese, B. (2023). A longitudinal study of late-life psychosis and incident dementia and the potential effects of race and cognition. Nature Mental Health, 1(4), 273–283. https://doi.org/10.1038/s44220-023-00043-x
- Ismail, Z., Leon, R., Creese, B., Ballard, C., Robert, P., & Smith, E. E. (2023). Optimizing detection of Alzheimer’s disease in mild cognitive impairment: A 4-year biomarker study of mild behavioral impairment in ADNI and MEMENTO. Molecular Neurodegeneration, 18(1), 50. https://doi.org/10.1186/s13024-023-00631-6
- Ismail, Z., Smith, E. E., Geda, Y., Sultzer, D., Brodaty, H., Smith, G., Agüera-Ortiz, L., Sweet, R., Miller, D., & Lyketsos, C. G. (2016). Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimer’s & Dementia, 12(2), 195–202. https://doi.org/10.1016/j.jalz.2015.05.017
- Johansson, M., Stomrud, E., Insel, P. S., Leuzy, A., Johansson, P. M., Smith, R., Ismail, Z., Janelidze, S., Palmqvist, S., van Westen, D., Mattsson-Carlgren, N., & Hansson, O. (2021). Mild behavioral impairment and its relation to tau pathology in preclinical Alzheimer’s disease. Translational Psychiatry, 11(1), 76. https://doi.org/10.1038/s41398-021-01206-z
- Kan, C. N., Cano, J., Zhao, X., Ismail, Z., Chen, C. L.-H., & Xu, X. (2022). Prevalence, clinical correlates, cognitive trajectories, and dementia risk associated with mild behavioral impairment in Asians. The Journal of Clinical Psychiatry, 83(3), 40123. https://doi.org/10.4088/JCP.21m14105
- Kassam, F., Chen, H., Nosheny, R. L., McGirr, A., Williams, T., Ng, N., Camacho, M., Mackin, R. S., Weiner, M. W., & Ismail, Z. (2023). Cognitive profile of people with mild behavioral impairment in Brain Health Registry participants. Int Psychogeriatr, 35(11), 643–652. https://doi.org/10.1017/S1041610221002878
- Korhonen, K., Einiö, E., Leinonen, T., Tarkiainen, L., & Martikainen, P. (2020). Midlife socioeconomic position and old-age dementia mortality: A large prospective register-based study from Finland. BMJ Open, 10(1), e033234. https://doi.org/10.1136/bmjopen-2019-033234
- Krell-Roesch, J., Syrjanen, J. A., Bezold, J., Trautwein, S., Barisch-Fritz, B., Kremers, W. K., Fields, J. A., Scharf, E. L., Knopman, D. S., Stokin, G. B., Petersen, R. C., Jekauc, D., Woll, A., Vassilaki, M., & Geda, Y. E. (2023). Mid- and late-life physical activity and neuropsychiatric symptoms in dementia-free older adults: Mayo clinic study of aging. The Journal of Neuropsychiatry and Clinical Neurosciences, 35(2), 133–140. https://doi.org/10.1176/appi.neuropsych.20220068
- Lang, S., Yoon, E. J., Kibreab, M., Kathol, I., Cheetham, J., Hammer, T., Sarna, J., Ismail, Z., & Monchi, O. (2020). Mild behavioral impairment in Parkinson’s disease is associated with altered corticostriatal connectivity. NeuroImage, 26, 102252. https://doi.org/10.1016/j.nicl.2020.102252
- Liang, Y.-J., Su, Q.-W., Sheng, Z.-R., Weng, Q.-Y., Niu, Y.-F., Zhou, H.-D., & Liu, C.-B. (2022). Effectiveness of physical activity interventions on cognition, neuropsychiatric symptoms, and quality of life of Alzheimer’s Disease: An update of a systematic review and meta-analysis. Frontiers in Aging Neuroscience, 14, 830824. https://doi.org/10.3389/fnagi.2022.830824
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., Brayne, C., Burns, A., Cohen-Mansfield, J., Cooper, C., Costafreda, S. G., Dias, A., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Kivimäki, M., Larson, E. B., Ogunniyi, A., … Mukadam, N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet, 396(10248), 413–446. https://doi.org/10.1016/S0140-6736(20)30367-6
- Lussier, F. Z., et al. (2020). Mild behavioral impairment is associated with beta-amyloid but not tau or neurodegeneration in cognitively intact elderly individuals. Alzheimers Dement, 16(1), 192–199. https://doi.org/10.1002/alz.12007
- Ma, Q. (2008). Beneficial effects of moderate voluntary physical exercise and its biological mechanisms on brain health. Neuroscience Bulletin, 24(4), 265–270. https://doi.org/10.1007/s12264-008-0402-1
- Marden, J. R., Tchetgen Tchetgen, E. J., Kawachi, I., & Glymour, M. M. (2017). Contribution of socioeconomic status at 3 life-course periods to late-life memory function and decline: Early and late predictors of dementia risk. American Journal of Epidemiology, 186(7), 805–814. https://doi.org/10.1093/aje/kwx155
- Matsuoka, T., Imai, A., & Narumoto, J. (2023). Neuroimaging of mild behavioral impairment: A systematic review. Psychiatry and Clinical Neurosciences Reports, 2(1), e81. https://doi.org/10.1002/pcn5.81
- Matsuoka, T., Ismail, Z., & Narumoto, J. (2019). Prevalence of mild behavioral impairment and risk of dementia in a psychiatric outpatient clinic.
- Matuskova, V., Ismail, Z., Nikolai, T., Markova, H., Cechova, K., Nedelska, Z., Laczó, J., Wang, M., Hort, J., & Vyhnalek, M. (2021). Mild behavioral impairment is associated with atrophy of entorhinal cortex and hippocampus in a memory clinic cohort. Frontiers in Aging Neuroscience, 13, 643271. https://doi.org/10.3389/fnagi.2021.643271
- Matuskova, V., Veverova, K., Jester, D. J., Matoska, V., Ismail, Z., Sheardova, K., Horakova, H., Cerman, J., Laczó, J., Andel, R., Hort, J., & Vyhnalek, M. (2024). Mild behavioral impairment in early Alzheimer’s disease and its association with APOE and BDNF risk genetic polymorphisms. Alzheimer’s Research & Therapy, 16(1), 21. https://doi.org/10.1186/s13195-024-01386-y
- McGirr, A., Nathan, S., Ghahremani, M., Gill, S., Smith, E. E., & Ismail, Z. (2022). Progression to dementia or reversion to normal cognition in mild cognitive impairment as a function of late-onset neuropsychiatric symptoms. Neurology, 98(21), e2132–e2139. https://doi.org/10.1212/WNL.0000000000200256
- Miao, R., Chen, H.-Y., Gill, S., Naude, J., Smith, E. E., & Ismail, Z. (2022). Plasma β-amyloid in mild behavioural impairment–neuropsychiatric symptoms on the Alzheimer’s continuum. Journal of Geriatric Psychiatry and Neurology, 35(3), 434–441. https://doi.org/10.1177/08919887211016068
- Miao, R., Chen, H.-Y., Robert, P., Smith, E. E., & Ismail, Z. (2021). White matter hyperintensities and mild behavioral impairment: Findings from the MEMENTO cohort study. Cerebral Circulation – Cognition and Behavior, 2, 100028. https://doi.org/10.1016/j.cccb.2021.100028
- Montero-Odasso, M., Ismail, Z., & Livingston, G. (2020). One third of dementia cases can be prevented within the next 25 years by tackling risk factors. The case "for" and "against”. Alzheimer’s Research & Therapy, 12(1), 81. https://doi.org/10.1186/s13195-020-00646-x
- Naude, J. P., Gill, S., Hu, S., McGirr, A., Forkert, N. D., Monchi, O., Stys, P. K., Smith, E. E., & Ismail, Z. (2020). Plasma Neurofilament Light: A marker of cognitive decline in Mild Behavioural Impairment. Journal of Alzheimer’s Disease, 76(3), 1017–1027. https://doi.org/10.3233/JAD-200011
- Naude, J., Wang, M., Leon, R., Smith, E., & Ismail, Z. (2024). Tau-PET in early cortical Alzheimer brain regions in relation to mild behavioral impairment in older adults with either normal cognition or mild cognitive impairment. Neurobiology of Aging, 138, 19–27. https://doi.org/10.1016/j.neurobiolaging.2024.02.006
- Pedersen, B. K. (2009). The diseasome of physical inactivity – And the role of myokines in muscle – Fat cross talk. The Journal of Physiology, 587(Pt 23), 5559–5568. https://doi.org/10.1113/jphysiol.2009.179515
- R Core Team, (2022). R: A language and environment for statistical computing. R.F.f.S. Computing.
- Ramezani, M., Ruskey, J. A., Martens, K., Kibreab, M., Javer, Z., Kathol, I., Hammer, T., Cheetham, J., Leveille, E., Martino, D., Sarna, J. R., Gan-Or, Z., Pfeffer, G., Ismail, Z., & Monchi, O. (2020). Association between BDNF Val66Met polymorphism and mild behavioral impairment in patients with Parkinson’s disease. Frontiers in Neurology, 11, 587992. https://doi.org/10.3389/fneur.2020.587992
- Rao, A., Thakral, M., Saini, M., Chatterjee, P., & Dey, A. (2020). Blood biomarkers in older subjects with mild behavioral impairment: A cross-sectional study from the memory clinic, all India Institute of medical sciences, India. Journal of the Indian Academy of Geriatrics, 16(3), 91. https://doi.org/10.4103/jiag.jiag_7_20
- Rouse, H. J., Ismail, Z., Andel, R., Molinari, V. A., Schinka, J. A., & Small, B. J. (2024). Impact of mild behavioral impairment on longitudinal changes in cognition. The Journals of Gerontology, 79(1), glad098. https://doi.org/10.1093/gerona/glad098
- Ruthirakuhan, M., Ismail, Z., Herrmann, N., Gallagher, D., & Lanctôt, K. L. (2022). Mild behavioral impairment is associated with progression to Alzheimer’s disease: A clinicopathological study. Alzheimer’s & Dementia, 18(11), 2199–2208. https://doi.org/10.1002/alz.12519
- Showraki, A., Murari, G., Ismail, Z., Barfett, J. J., Fornazzari, L., Munoz, D. G., Schweizer, T. A., & Fischer, C. E. (2019). Cerebrospinal fluid correlates of neuropsychiatric symptoms in patients with Alzheimer’s Disease/Mild Cognitive Impairment: A systematic review. Journal of Alzheimer’s Disease, 71(2), 477–501. https://doi.org/10.3233/JAD-190365
- Son, S., Speechley, M., Zou, G., Kivipelto, M., Mangialasche, F., Feldman, H., Chertkow, H., Belleville, S., Nygaard, H., Hachinski, V., Pieruccini-Faria, F., & Montero-Odasso, M. (2024). Potentially modifiable dementia risk factors in Canada: An analysis of canadian longitudinal study on aging with a multi-country comparison. medRxiv, p. 2024.02. 20.24303090.
- Soto, M., Rosenberg, P., Ballard, C., Vellas, B., Miller, D., Gauthier, S., Carrillo, M. C., Lyketsos, C., & Ismail, Z. (2024). CTAD Task Force Paper: Neuropsychiatric Symptoms in AD: Clinical trials targeting mild behavioral impairment: A report from the international CTAD task force. The Journal of Prevention of Alzheimer’s Disease, 11(1), 56–64. https://doi.org/10.14283/jpad.2023.125
- Stewart, A. L., Mills, K. M., King, A. C., Haskell, W. L., Gillis, D., & Ritter, P. L. (2001). CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Medicine and Science in Sports and Exercise, 33(7), 1126–1141. https://doi.org/10.1097/00005768-200107000-00010
- Sun, Y., Xu, W., Chen, K.-L., Shen, X.-N., Tan, L., & Yu, J.-T. (2021). Mild behavioral impairment correlates of cognitive impairments in older adults without dementia: Mediation by amyloid pathology. Translational Psychiatry, 11(1), 577. https://doi.org/10.1038/s41398-021-01675-2
- Teri, L., Logsdon, R. G., & McCurry, S. M. (2008). Exercise interventions for dementia and cognitive impairment: The Seattle Protocols. The Journal of Nutrition Health and Aging, 12, 391–394. https://doi.org/10.1007/BF02982672
- Tortosa-Martínez, J., & Clow, A. (2012). Does physical activity reduce risk for Alzheimer’s disease through interaction with the stress neuroendocrine system? Stress, 15(3), 243–261. https://doi.org/10.3109/10253890.2011.629323
- Vellone, D., Ghahremani, M., Goodarzi, Z., Forkert, N. D., Smith, E. E., & Ismail, Z. (2022). Apathy and APOE in mild behavioral impairment, and risk for incident dementia. Alzheimer’s & Dementia, 8(1), e12370. https://doi.org/10.1002/trc2.12370
- Veronese, N., Solmi, M., Basso, C., Smith, L., & Soysal, P. (2019). Role of physical activity in ameliorating neuropsychiatric symptoms in Alzheimer disease: A narrative review. International Journal of Geriatric Psychiatry, 34(9), 1316–1325. https://doi.org/10.1002/gps.4962
- Vidoni, E. D., Perales, J., Alshehri, M., Giles, A.-M., Siengsukon, C. F., & Burns, J. M. (2019). Aerobic exercise sustains performance of instrumental activities of daily living in early-stage Alzheimer’s disease. Journal of Geriatric Physical Therapy, 42(3), E129–E134. https://doi.org/10.1519/JPT.0000000000000172
- Wang, M., Zhang, H., Liang, J., Huang, J., & Chen, N. (2023). Exercise suppresses neuroinflammation for alleviating Alzheimer’s disease. Journal of Neuroinflammation, 20(1), 76. https://doi.org/10.1186/s12974-023-02753-6
- Wolfova, K., Creese, B., Aarsland, D., Ismail, Z., Corbett, A., Ballard, C., Hampshire, A., & Cermakova, P. (2022). Gender/sex differences in the association of mild behavioral impairment with cognitive aging. Journal of Alzheimer’s Disease, 88(1), 345–355. https://doi.org/10.3233/JAD-220040
- Yaffe, K., Barnes, D., Nevitt, M., Lui, L. Y., & Covinsky, K. (2001). A prospective study of physical activity and cognitive decline in elderly women: Women who walk. Archives of Internal Medicine, 161(14), 1703–1708. https://doi.org/10.1001/archinte.161.14.1703
- Yoon, E. J., Ismail, Z., Hanganu, A., Kibreab, M., Hammer, T., Cheetham, J., Kathol, I., Sarna, J. R., Martino, D., Furtado, S., & Monchi, O. (2019). Mild Behavioral Impairment is linked to worse cognition and brain atrophy in Parkinson’s disease. Neurology, 93(8), e766–e777. https://doi.org/10.1212/WNL.0000000000007968
- Yoon, E. J., Lee, J.-Y., Kwak, S., & Kim, Y. K. (2022). Mild behavioral impairment linked to progression to Alzheimer’s disease and cortical thinning in amnestic mild cognitive impairment. Frontiers in Aging Neuroscience, 14, 1051621. https://doi.org/10.3389/fnagi.2022.1051621
- Zheng, X., Takatsu, S., Ishikawa, R., & Hasegawa, H. (2018). Moderate intensity, exercise-induced catecholamine release in the preoptic area and anterior hypothalamus in rats is enhanced in a warm environment. Journal of Thermal Biology, 71, 123–127. https://doi.org/10.1016/j.jtherbio.2017.11.003
- Zotcheva, E., Bratsberg, B., Strand, B. H., Jugessur, A., Engdahl, B. L., Bowen, C., Selbæk, G., Kohler, H.-P., Harris, J. R., & Weiss, J. (2023). Trajectories of occupational physical activity and risk of later-life mild cognitive impairment and dementia: The HUNT4 70+ study. The Lancet Regional Health–Europe, 34.
