Abstract
The use of controlled release fertilizer (CRF) has become a new trend to minimize environmental pollution. In this study, urea–kaolinite containing 20 wt% urea after one hour dry grinding was mixed with different concentrations of chitosan as a binder to prepare nitrogen-based CRF. Fourier transform infrared spectroscopy confirmed the hydrogen bonding between urea and kaolinite. Covalent interaction between urea–kaolinite and chitosan make the granules stronger. The nitrogen release was measured in 5 days interval using a diacetylmonoxime calorimetric method at a wavelength of 527 nm. The results illustrated that by increasing the chitosan concentration from 3 to 7.5%, nitrogen release decreased from 41.23 to 25.25% after one day and from 77.31 to 59.27% after 30 days incubation in water. Compressive stress at break tests confirmed that granules with chitosan 6% had the highest resistance and were chosen for ammonia volatilization tests. Ammonia volatilization was carried out using the forced-draft technique for a period of 10 weeks. The results showed that the total amount of ammonia loss for conventional urea fertilizer and urea–kaolinite–chitosan granules was 68.63 and 56.75%, respectively. This controlled release product could be applied in agricultural crop production purpose due to its controlled solubility in the soil, high nutrient use efficiency and potential economic benefits.
Introduction
Fertilizers are chemical compounds used to enhance the agricultural crop growth through their ability to easy supply of plant requirement nutrients.[Citation1] Urea is the most widely used N fertilizers which contains 46% nitrogen. A large proportion of urea hydrolyzes before plant uptake.[Citation2] Denitrification is generally reported as a major reason for the low efficiency of applied nitrogen fertilizers.[Citation3,4] Controlled release compounds are applied in biotechnology, medical purpose and especially as fertilizers in agricultural crop production.[Citation5,6] Controlled release formulation fertilizers compared to conventional fertilizers can overcome the loss of nutrients constraints, such as off stream losses, leaching, volatilization due to their more sustainable release characters and better uptake and stability.[Citation7]
A number of commercial coated fertilizer products with alkyd resin, polyurethane and polyolefin coating materials cannot degrade properly in soil solution phases, and thereby their accumulation in the soil destructs soil structure and consequently it fulminates the sustainable agricultures of arable lands.[Citation8]
Chitosan, the second great amount of natural polysaccharide on earth crust, as a compound of chitin deacetylation is a biodegradable and non-toxic material for environment. Chitosan is widely used to produce controlled release materials in various fields, particularly in controlled release fertilizer (CRF) manufacturing.[Citation9,10] Chitosan can be successfully processed by a lot of chemical reactions because of its hydroxyl and amino groups.[Citation11]
The intercalation of kaolinite by urea has been studied in numerous works.[Citation12–16] However, the previous studies have focused only on the intercalation of urea as the guest molecule between the clay nanolayers rather than on the nitrogen release behaviour.
Few works have applied chitosan particles in the agricultural field such as incorporation of NPK fertilizer into the chitosan nanoparticles to make fertilizer consumption more efficient.[Citation17] Furthermore, chitosan-coated NPK compound fertilizers have demonstrated controlled release properties.[Citation18]
In this work, intercalated urea–kaolinite mixture was used as the fertilizer base and chitosan was used as the binder to prepare CRF. Nitrogen release properties of the CRF were then investigated.
Although some efforts have been done to investigate features of nanoclay minerals and chitosan biodegradable polymer in improving slow release properties, these works did not focus on nitrogen release properties.[Citation19,20] The process used for coating the granules was generally complicated and cannot be assumed as a cost-effective process.[Citation18] Additionally, there are no attempts to explore the effect of different chitosan concentration on controlled release properties of nitrogen fertilizers. In this study, urea-intercalated kaolinite, which contains the highest urea concentration (20%) was mixed with different concentrations of chitosan as the binder and then was granulated to prepare nitrogen-based CRF.
Methodology
Materials
In the current study, the kaolinite (Al2 (Si2O5)(OH)4) as a hydrated aluminium silicate was obtained from KAOLIN Company in Malaysia. Urea (CON2H4) was analytical grade from Aldrich. Chitosan was used as the binder and purchased from Segar Alatan Sains (M) Sdn. Bhd. Other reagents for urea release measurement, such as phenylmercuric acetate, thiosemicarbazide, potassium chloride, diacetylmonoxime, sulphuric acid and phosphoric acid were of analytical grade from Sigma–Aldrich. For ammonia volatilization, boric acid, bromocresol green, methyl red and HCl were needed and purchased from Lee Hung Scientific Sdn. Bhd. The most important physicochemical properties of the selected soil used for determination of ammonia volatilization are listed in Table .
Table 1. The selected soil physicochemical properties.
Preparation of nitrogen CRF
The highest degree of intercalation which contains the most urea content (20%) [Citation21]) was used to prepare nitrogen-based controlled release fertilizer granules (NCRFs) and compared with nitrogen release behaviour of conventional urea fertilizer (CUF) and non-intercalated urea fertilizer (NIUF). The chitosan concentration of both samples of intercalated (NCRF) and non-intercalated (NIUF) granules was similar (6%). All samples were prepared in the same size (3.67 ± 0.07 mm).
To study the effect of weight percentage of chitosan on the urea release behaviour of granules, 10 g urea–kaolinite mixture (UK) after 1 h dry grinding was mixed with different percentages of chitosan and granulated to prepare urea-intercalated kaolinite fertilizer (UIKF) with controlled nitrogen release properties. Four different samples of granules (UIKF-chitosan 3%), (UIKF-chitosan 4.5%), (UIKF-chitosan 6%) and (UIKF-chitosan 7.5%) were prepared in the same size (3.67 ± 0.07 mm). The prepared granules were dried at 50 °C overnight.
Determination of urea release using UV–vis spectroscopy
To investigate the controlled release behaviour of prepared granules, 1 g of each sample was put in a beaker with 200 ml water. The beaker was covered with parafilm and kept for 30 days in room temperature (25 °C).[Citation22] Furthermore, all the aforementioned procedures were done in three replications. After 1, 5, 10, 15, 20, 25 and 30 days of incubation,[Citation2,23] the urea content of 0.1 ml of each sample was measured using UV–vis spectroscopy technique. Determination of released urea was carried out according to the diacetylmonoxim (DAM) colorimetric method using UV–vis spectrometer.[Citation24] This method is based on reaction of urea with diacetylmonoxime under acidic conditions provided by reagents and measuring the colour absorbency of the reaction product by UV–vis spectrophotometer at a maximum absorbance of 527 nm. After preparing the calibration curve of absorption versus concentration (ppm), the absorption related to the urea content of 0.10 ml of each sample was measured using UV–vis spectrometer and then the concentration of urea was calculated through an equation provided by calibration curve. The concentration was ultimately reported by the percentage based on the primary concentration of urea in each sample.
Crush strength of granular fertilizer
To study the effect of binder on the crush strength of granules, the crush strength of CUF, non-intercalated urea fertilizer with chitosan 6% (NIUF) and urea-intercalated kaolinite fertilizer with chitosan 6% (UIKF) were investigated. All samples were prepared in the same size (3.67 ± 0.07 mm).
To study the effect of binder concentration on crush strength of granular fertilizer, three different sizes of 3.66 mm (UIKF-S1), 5.49 mm (UIKF-S2) and 6.60 mm (UIKF-S3) were prepared for fertilizers with various concentrations of chitosan. Also, the effect of granule size on breaking force was investigated. The crush strength of the granular fertilizer was determined using the Universal testing machine.
Characterization of selected urea–kaolinite fertilizer with chitosan binder
Urea–kaolinite mixture after 1 h dry grinding with 6% chitosan was characterized through Fourier transform infra-red spectroscopy (FTIR) and scanning electron microscopy (SEM). In current work, FTIR was used to explore the interaction of urea with chitosan and kaolinite. The FTIR technique can be performed with an infrared spectrum of absorption, emission and photoconductivity or Raman scattering of a solid, liquid or gas.[Citation13,19,20] The morphology of kaolinite structure before and after dry grinding was studied by SEM. To investigate the dissolved urea which diffuses out from the barriers, the X-ray diffraction (XRD) pattern of urea–kaolinite with chitosan 6% was investigated before and after 30 days immersion in water.
Ammonia volatilization study
Ammonia volatilization was measured for CUF and UIKF-chitosan 6% using the forced-draft technique for 10 weeks. A closed-dynamic (aerobic) air flow system [Citation25] comprised an air exchange chamber (ground glass Erlenmeyer 500 ml) and trapping flask (ground glass Erlenmeyer 250 ml), both stoppered and fitted with an inlet/outlet facility. The inlet of the chamber was connected to a heavy duty air pump, while the outlet connected by polyethylene tubing to the trapping flask was fitted with a glass distribution rod immersed in trapping solution (Figure ). The chamber was filled with soil equivalent to 300 g of air-dried soil. The soil was wetted to 40% moisture holding capacity and equivalent of 400 μg Ng−1 of urea was added. The water content was kept at field capacity.
In this method, an aliquot of selected soil was put in the air exchange chamber leaving some volume above the soil surface for air exchange to take place. The chamber was weighed every 2 days and distilled water was added to compensate the loss due to evaporation. Air was pumped using air-pump and the flow rate was adjusted to an appropriate rate (2.5–3.5 V/min).
The air was passed through the chamber and through the trapping flask subsequently containing 75 ml of 2% boric acid-mixed indicator (bromocresol green and methyl red) to capture the release of NH3-N. After changing the colour of the indicator from purple to green, it was titrated using a 0.1 N HCl acid solution. The end point is reached when the colour changes from green to purple. The amount of urea lost by volatilization of NH3-N was then determined. Titration with 0.1 N HCl was used to determine the percentage of NH3-N volatilized.
The effect of soil pH was monitored daily for 10 days after application of CUF and urea-intercalated kaolinite with chitosan 6% to the soil by adding 0.40 g NKg−1 soil.
Statistical method
Using the SPSS software, descriptive statistics (mean and standard deviation (SD)) of all treatment in experiments was calculated. As the measurement was done for three replicates for each sample, the SD values show the existence of variation. A low SD explained the tendency of the data to the mean, whereas high SD illustrates the data tendency to spread out far from the mean.[Citation26]
Results and discussion
Effect of mixing urea and kaolinite on release behaviour of fertilizer granules
The mean comparison between nitrogen release characteristics of conventional urea granules, non-intercalated urea fertilizer and urea-intercalated kaolinite with chitosan 6% was done to study the effect of 1 h dry grinding on slow release properties of CRF (Figure ).
Figure 2. The urea release behaviour of CUF, NIUF and UIKF-chitosan 6% granules (error bars represented the SD of three replications).
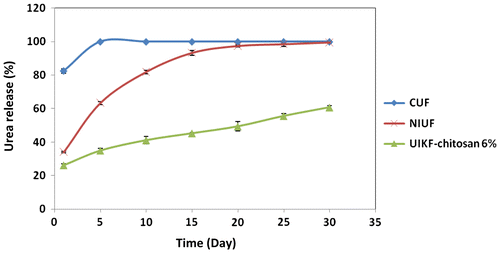
The results indicated that 80% and all of urea of CUF released into the water during the first and second days of incubation, respectively. Non-intercalated urea fertilizer granules released around 34 and 99% urea after 1 day and 30 days, respectively, which is the similar to the previous report.[Citation21] These results showed that slow release properties of non-intercalated kaolinite sample did not confirm to the standard of slow release fertilizers of Comité Européen de Normalisation (CEN). According to CEN, a fertilizer can be described as having controlled release properties if the nutrient release is not more than 15% after 1 day or not more than 75% after 28 days.[Citation22] Mixture of urea–kaolinite after 1 h dry grinding with chitosan binder released around 26.28 and 60.82% urea after 1 day and 30 days, respectively, which follows the CEN standard. The results were in line with He et al. [Citation27], Lan and Mingzhu [Citation23] and Boli and Mingzhu [Citation2]’s findings who reported the results of urea release according to this standard.
Effect of chitosan concentration on release behaviour of fertilizer granules
The result of the urea release from granules with different concentrations of chitosan is shown in Figure .
Figure 3. Urea release behaviour of granules with different binder concentrations (error bars represent the SD of three replicates).
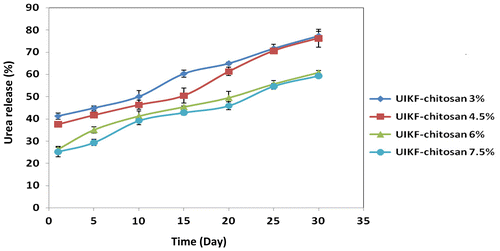
According to the results, the increasing of the chitosan concentration from 3 to 7.5% caused decrease in the urea release from granules. The percentage of release from fertilizers with chitosan 3, 4.5, 6 and 7.5% after 24 h was 41.23, 37.62, 26.28 and 25.25%, and after 30 days were 77.31, 76.28, 60.82 and 59.27%, respectively. From the results, it is clear that just fertilizer with chitosan 6 and 7.5% follows the standard of CRF. The effect of chitosan doses on plant growth was investigated by El-Tanahy et al. [Citation28]. They observed a positive relationship between increasing the applied concentration of chitosan and the response of all plant growth and yield parameters.
Crush strength of granules
Crush strength of three different fertilizers
Table shows the crush strength of CUF, non-intercalated urea–kaolinite fertilizer with chitosan 6% and urea-intercalated kaolinite fertilizer with chitosan 6%. The diameter of the three fertilizers is 3.673 ± 0.07 mm. The fertilizers with chitosan binder compared to the conventional fertilizer are more robust. The same measurement was done by Mahdavi et al. [Citation21]. They investigated the crush strength of conventional fertilizer, urea-intercalated kaolinite and non-intercalated urea fertilizer with the same percentages of HPMC as a binder.
Table 2. Crush strength of three different fertilizers.
Effect of chitosan concentration on crush strength of granules
Figure illustrates the effect of various amounts of binder concentration on the force required to break fertilizer granules. The granule sizes were varied as 3.66, 5.49 and 6.60 mm.
Figure 4. Effect of different amounts of chitosan on force required to crush for three different sizes of granules (error bars represent the SD of three replicates).
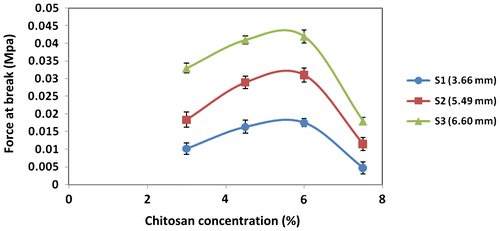
It can be seen that for all granule sizes, the optimum binder concentration was roughly 6%. Increasing the binder concentration further led to a significant reduction in the force at break. This is most probably due to the granules becoming more brittle if the chitosan concentration is too high.
Effect of granule size on crush strength of granules
Figure shows the effect of granule size on force required to break fertilizer granules with different binder concentrations.
Figure 5. The effect of granule size of force required to break fertilizer granules with various binder concentrations (error bars represent the SD of three replicates).
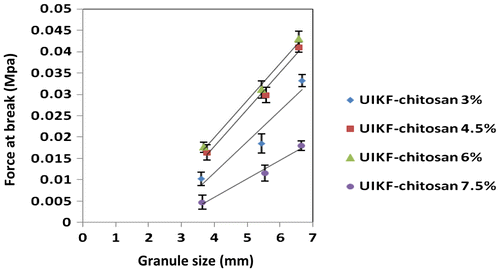
The results indicated that there is a direct relationship between granule size and compressive stress. As it can be seen in Figure , by increasing the granule size, the force required to crush it increases until a fragment occurs. The same phenomenon was observed by Walker et al. [Citation29].
Characterization of urea–kaolinite mixing with chitosan
Chemical interaction of urea, kaolinite and chitosan
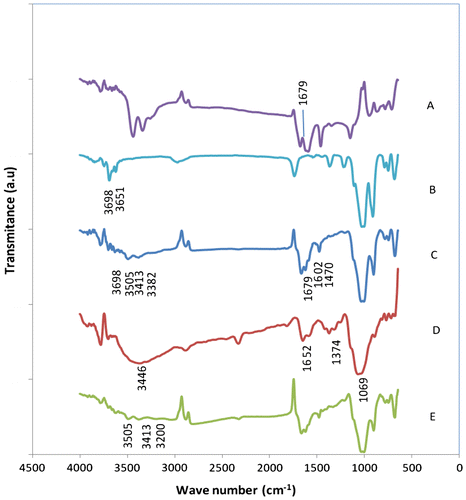
Urea–kaolinite mixture with chitosan binder was characterized using FTIR to determine the interaction of urea with kaolinite and chitosan. Figure shows the FTIR spectra for urea, kaolinite, urea-intercalated kaolinite using dry grinding chitosan and urea-intercalated kaolinite with chitosan binder. The pure urea (Figure (A)) has a characteristic absorption at 1679 cm−1 (C=O).[Citation12,19,20] The spectra for the original kaolinite (Figure (B)) shows characteristic O–H stretching bands at 3698 and 3651 cm−1 attributed to the inner surface hydroxyl groups which is reported in the literatures.[Citation21,30] After intercalation (Figure (C)), the peak at 3651 cm−1 completely disappeared and a new peak appears at 3505 cm−1 which is attributed to the formation of hydrogen bond between –NH2 groups and the oxygen of the tetrahedral sheets. The bands at 3413 and 3382 cm−1 confirm the asymmetric and symmetric –NH2 stretching frequencies involved in weak H-holding with the inner surface hydroxyls.[Citation14,31,32] The absorption bands at 1679 and 1602 cm−1 resulted from contributions of both –CO stretching and –NH2 bending motions. Also, the band which appeared at 1470 cm−1 is attributed to –CN stretching.[Citation33] A broadband around 3449 cm−1 in Figure (D) was assigned as the stretching vibration of the inter and intramolecular hydrogen bonds from the –NH2 and –OH groups of the chitosan molecules,[Citation34,35] 1652 cm−1 (amide) and 1069 cm−1 (C–O–C stretching vibration).[Citation36] Figure (E) exhibits the peak at 3200 cm−1 for –NH stretching vibration. It can be deduced that the formation of some chemical bonds makes the granules more stronger.
Morphology of urea, kaolinite and mixture of urea–kaolinite after 1 h dry grinding
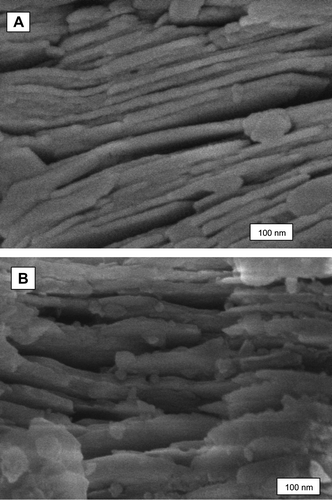
According to CEN, nitrogen release behaviour of granules with urea–kaolinite mixture after 1 h dry grinding could be described as CRF. Figure illustrates the SEM images of pure kaolinite and mixture of urea–kaolinite which was obtained by dry grinding of urea and kaolinite (U/K = 1/4) for 1 h at room temperature. The elementary nanolayers of kaolinite can be seen in these two images. Using SEM, it is difficult to determine the actual interlayer spacing of kaolinite before and after intercalation. In order to determine the d-spacing, high-resolution transmission electron microscopy would be needed.
Figure shows the SEM images of (A) urea, (B) kaolinite and (C) urea–kaolinite after 1 h dry grinding in different magnifications from Figure . It can be seen that dry grinding caused reduction in size of the kaolinite particles which has been mentioned in previous studies.[Citation12,37]
Mechanism of urea release from nitrogen CRF
The proposed mechanism of releasing the urea from NCRF can be described by the following steps: First, the water penetrates inside of the granules through tiny porosities.[Citation2] The weight of granules with chitosan 6% before (0.41 g) and after immersion (0.46 g) in water indicates the penetration of water. The penetrated water enters into the mixture of urea–kaolinite mixing with binder and dissolves the soluble urea molecules which are bonded to nanolayer surfaces and chitosan through hydrogen bonds. The nanolayers of kaolinite and also chitosan are the barriers against penetrating water.
In the second step, the dissolved urea diffuses out from the barriers. To investigate this phenomenon, the XRD pattern of urea–kaolinite with chitosan as a binder is shown before and after immersion in water for 30 days (Figure ).
Figure 9. XRD patterns of UIKF-chitosan 6% sample before immersion and after 30 days immersion in water.
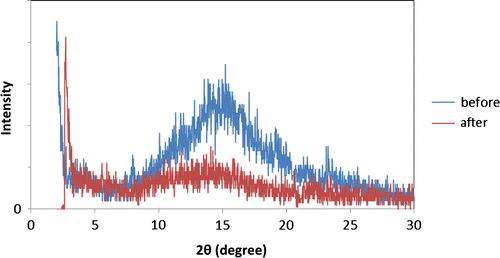
Comparing the two curves, it can be illustrated that the peak at 2θ = 15° was completely removed after 30 days immersion in water. Removal of this peak is due to the urea moving out of the barriers. It releases out into the free water through the porosities.[Citation2,23]
Volatilization of NH3-N
The cumulative NH3-N loss with time from conventional urea and urea-intercalated kaolinite with chitosan 6% fertilizers was curvilinear (Figure ).
Figure 10. Cumulative ammonia volatilization from CUF and UIKF with chitosan 6% (error bars represent the SD of three replicates).
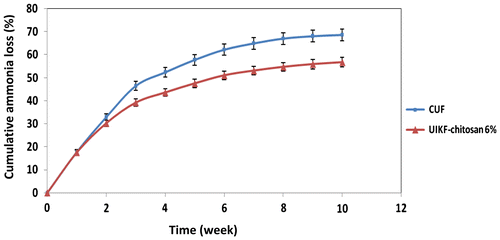
Ammonia volatilization was investigated during 10 weeks after fertilizing the soil with conventional urea and urea-intercalated kaolinite fertilizer. Compared with CUF, the ammonia volatilization decreased for urea-intercalated kaolinite–chitosan 6%. The total amount of NH3-N loss for CUF and UIKF-chitosan 6% was 68.63 and 56.75%, respectively. It is evident that the efficacy of fertilizer with chitosan 6% is higher than that of common urea. Similar results were obtained by Lin et al. [Citation38] and Zhaohui et al. [Citation39].
Changing in pH
Soil pH after fertilizer application was monitored daily for 10 days. Figure shows changes in soil pH after CUF and UIKF-chitosan 6% application to soil by adding 0.40 g NKg−1 soil.
Figure 11. Changes in soil pH after CUF and UIKF-chitosan 6% applies to soil by adding 0.40 g NKg−1 soil (error bars represent the SD of three replicates).
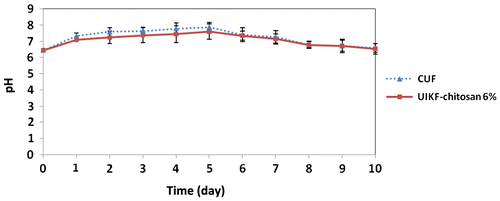
The highest soil pH can be seen at day 5 after application of both fertilizers. By comparison, the pH values were lower in UIKF-chitosan 6% and this process favoured reduction of ammonia volatilization. This indicates that the hydrolysis rate of the CRF was lower than the conventional urea, showing that this fertilizer can increase its urea efficacy. The same phenomenon was also observed in laboratory incubation experiment.[Citation40]
Conclusion
This work revolves around the use of chitosan as a binder to prepare urea–kaolinite CRF granules. The concentration of chitosan was varied from 3 to 7.5% and it was found that chitosan concentration of 6% and above was able to reduce urea release up to CRF standards. Crush tests revealed that roughly 6% chitosan was the optimum concentration to yield granules which had the highest resistance. The effect of granule size was also investigated. There is a direct relationship between granule size and compressive stress. Ammonia volitilization tests revealed that the CRF was able to reduce nitrogen losses compared to conventional urea granules by roughly 12%. This work demonstrates the feasibility of using chitosan as the binder to prepare urea–kaolinite CRF granules.
Intercalation of other essential elements, including P and K, could be studied in future works. In current work, 20% wt urea was loaded on the kaolinite surface which contains 10% N. Other materials could be evaluated to load more urea. By doing this, the quantity of applied CRF will be decreased in each application.
Notes on Contributors
Bita Roshanravan holds a MSc degree in Environmental engineering from the University Putra Malaysia 43400, Serdang, Selangor. She has published two research papers and has attended over five local and international conferences. Her research interest is environmental pollution and monitoring.
Shahram Mahmoud Soltani received his master’s degree in Soil Sciences at Shiraz University, Iran. He currently works as an academic staff in the Department of Soil and Plant Nutrition, Rice Research Institute of Iran. He has published two international and eight national congress papers, as well as four international and four national journal papers.
Suraya Abdul Rashid is an associate professor at the Department of Chemical and Environmental Engineering, Universiti Putra Malaysia. She is currently the Head of the Materials Processing and Technology Laboratory, Institute of Advanced Technology, University Putra Malaysia. Her main research interest is in the area of nanomaterials synthesis and applications, particularly green nanotechnology. She has published more than 40 publications in ISI journals.
Fariba Mahdavi received her bachelor’s degree in Chemical Engineering from University of Kashan, Iran, and her master’s degree in Nanotechnology and Nanomaterials from University Putra Malaysia. She is currently a PhD student in Deakin University of Australia working on organic coating disbondment properties. She has six published journal papers and several conference papers in different areas related to nanotechnology and coatings.
Mohd Khanif Yusop is a professor at the Department of Land Management, Faculty of Agriculture, University Putra Malaysia. He has supervised more than 20 PhD and Master’s students in the field of Soil Sciences. He is actively involved in research on nitrate pollution, improving efficiency of urea fertilizer, increasing productivity of rice, kenaf in BRIS soil and micronutrient studies. He has published more than 130 publications in international and local journals.
References
- Behera SK, Panda RK. Integrated management of irrigation water and fertilizers for wheat crop using field experiments and simulation modeling. Agric. Water Manage. 2009;96:1532–1540.10.1016/j.agwat.2009.06.016
- Boli N, Mingzhu L. Multifunctional slow release urea fertilizer from ethylcellulose and superabsorbent coated formulations. Chem. Eng. J. 2009;155:892–898.
- Kottegoda N, Munaweera I, Madusanka N, Karunaratne V. A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr. Sci. (00113891) 2011;101:73–78.
- Kottegoda N, Munaweera I, Madusanka AN, Karunaratne V. Compositions for sustained release of agricultural macronutrients and process thereof. In Google Patents US20110296885 A1; 2013.
- Hussain MR, Devi RR, Maji TK. Controlled release of urea from chitosan microspheres prepared by emulsification and cross-linking method. Iran. Polym. J. 2012;21:473–479.10.1007/s13726-012-0051-0
- Jarosiewicz A, Tomaszewska M. Controlled-release NPK fertilizer encapsulated by polymeric membranes. J. Agric. Food Chem. 2003;51:413–417.10.1021/jf020800o
- Ghormade V, Deshpande MV, Paknikar KM. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotech. Adv. 2011;29:792–803.10.1016/j.biotechadv.2011.06.007
- Shoji S, Kanno H. Use of polyolefin-coated fertilizers for increasing fertilizer efficiency and reducing nitrate leaching and nitrous oxide emissions. Fertilizer Res. 1994;39:147–152.10.1007/BF00750913
- Muzzarelli RA, Morganti P, Morganti G, Palombo P, Palombo M, Biagini G, Belmonte MM, Giantomassi F, Orlandi F, Muzzarelli C. Chitin nanofibrils/chitosan glycolate composites as wound medicaments. Carbohydr. Polym. 2007;70:274–284.10.1016/j.carbpol.2007.04.008
- Wu L, Liu M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008;72:240–247.10.1016/j.carbpol.2007.08.020
- Osifo PO, Masala A. Characterization of direct methanol fuel cell (DMFC) applications with H2SO4 modified chitosan membrane. J. Power Source. 2010;195:4915–4922.10.1016/j.jpowsour.2009.12.093
- Frost RL, Kristof J, Rintoul L, Kloprogge JT. Raman spectroscopy of urea and urea-intercalated kaolinites at 77 K. Spectrochim. Acta A. 2000;56:1681–1691.10.1016/S1386-1425(00)00223-7
- Letaief S, Elbokl TA, Detellier C. Reactivity of ionic liquids with kaolinite: Melt intersalation of ethyl pyridinium chloride in an urea–kaolinite pre-intercalate. J. Colloid Interface Sci. 2006;302:254–258.10.1016/j.jcis.2006.06.008
- Orzechowski K, Słonka T, Głowinski J. Dielectric properties of intercalated kaolinite. J. Phys. Chem. Solids. 2006;67:915–919.10.1016/j.jpcs.2006.03.001
- Valášková M, Rieder M, Matějka V, Čapková P, Slíva A. Exfoliation/delamination of kaolinite by low-temperature washing of kaolinite–urea intercalates. Appl. Clay Sci. 2007;35:108–118.
- Rutkai G, Mako E, Kristof T. Simulation and experimental study of intercalation of urea in kaolinite. J. Colloid. Interf. Sci. 2009;334:56–69.
- Corradini E, Moura MRD, Mattoso LHC. A preliminary study of the incorporation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010;4:509–515.10.3144/expresspolymlett.2010.64
- Wu L, Liu MZ, Liang R. Preparation and properties of a double coated slow release NPK compound fertilizer with superabsorbent and water retention. Bioresour. Technol. 2008;99:547–554.10.1016/j.biortech.2006.12.027
- Tsunematsu K, Ttateyama H. Delamination of urea–kaolinite complex by using intercalation procedures. J. Am. Ceram. Soc. 1999;82:1589–1591.
- Valaskova M, Rieder M, Matejka V, Capkova P, Sliva A. Exfoliation/delamination of kaolinite by low-temperature washing of kaolinite–urea intercalates. Appl. Clay Sci. 2007;35:108–118.10.1016/j.clay.2006.07.001
- Mahdavi F, Suraya AR, Yusop MK. Intercalation of urea into kaolinite for preparation of controlled release fertilizer. Chem. Ind. Chem. Eng. 2014;20:207–213.10.2298/CICEQ121004001M
- Trenkel ME. Controlled release and stabilized fertilizers in agriculture. Paris: International fertilizer industry association; 2010.
- Lan W, Mingzhu L. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008;72:240–247.
- Douglas JJ, Bremner JM. Colorimetric determination of microgram quantities of urea. Anal. Lett. 1970;3:79–87.10.1080/00032717008067782
- Fenn LB, Kissel DE. Ammonia volatilization from surface applications of ammonium compounds on calcareous soils: I. General theory. Soil Sci. Soc. Am. J. 1973;37:855–859.10.2136/sssaj1973.03615995003700060020x
- Standard deviation. In Wikipedia. 2012. Available from http://en.wikipedia.org/wiki/Standard_deviation
- He X, Liao Z, Huang P, Duan J, Ge R, Li H, Geng Z. Characteristics and performance of novel water-absorbent slow release nitrogen fertilizers. Agric. Sci. China. 2007;6:338–346.10.1016/S1671-2927(07)60054-6
- El-Tanahy AMM, Mahmoud AR, Abde-Mouty MM, Ali AH. Effect of chitosan doses and nitrogen sources on the growth, yield and seed quality of cowpea. Aust. J. Basic Appl. Sci. 2012;4:115–121.
- Walker GM, Magee TRA, Holland CR, Ahmad MN, Fox N, Moffatt NA. Compression testing of granular NPK fertilizers. Nutr. Cycl. Agroecosyst. 1997;48:231–234.10.1023/A:1009714306464
- Gardolinski JE, Carrera LCM, Cantão MP, Wypych F. Layered polymer-kaolinite nanocomposites. J. Mater. Sci. 2000;35:3113–3119.10.1023/A:1004820003253
- Ledoux RL, White JL. Infrared studies of hydrogen bonding interaction between kaolinite surfaces and intercalated potassium acetate, hydrazine, formamide, and urea. J. Colloid Interface Sci. 1966;21:127–152.10.1016/0095-8522(66)90029-8
- Xiaoyan Z, Chunjie Y, Jieyu C. Application of urea-intercalated kaolinite for paper coating. Appl. Clay Sci. 2012;55:114–119.
- Valášková M, Barabaszová K, Hundáková M, Ritz M, Plevová E. Effects of brief milling and acid treatment on two ordered and disordered kaolinite structures. Appl. Clay Sci. 2011;54:70–76.
- Van de Velde K, Kiekens P. Structure analysis and degree of substitution of chitin, chitosan and dibutyrylchitin by FT-IR spectroscopy and solid state C NMR. Carbohydr. Polym. 2004;58:409–416.10.1016/j.carbpol.2004.08.004
- Zhao Z, Zheng J, Wang M, Zhang H, Han C. High performance ultrafiltration membrane based on modified chitosan coating and electrospun nanofibrous PVDF scaffolds. J. Membr. Sci. 2012;394:209–217.10.1016/j.memsci.2011.12.043
- Dorniani D, Hossein MZB, Kura AU. Preparation and characterization of 6-mercaptopurine-coated magnetite nanoparticles as a drug delivery system. Drug Design, Develop. Therapy. 2013;7:1015–1026.10.2147/DDDT
- Sanchez-Soto PJ, Haro MC, Maqueda LA, Varona I, Rodriguez JL. Effect of dry grinding on the structural changes of kaolinite powders. J. Am. Ceram. Soc. 1999;83:1649–1657.
- Lin DX, Fan XH, Feng HU, Zhao HT, Luo JF. Ammonia volatilization and nitrogen utilization efficiency in response to urea application in rice fields of the Taihu lake region, China. Soil Sci. Soc. China. 2007;17:639–645.
- Zhaohui J, Qingru Z, Boqing T, Bohan L, Hejie P, Xiaoyou F, Yulin S. Ammonia volatilization and availability of Cu, Zn induced by application of urea with and without coating in soils. J. Environ. Sci. 2012;24:177–181.
- Sun KJ, Mao XY, Lu QM, Jia AP, Liao ZW. Mitigation effect of several controlled-release N fertilizers on ammonia volatilization and related affecting factors. Chinese J. Appl. Ecol. 2004;15:2347–2350.