Abstract
Biochars converted from agricultural residuals can effectively remove ammonium from water. This work further improved the sorption ability of biochars to aqueous ammonium through alkali modification. Three modified biochars were prepared from agricultural residuals pre-treated with NaOH solution through low-temperature (300 °C) slow pyrolysis. The modified biochars effectively removed ammonium ions from water under various conditions with relatively fast adsorption kinetics (reached equilibrium within 10 h) and extremely high adsorption capacity (>200 mg/g). The Langmuir maximum capacity of the three modified biochars were between 313.9 and 518.9 mg/g, higher than many other ammonium adsorbents. Although the sorption of ammonium onto the modified biochar was affected by pH and temperature, it was high under all of the tested conditions. Findings from this work indicated that alkali-modified biochars can be used as an alternative adsorbent for the removal of ammonium from wastewater.
1. Introduction
Environmental application of biochar is a hot research topic that has attracted much attention in the science communities.[Citation1,2] As a low-cost adsorbent, biochars made from different types of feedstock show strong sorption ability to a variety of pollutants in aqueous solutions.[Citation3,4] Several engineering methods have been developed to modified biochars to further improve their sorptive abilities and promote the applications of biochar technology in environmental remediation.[Citation5–9] Recent studies have shown that surface modification of biochars such as alkali treatment or acid oxidation can greatly enhance their removal of cationic contaminants, particularly heavy metals, from aqueous solutions.[Citation10–12]
While most of the previous studies on biochar-based sorbents mainly focus on heavy metal and organic contaminants,[Citation3,4,13] relatively less research attention has been paid to the removal of aqueous nutrients.[Citation14–16] Recently, Gao et al. [Citation17] found that biochars derived from agricultural residuals can effectively removal ammonium, a nutrient compound, from aqueous solutions. As a follow-up, this work was designed to examine the adsorption of ammonium onto modified biochars. Because biochar surfaces are often negatively charged,[Citation18,19] it can adsorption cations such as ammonium through electrostatic interactions.[Citation17,20] Thus it is anticipated that alkali treatment of the biochar would increase the negative charges on its surfaces to enhance the removal of ammonium. The specific objectives of this work were to: (1) develop surface modified biochars with alkali treatment; (2) measure the sorption kinetics and isotherms of ammonium onto the modified biochars; and (3) determine the effects of temperature, pH, and ionic strength on ammonium sorption.
2. Materials and methods
2.1. Biochar modification
Three types of modified biochars were produced from peanut shells (PS), corncobs (CC), and cotton stalks (CS). The feedstock materials were grounded and then treated with 1.0 M NaOH solution at a solid-liquid ratio of 1.0 g to 2.5 mL for 2 h. This NaOH concentration was selected through a pre-experiment that showed it produced the modified biochar with higher removal of ammonium from aqueous solutions than other two NaOH concentrations (Figure S1, Supporting Information). Each of the alkali-treated feedstock materials was oven dried and then converted into the modified biochar through slow pyrolysis at 300 °C following the procedures of Gao et al. [Citation17]. The resulting biochar samples were ground and sieved into a size range of 0.9–1.2 mm, washed with DI water, and then oven dried. The six unmodified biochar and modified biochar samples were labeled as CS, PS, CC, mCS, mPS, and mCC based on their feedstock types.
2.2. Physical and chemical properties of biochar
In order to analyze the type of the functional groups, the six biochars (CS, PS, CC, mCS, mPS, and mCC) in this experiment were measured by Nicolet 6700 Fourier transform-infrared spectrometer manufacture.
The specific surface area and pore size distribution of the six biochars (CS, PS, CC, mCS, mPS, and mCC) were determined from N2 adsorption isotherms at 77 K with United States Michael instruments, TriStar II automatic specific surface and pore size distribution analyzer.
2.3. Batch sorption experiments
Adsorption kinetics of ammonium onto the modified biochars were determined by adding about 0.1 g of each sorbent into 50 mL ammonium nitrate solutions (50 ppm) in centrifuge tubes. The mixtures were then shaken in the mechanical shaker at 120 r/min at 25 °C. The tubes were withdrawn from the shaker for analyzing ammonium concentration at different time intervals (i.e. 0.5, 1, 2, 5, 10, 20, and 24 h). Each sample was immediately filtered through 0.22 μm membrane filters to determine the ammonium concentrations in the supernatant using the phenate method through a UV/Vis spectrophotometer.[Citation14] Sorbed ammonium concentrations on the biochar were calculated based on difference between the initial and final aqueous concentrations. All the sorption experiments were performed in triplicate and the average values were reported.
Similar procedures were used to determine the adsorption isotherms of ammonium onto the modified biochar. Instead of different time intervals, the sorbents (0.1 g) were mixed with 50 mL ammonium solutions of different initial concentrations (i.e. 10, 25, 50, 100, 200, 300, and 500 mg/L). The mixtures were then shaken in the mechanical shaker (120 r/min) at 25 °C for 24 h. After that, the same method was used to determine the ammonium concentrations in the supernatants and on the sorbents. Various mathematical models including Pseudo-first-order, pseudo-second-order, and Elovich models for adsorption kinetics and Langmuir and Freundlich models for adsorption isotherms were used to describe the experimental data.[Citation15] Details about the models can be found in the supporting information (S1).
Based on the isotherms, the modified biochar with the highest sorption of ammonium was selected to test the effects of pH and temperature. For each condition, the biochar (0.1 g) was mixed with 50 mL ammonium solutions (50 ppm) in the centrifuge tube and the mixture was shaken in the mechanical shaker (120 r/min) for 24 h. The experimental conditions were similar to that of Gao et al. [Citation17].
3. Results and discussion
3.1. Physical and chemical properties of biochar
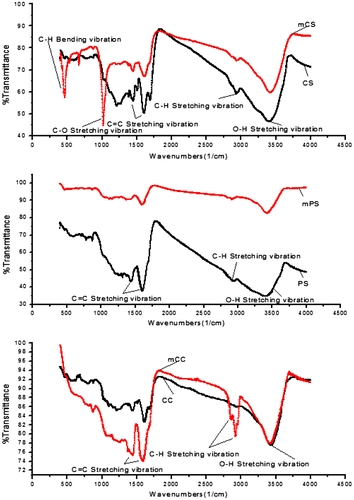
Because of the different modes of vibration between the functional group atom (stretching, rotation, symmetric, and asymmetric), oxygen-containing functional group of a surface of the biochar have different peak positions in the infrared spectrum, which will cause the difference in infrared absorption wavelength. The FT-IR spectra of the six biochars are illustrated in Figure . Different spectra reflected changes in the surface functional groups of six biochars. Compared with CS, there were some differences in the FT-IR spectrum of mCS, which appeared new functional groups of C-O Stretching vibration and C-H Bending vibration. The maximum adsorption capacities increased rapidly from 202.5 mg/g for CS to 518.9 mg/g for mCS. As seen from the FT-IR spectra in PS and mPS, the surface functional groups changed little. The maximum adsorption capacities slowly increased from 243.3 mg/g for PS to 313.9 mg/g for mPS, the increase of adsorption capacity was mainly due to the increase of surface area and total pore volume after modification. Compared with CC, mCC increased functional groups of C–H Stretching vibration. The maximum adsorption capacities increased acutely from 217.4 mg/g for CC to 373.1 mg/g for mCC. This showed that the number of surface functional groups were increased after modification, therefore enhancing the absorbance.[Citation21]
Comparison of unmodified biochars and modified biochars (Table ) showed that surface area and total pore volume after modification were increased. This indicated that raw materials modified by NaOH solution can be further increased surface area and total pore volume in a certain extent, thereby also enhancing the biochars’ adsorption effect of ammonia nitrogen.
Table 1. The specific surface area and void structures of different biochars.
3.2. Adsorption kinetics and isotherms
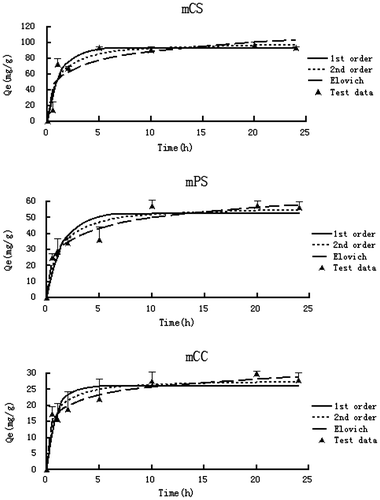
In the previous work, the adsorption kinetics curves of CS and CC were better fitted to the Elovich model, while the adsorption kinetics curve of PS was better fitted to the first-order model. The Langmuir maximum adsorption capacities of the three biochars were 202.5, 243.3, and 217.4 mg/g for CS, PS, and CC, respectively.[Citation17]
Similar to that of the unmodified biochars, the three modified biochars also sorbed the ammonium in aqueous solutions in relatively fast ways (Figure ). It only took 5–10 h for the adsorption kinetic curves to reach the equilibrium, which was slightly faster than that of the unmodified biochars. This result suggested that alkali modification can improve the sorptive properties of the biochar, which is consistent with findings from previous studies.[Citation11,22] The three kinetic models were used to simulate the experimental data and fittings were reasonably well with R2 values larger than 0.85 (Table ). According to the fitting results, the adsorption kinetics curves of mCC and mPS were better fitted to the Elovich model, while the adsorption kinetics curve of mCS was better fitted to the first-order model.
Table 2. Best model parameters of ammonia sorption onto alkali-modified biochars.
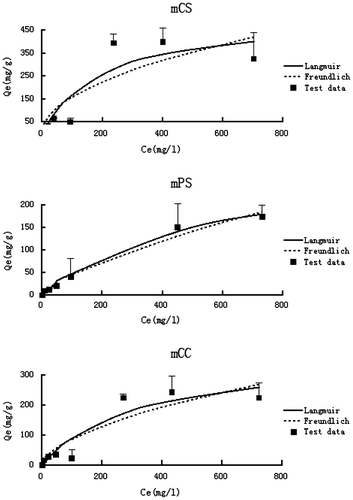
The adsorption isotherms demonstrated that the three alkali-modified biochars had large sorption capacities to aqueous ammonium (Figure ), suggesting they can be used as alternative adsorbents for the treatment of ammonium contamination. While all the modified biochars showed higher than 300 mg/g sorption of ammonium, the mCS was the best with the sorption capacity around 500 mg/g (Figure ), much higher than that of the unmodified biochars.[Citation17] The ammonium sorption ability of the three alkali-modified biochars were comparable to that of many other sorbents as reported in the literature,[Citation23–25] further suggesting that they can be used as alternative adsorbent for the removal of aqueous ammonium. Both the Langmuir and the Freundlich models simulated the experimental isotherms well with good R2 values (Table ). In general, simulations of the Langmuir model were better than that of the Freundlich models, which is different from the modeling results of the unmodified biochars in Gao et al. [Citation17].This indicated that alkali modification alter the surfaces of the modified biochars and might made them more uniform than that of the unmodified biochars. The Langmuir maximum adsorption capacities of the three modified biochars were 518.9, 313.9, and 373.1 mg/g for mCS, mPS, and mCC, respectively, indicating the modified biochars were effective adsorbents for ammonium in aqueous solution.
3.3. Effects of pH and temperature
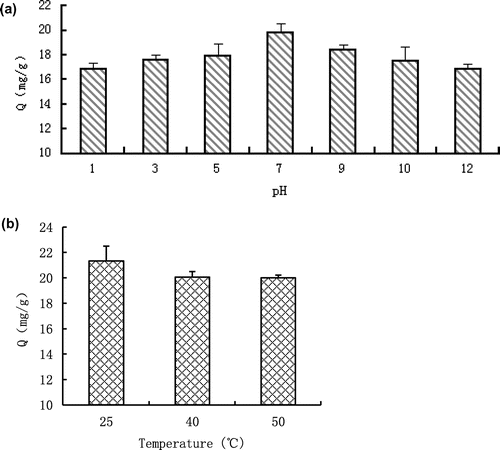
Because the sorption of ammonium onto the biochar (mCS) surface was mainly controlled by the electrostatic interactions, the removal rate was pH dependent (Figure (a)). The optimum pH for adsorption was found to be at 7, which was a little lower than that of the unmodified biochar.[Citation17] This is probably because the alkali treatment altered the surface chemistry and charge potential of the biochar. At pH > 7, the adsorption took a decreasing trend. The decrease in adsorption at pH > 7 could be attributed to the fact that most of the ammonium is converted to NH3·H2O which cannot be adsorbed onto the adsorbent.[Citation26] On the other hand, the decreased ammonium removal at lower pH could be due to the competition between ammonium ions and hydrogen ions. The negatively charged functional groups on the biochar surfaces adsorbed more hydrogen ions and repelled the polar attraction of ammonium ions in aqueous solution.[Citation27] Similar to that of the unmodified biochar,[Citation17] increase in temperature also reduce the removal of ammonium by the modified biochar (Figure (b)), indicating both adsorption processes were exothermic. The modified biochar showed extremely good adsorption ability to ammonium at high temperature (50 °C) with a sorption rate higher than 20 mg/g, indicating it can be used in high temperature environment for ammonium removal.
4. Conclusions
Alkali-modified biochars were produced from NaOH treated biomass through low temperature pyrolysis. The modified biochars showed strong sorption ability to ammonium in aqueous solutions with fast adsorption kinetics and extremely high adsorption capacities. The Langmuir maximum sorption capacities of the modified biochars were all higher than 300 mg/g, which is comparable to that of many other high efficiency adsorbents. Findings from this work showed that alkali-modified biochars derived from agricultural residuals can be used as an alternative adsorbent for the treatment of ammonium pollution in water.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/09542299.2016.1142833.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on Contributor
Zhigang Liu is a graduate student in the School of Civil Engineering at the Wuhan University in China. His research mainly focuses on water treatment and waste water treatment.
Yingwen Xue is an associate professor in the School of Civil Engineering at the Wuhan University in China. He earned his PhD degree in Disaster Prevention and Reduction Engineering and Protective Engineering at Wuhan University. Xue has published more than 32 peer-reviewed journal articles. His research mainly focuses on water treatment, biochar, and nanotechnology for water treatment.
Fei Gao is a graduate student in the School of Civil Engineering at the Wuhan University in China. Her research mainly focuses on water treatment and waste water treatment.
Xiaoru Cheng is a professor in the School of Civil Engineering at the Wuhan University in China. He earned his Master degree in Municipal engineering at Wuhan University of Technology. He has published more than 16 peer-reviewed journal articles. His research mainly focuses on water treatment, biochar, and nanotechnology for water treatment.
Kai Yang is a professor in the School of Civil Engineering at the Wuhan University in China. He earned his PhD degree in Hydrology and Water Resources at Wuhan University. Yang has published more than 43 peer-reviewed journal articles. His research mainly focuses on water treatment, water restoration, and water quality.
Funding
This work was partially supported by the National “Twelfth Five-Year” Plan for Science & Technology Pillar Program [grant number 2014BAL04B04], [grant number 2015BAL01B02] and the Wuhan Water Engineering & Technology Co. Ltd.
References
- Sohi SP. Carbon storage with benefits. Science 2012;338:1034–1035.10.1126/science.1225987
- Lehmann J. A handful of carbon. Nature 2007;447:143–144.
- Inyang M, Gao B, Yao Y, et al. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2015:1–28.
- Xie T, Reddy KR, Wang CW, et al. Characteristics and applications of biochar for environmental remediation: a review. Crit. Rev. Environ. Sci. Technol. 2015;45:939–969.10.1080/10643389.2014.924180
- Inyang M, Gao B, Zimmerman A, et al. Synthesis, characterization, and dye sorption ability of carbon nanotube-biochar nanocomposites. Chem. Eng. J. 2014;236:39–46.10.1016/j.cej.2013.09.074
- Zhou YM, Gao B, Zimmerman AR, et al. Biochar-supported zerovalent iron reclaims silver from aqueous solution to form antimicrobial nanocomposite. Chemosphere 2014;117:801–805.10.1016/j.chemosphere.2014.10.057
- Hu X, Ding ZH, Zimmerman AR, et al. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2015;68:206–216.10.1016/j.watres.2014.10.009
- Zhang M, Gao B. Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite. Chem. Eng. J. 2013;226:286–292.10.1016/j.cej.2013.04.077
- Zhang M, Gao B, Yao Y, et al. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012;210:26–32.10.1016/j.cej.2012.08.052
- Wang H, Gao B, Wang S, et al. Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour. Technol. 2015;197:356–362.10.1016/j.biortech.2015.08.132
- Ding Z, Hu X, Wan Y, et al. Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: batch and column tests. J. Ind. Eng. Chem. 2015;33:239–245.
- Xue Y, Gao B, Yao Y, et al. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: batch and column tests. Chem. Eng. J. 2012;200–202:673–680.10.1016/j.cej.2012.06.116
- Mohan D, Sarswat A, Ok YS, et al. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent – a critical review. Bioresour. Technol. 2014;160:191–202.10.1016/j.biortech.2014.01.120
- Yao Y, Gao B, Zhang M, et al. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012;89:1467–1471.10.1016/j.chemosphere.2012.06.002
- Yao Y, Gao B, Inyang M, et al. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard. Mater. 2011;190:501–507.10.1016/j.jhazmat.2011.03.083
- Yao Y, Gao B, Inyang M, et al. Biochar derived from anaerobically digested sugar beet tailings: characterization and phosphate removal potential. Bioresour. Technol. 2011;102:6273–6278.10.1016/j.biortech.2011.03.006
- Gao F, Xue Y, Deng P, et al. Removal of aqueous ammonium by biochars derived from agricultural residuals at different pyrolysis temperatures. Chem. Speciation Bioavailability 2015;27:92–97.
- Yao Y, Gao B, Chen JJ, et al. Engineered carbon (biochar) prepared by direct pyrolysis of Mg-accumulated tomato tissues: characterization and phosphate removal potential. Bioresour. Technol. 2013;138:8–13.10.1016/j.biortech.2013.03.057
- Yao Y, Gao B, Chen JJ, et al. Engineered biochar reclaiming phosphate from aqueous solutions: mechanisms and potential application as a slow-release fertilizer. Environ. Sci. Technol. 2013;47:8700–8708.
- Kizito S, Wu S, Kipkemoi Kirui W, et al. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015;505:102–112.10.1016/j.scitotenv.2014.09.096
- Zheng Y, Yu Q, Wang H, et al. Preparation of biochars from biogas residue and adsorption of ammonia-nitrogen in biogas slurry. CIESC J. 2014;65:1856–1861.
- Yin CY, Aroua MK, Daud WMAW. Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep. Purif. Technol. 2007;52:403–415.10.1016/j.seppur.2006.06.009
- Caichun WEI, Zhang X, Zhang B, et al. Removing ammonia nitrogen from by modified zeolite. J. Guilin Inst. Technol. 2006;26:28–32.
- Rahmani, AR, Mahvi, AH. Use of ion exchange for removal of ammonium: a biological regeneration of zeolite. In: Lekkas TD, editor. Proceeding of the 9th International Conference on Environmental Science and Technology Vol B – Poster Presentations.; 2005. p. B755–B760.
- Liu Q, Tan X, Zhao L. Experimental study on the ammonium ion-exchange material and its removal of ammonia nitrogen from water. In: Shi YG, Zuo JL, editors. Environmental biotechnology and materials engineering, Pts 1–3. Advanced materials research. Stafa: Trans Tech Publications Ltd; 183–185; 2011. p. 1558–1562.
- Huang HM, Xiao XM, Yan B, et al. Ammonium removal from aqueous solutions by using natural Chinese (Chende) zeolite as adsorbent. J. Hazard. Mater. 2010;175:247–252.10.1016/j.jhazmat.2009.09.156
- Novak JM, Busscher WJ, Watts DW, et al. Short-term CO2 mineralization after additions of biochar and switchgrass to a Typic Kandiudult. Geoderma 2010;154:281–288.10.1016/j.geoderma.2009.10.014