Abstract
The consumption of citrus flavonoid, hesperidin may inhibit the bone loss. The purpose of this study was to evaluate the effect of hesperidin on the bioavailability of Ca, a probable reason to prevent bone loss. Citrus flavonoid (hesperidin) in combination with citric acid and ascorbic acid was scrutinized to estimate the bioavailability of micronutrients from chicken egg shells using in vitro method. Effect of citric acid, ascorbic acid and hesperidin on the bioavailability of minerals (Zn, Fe) and macro elements (Ca, Mg, P) was evaluated and the amounts required to get maximum bioavailability were concluded. The highest bioavailability of Ca, Mg, P, Fe and Zn was 89.25 ± 2.13, 92.28 ± 1.87, 40.32 ± 3.09, 32.81 ± 1.24 and 46.19 ± 0.83%, respectively after the addition of 3 g of citric acid, 100 mg of ascorbic acid and 4 mg of hesperidin per gram of chicken eggshell powder. Citric acid greatly affects the bioavailability of Ca, Mg, P, and Zn, whereas addition of ascorbic acid enhances the bioavailability of Fe, and hesperidin boosts the bioavailability (p < 0.05) of all micronutrients of the chicken eggshells.
Introduction
Hesperidin is a bioflavonoid abundantly found in citrus fruits and juices [Citation1] is reported to protect against DNA damage and lipid peroxidation, modify several antioxidant enzymes, reduced body weight in overweight or obese nonsmoking individuals and have antioxidant, anti-inflammatory, anti-viral, anti-carcinogenic activities.[Citation2,3] Deficiency can cause vascular degeneration, vascular bleeding, nose bleeds and periodontal bleeding. Hesperidin alone, or in combination with other citrus bioflavonoids like diosmin is most often used for blood vessel conditions such as hemorrhoids, varicose vein, and venous stasis. It is also used to treat lymphedema.[Citation4] Studies show that hesperidin inhibits bone loss in androgen-deficient male mice,[Citation5] prevent bone loss [Citation1] and bone loss in ovariectomized mice.[Citation6] To evaluate the role of hesperidin in preventing bone loss, the role of hesperidin in bioavailability of Ca was evaluated. Egg shells were chosen as a source of Ca and other minerals and macro-elements.
Egg shells have multidimensional uses in medicine and nutrition [Citation7] as they contain logically active compounds.[Citation8] Eggshells are a good source of various minerals and metals as they contain 98.2% calcium carbonate, 0.9% magnesium, and 0.9% phosphorous.[Citation9] Egg shells are also blessed with total and essential amino acids in the range of 189–353 and 98.1–188 mg/g respectively, [Citation10] 10% collagen [Citation7] and trace elements.[Citation11] Egg shells are enriched with micronutrients as strontium, fluorine, and selenium [Citation10] can be a good supplement of calcium and other minerals. Chicken egg shell powder enriched supplement increases bone mineral density of hip bone in healthy late menopausal women within 12 months.[Citation12]
Egg shells are massive waste from bakeries, hatcheries and fast food restaurants. According to ADAS consulting Ltd. UK approximately 10,000–11,000 tons of egg shells have to be disposed-off each year which is quite problematic, costly and also a cause of environmental pollution. So there is a dire need to recycle this waste.[Citation13] Recent uses of egg shells waste included soil stabilizing agents like lime and bitumen,[Citation14] incineration with waste to energy and land fill etc.[Citation13] Wasted egg shells have a potential in pharmaceutical industry due to their unique composition which is similar to human teeth and bones. Several studies support its utilization as calcium supplement for human [Citation15,16] but the bioavailability of calcium and other minerals and elements have not been well characterized.
The aim of work was to study the role of citrus flavonoid in the bioavailability of calcium and other macro-elements of eggshells and to evaluate the role of hesperidin in inhibition of bone loss.
Materials and methods
Chemicals and reagents
All chemicals were of analytical grade. Hesperidin (Acros Organics, USA), citric acid (E. Merk Darmstadt, Germany), L-ascorbic acid (Wilfrid Smith Ltd. U.K), pepsin (P-7000, from porcine stomach mucosa), bile salt (B-8361, porcine) and pancreatine (P-3292, from porcine pancreas) were purchased from Sigma Aldrich. Regenerated cellulose tubular membrane (flat width: 45 mm, wall thickness: 20 μm, vol./cm: 6.42 mL & dry cylinder diameter: 28.6 mm) were purchased from Cellu Sep Company, U.S.A.
Preparation of reagents and solutions
Pepsin
Sixteen grams of pepsin (P-7000, from porcine stomach mucosa) were suspended in 0.1 M HCl and brought to 100 mL with 0.1 M HCl.
Pancreatin-bile extracts mixture
Four grams pancreatin (P-3292, from porcine pancreas) and 25 g bile extract (B-8361, porcine) was dispersed in 0.1 M NaHCO3 and the mixture was brought to 1 L with 0.1 M NaHCO3.
Hesperidin solution
Hundred miligrams of hesperidin (Acros Organics, USA) was dispersed in small amount of 0.1 M HCl, shake well and volume was made up to 100 mL with 0.1 M HCl.
Sampling
Chicken egg shells were collected randomly during the period of April through June 2013 from local markets of Lahore-Pakistan.
Sample preparation
Washing
Various dipping, washing and boiling treatments were done as indicated in Table and eggshells powder was tested for Staphylococcus aureus (Steph. aureus), Salmonilla sp. and Escherichia coli (E. coli) according to FAO protocol.[Citation17] Salmonilla sp. and E. coli was not detected in the eggshells powder. Steph. aureus was detected and cannot be removed with simple washing and dipping methods, so sample was autoclaved for 1–15 min and again tested. Steph. aureus was not detected in samples which were autoclaved at 0.2 mega paskel and 121 °C for one min or more in an autoclave unit (Hirayama, Japan).
Table 1. Various washing treatments and their effect on the microbial load of chicken eggshells.
Grinding
Chicken eggshells were washed, dried and ground with grinding disk mill (FFC-23 A, China) to turn them into fine powder of 149 micron by passing through 100 mesh.
Preliminary study
During our preliminary study it was found that hesperidin alone did not enhance the bioavailability of Ca and other minerals while when experiments were performed with lemon juice bioavailability increased manifold. So based on the composition of lemon juice, major components of lemon juice like citric acid and ascorbic acid were also included in study along with the major citrus flavonoid.
In vitro bioavailability
Five hundred milligram of egg shell powder was weighed and suspension was prepared by adding 15 ml water. pH of the contents was adjusted to 2.00 ± 0.1 with 6 N HCl. Three milliliter of pepsin solution (10% W/V in 0.1 N HCl) was added to prepare a gastric mixture. Contents were shaken for two hours in an incubator (WIS 20R Dathan Scientific, Korea) at 37 °C. After incubation, samples were removed for measurement of titratable acidity. Aliquots (25 g) of the pepsin digest were taken in triplicate, transferred to 500 ml conical flask individually. Segments of dialysis tubing, containing 25 ml water and an amount of NaHCO3 equivalent to the titratable acidity, were closed at both ends and were placed in conical flasks containing gastric digests. The conical flasks were sealed with parafilm and incubated in a shaking incubator (WIS 20R Dathan Scientific, Korea) at 37 °C until the pH reached about 7.5 (~30 min). Fifteen milliliter of pencreatin-bile extract mixture was added to each conical flask and incubation was continued for another two hours. At the end of the incubation period, the dialysis tubes were removed, rinsed with distilled water, and the contents (dialysates) weighed and analyzed for Ca, Mg, Zn and Fe by atomic absorption spectrophotometer while phosphorus contents were determined spectrophotometrically according to the method of AOAC.[Citation18] Bioavailability was calculated according to the method described by Miller.[Citation19]
Effect of citric acid, L-ascorbic acid and hesperidin
To aliquots of egg shell powder varying amounts of citric acid were added individually and the above mentioned protocol was applied till maximum bioavailability of Ca, Mg, P, Fe and Zn was achieved. When the amount of citric acid (required for maximum bioavailability) was optimized the varying amounts of L-ascorbic acid were added to the aliquots of egg shell along with citric acid and the above protocol was followed and the amount of ascorbic acid required for maximum bioavailability was optimized. Similarly with the optimum amounts of citric acid and ascorbic acid, varying amounts of hesperidin (100 mg/100 mL in 0.1 M HCl) were added to the aliquots of egg shells individually and the test protocol was followed to scrutinize the amount of hesperidin required to achieve maximum bioavailability.
Analysis by flame atomic absorption spectrophotometer
A pye Unicam atomic absorption spectrometer was used for the determination of Mg, Fe, Zn, Co, and Mn. It was equipped with appropriate hollow cathode lamp and air acetylene burner. SOLLAR software was used for data acquiring and processing. Ca, Mg, Fe and Zn hollow cathode lamps were used as radiation source which was operated at 422.7 nm, 285.2 nm, 248.3 nm and 213.9 nm, respectively and the lamp current was set at 6 mA for Ca & Mg, 15 mA for Fe and 10 mA for Zn. The flame atomic absorption spectrophotometer was calibrated with standard stock solutions of Ca (1000 ± 2 mg/L), Mg (1000 ± 2 mg/L), Fe (1001 ± 2 mg/L) and Zn (1001 ± 2 mg/L) purchased from Merck (Darmstadt, Germany). To avoid interferences from phosphorus in Ca determinations, lanthanum chloride (1%) was added to the sample.
Phosphorous determination
Phosphorous was determined by AOAC [Citation18] using ammonium-vanido-molybdate as coloring reagent and absorption of the developed color was measured at 420 nm. Standard curve was drawn using phosphorus standard (1000 ± 2 mg/L) solution (Fisher Scientific, UK) and concentration of phosphorus was determined from the calibration curve.
Statistical analysis
All determinations were carried out in triplicate and mean ± standard deviations were calculated using Microsoft XL 2010. Data was assessed by analysis of variance using SPSS 11.0 SPSS. Significance was considered at probability p < 0.05.
Results
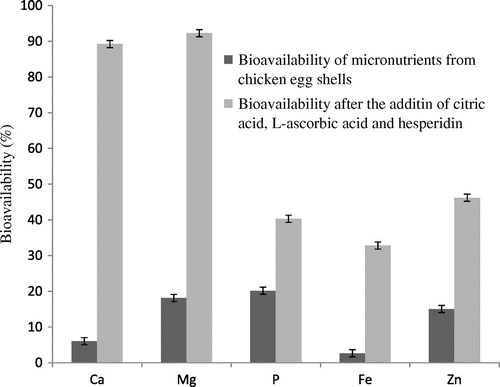
Table indicated the nutritional profile, amount of various minerals and macro elements present in the egg shells and the bioavailability of Ca, Mg, P, Fe and Zn in its natural form present in the egg shells. Bioavailability of Ca, Mg, P, Fe and Zn was 6.05 ± 0.84, 18.13 ± 1.54, 20.17 ± 2.37, 15.04 ± 0.98 and 2.64 ± 0.17% respectively, on the basis of amount of these components present.
Table 2. Composition of egg shells powder proceeded for bioavailability studies
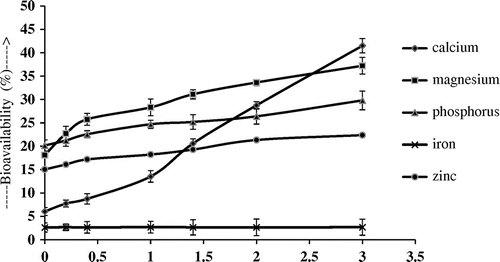
Effect of citric acid on bioavailability
The effect of citric acid on the bioavailability of Ca, Mg, P, Fe and Zn is shown in Figure . After the addition of citric acid the bioavailability of Ca, Mg, P, and Zn increases with the concentration of citric acid, while addition of citric acid had little or no effect on the bioavailability of iron. Bioavailability of Ca which was 6.05 ± 0.84%, before the addition of citric acid, increases to 41.5 ± 1.53% after the addition of 3 g/g of citric acid. There is a linear correlation between the concentrations of citric acid and bioavailable minerals and macro elements except Fe (r2 = 0.98, 0.91, 0.97, 0.16 and 0.96 for Ca, Mg, P, Fe and Zn, respectively.) Further addition of citric acid did not change the bioavailability, however precipitation inside the intestinal digest was observed.
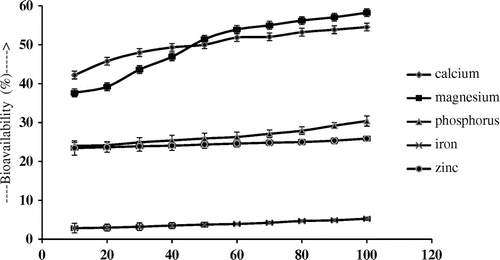
Effect of L-ascorbic acid
As shown in Figure “L-ascorbic acid” also has a positive effect on the bioavailability of Ca, Mg, Zn and Fe. Bioavailability of Ca increases from 42.21 ± 1.13 to 54.55 ± 1.12%, Mg from 37.64 ± 2.17 to 58.24 ± 1.92%, P from 24.00 ± 0.88 to 30.40 ± 1.32%, Fe from 2.85 ± 1.23% to 5.26 ± 0.39 and Zn from 23.45 ± 1.85 to 25.87 ± 0.63% after the addition of 100 mg of L-ascorbic acid.
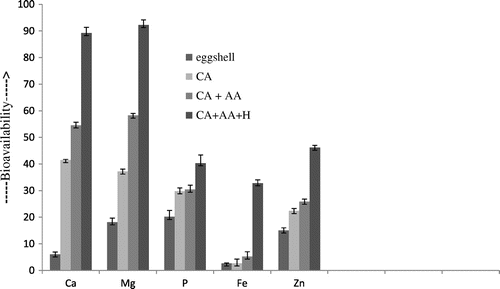
Effect of hesperidin
Figure shows the percent bioavailable Ca, Mg, P, Fe and Zn after the addition of hesperidin to the egg shells. Bioavailability of all mineral and macro elements of the egg shells showed the highest value that is 89.25 ± 2.13, 92.28 ± 1.87, 40.32 ± 3.09, 32.81 ± 1.24 and 46. 19 ± 0.83%, respectively for Ca, Mg, P, Fe and Zn. There is a linear correlation between the concentration of hesperidin and bioavailable minerals and macro elements (r2 = 0.95, 0.94, 0.94, 0.99 and 0.989) till the addition of 4 mg/g of hesperidin above this concentration hesperidin has no effect on the bioavailability of these mineral and macro-elements.
Figure 3. Effect of hesperidin (1–4 mg/g of chicken eggshell powder) on the bioavailability micronutrients of eggshells.
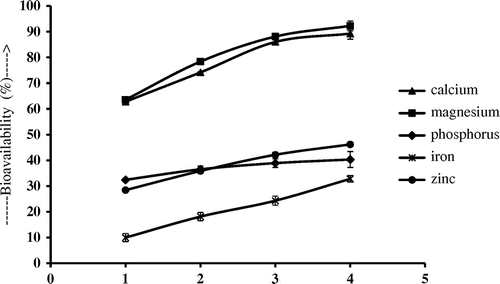
The cumulative effect of the whole study is shown in Figures and . It is evident that bioavailability of minerals and macro elements of egg shells can be increased manifold with the proposed combination. Bioavailability of Ca, Mg, P, Fe and Zn increases from 6.05 ± 0.84 to 89.25 ± 2.13%, 18.13 ± 1.54 to 92.28 ± 1.87%, 20.17 ± 2.37 to 40.32 ± 3.09%, 2.64 ± 0.17 to 32.81 ± 1.24% and 15.04 ± 0.98 to 46.19 ± 0.83%, respectively.
Figure 4. Cummulative effect of 3 g citric acid, 100 mg L-ascorbic acid and 4 mg hesperidin on the bioavailability of Ca, Mg, P, Fe and Zn from 1 g of chicken egg shells.
Discussion
Hesperidin had got much attention in the recent decade in relation to prevent bone loss.[Citation1,5,6] In our ensuing article we tried to comprehend the cause that how hesperidin played role in androgen deficiency-induced bone loss in male mice and overiectimized mice.[Citation5,6] Our study suggested that hesperidin increases the bioavailability of Ca, P and other micronutrients. It was also concluded that hesperidin alone did not played any role in the bioavailability of theses mineral and macro elements but it worked in the presence of citric acid and vitamin C. In our study we had chosen egg shells as source of Ca, P and other micro-nutrients although calcium carbonate give equivalent results.
The in vitro method of Miller [Citation19] was used to assess the bioavailability of Ca, Mg, P, Fe and Zn from egg shells powder. A lower absorption of calcium from egg shell powder had been reported by Chang.[Citation20] Low bioavailability of calcium from calcium carbonate as compared to other salt of calcium like calcium phosphate, calcium format and calcium lactate has also been reported.[Citation21,22] When citric acid was added to the egg shells the bioavailability increase. The reason for this increase in bioavailability was that calcium carbonate present in the egg shell converted to calcium citrate which has higher bioavailability as compared to calcium carbonate as concluded by other studies.[Citation22–24] Similarly the bioavailability of Mg and Zn on the addition of citric acid increases from 18.13 ± 1.54 to 37.21 ± 2.01% and 15.04 ± 0.98 to 22.38 ± 1.70%, respectively. Lindberg [Citation25] concluded that 65% of magnesium citrate complexes are water soluble compounds, while other compounds like magnesium oxide did not, hence more solubility of magnesium citrate caused to increase the bioavailability of magnesium.
It was evaluated that ascorbic acid also played role in the bioavailability of micronutrients. WHO/FAO [Citation26] proposed highest bioavailability of iron from ascorbic acid rich food. Ascorbic acid is a potent antioxidant and enzyme cofactor, it converts ferric form to ferrous and hence increases the bioavailability of Fe,[Citation27] and the other reason is the potential of chelation, when a mineral is compounded with a weak dietary ligand like ascorbate, it protects it from binding with a stronger ligand and hence improves its absorption.[Citation28]
The probable reason for much enhanced bioavailability of minerals/macro elements of chicken egg shells could be that the Ca is present in egg shells as calcium carbonate, Mg as magnesium oxide/carbonate, Zn chloride/oxide is converted to their relative citrate which has more bioavailability as compared to carbonates and oxides.[Citation22–24] When L-ascorbic acid was added, citrates convert to ascorbates which show slightly higher bioavailability. Experimental results showed that carbonate or oxides of these minerals and metals cannot be converted to ascorbate directly, so addition of citric acid is necessary to convert carbonates/oxides to ascorbate. Though the solubility of hesperidin is low in water, but it is finely dispersible in acidic solution like citric acid and 0.1 M HCl. When hesperidin was added in the presence of citric acid it enhanced its solubility and hence increased the absorption of ascorbates. Some previous studies support our findings as bioavailability of ascorbic acid increased 35% when administered with citrus extract.[Citation29] Iron on the other hand may be present in the form of chloride or phosphate did not show the effect of citric acid, whereas when it is converted to ascorbate, hesperidin facilitates the absorption of iron ascorbate and hence increases the bioavailability of iron. Bioavailability enhancing effect of citrus fruit juices on iron,[Citation30] beneficial effect of citrus flavonoids on bone and lipid for inhibition of bone loss [Citation5,6] sustenance the findings that bioavailability of Ca, P and other micronutrients of egg shells significantly (p < 0.05) increased in the presence of hesperidin.
Conclusions
The in vitro method to measure bioavailability of Ca, Mg, P, Fe and Zn from egg shell leads us to conclude that citrus flavonoids have a determining effect on the bioavailability of these elements. Maximum bioavailability of Ca can justified the role of hesperidin to prevent bone lose. On the other hand eggshell a massive waste of various industries can be a good natural source of many mineral and macro elements when administered with citric acid, ascorbic acid and hesperidin. A significant increase in bioavailability was evaluated when egg shells mixed with these organic acids and citrus flavonoid. This product could have a good potential for marketing with the objective of getting health benefit in managing osteoporosis and general deficiency of mineral and macro elements. In vivo studies are also required to validate the use of hesperidin and ascorbic acid as a carrier for mineral and macro elements.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding
This work was supported by the Higher Education Commission, Pakistan [074-2376-PS4-369].
Acknowledgment
We gratefully acknowledge the grant from Higher Education Commission of Pakistan for a PhD student who utilized her funds for the commencement of this work.
References
- Nielsen IL, Chee WS, Poulsen L, et al. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: a randomized, double-blind, crossover trial. J. Nutr. 2006;136:404–408.
- Oscar DR, Concepcion MA, Maria VM, et al. Normal or high polyphenol concentration in orange juice affects antioxidant activity, blood pressure, and body weight in obese or overweight adults. J. Nutr. 2015;145:1806–1816. doi:10.3945/jn.115.213660.
- Naiki T, Kurose Y, Hayashi K, et al. Effects of long-term feeding of α-glucosylhesperidin on the mechanical properties of rabbit femoral arteries. Biorheology. 2012;49:353–363. doi:10.3233/BIR-2012-0619.
- Hnatek L. Therapeutic potential of micronized purified flavonoid fraction (MPFF) of diosmin and hesperidin in treatment chronic venous disorder. Vnitr Lek. 2015;61:807–814.
- Chiba H, Kim H, Matsumoto A, et al. Hesperidin prevents androgen deficiency-induced bone loss in male mice. Phytother. Res. 2014;28:289–295.10.1002/ptr.v28.2
- Chiba H, Uehara M, Jian WX, et al. Hesperidin a citrus flavonoid, inhibit bone loss and decreases serum and hepatic lipids in overiectomized mice. J. Nutr. 2003;133:1892–1897.
- King’ori AM. A review of the uses of poultry eggshells and shell membrane. Int. J. Poult. Sci. 2011;10:908–912.
- Nakano T, Ikama NI, Ozimek L. Chemical composition of chicken eggshell and shell membranes. Int. J. Poult. Sci. 2003;82:510–514.10.1093/ps/82.3.510
- Schaafsma A, Pakan I, Hofstede GJH, et al. Minerals, amino acid and hormonal composition of chicken eggshell powder and evaluation of its use in human nutrition. Poult. Sci. 2000;79:1833–1838.10.1093/ps/79.12.1833
- Adeyeye E. Comparative study on the characteristics of egg shells of some birds species. Bull. Chem. Soc. of Ethop. 2009;23:159–166.
- Burger J, Gibbons JW. Trace elements in egg contents and egg shells of slider turtles (Trachemys scripta) from Savannah River site. Arch. Environ. Contam. Toxicol. 1998;34:382–386.10.1007/s002449900334
- Schaafsma A, Van Doormaal JJ, Muskiet FAJ, et al. Positive effects of a chicken eggshell powder-enriched vitamin–mineral supplement on femoral neck bone mineral density in healthy late post-menopausal Dutch women. Br. J. Nut. 2002;87:267–275.10.1079/BJN2001515
- Gittins J, Drakley C. Utilization of egg shell waste from UK egg processing and hatchery establishments. ADAS Consulting Ltd; 2002 [cited 2015 Aug 13]. Available from: https://www.academia.edu/10566648/ADAS_UTILISATION_OF_EGG_SHELL_WASTE_FROM_UK_EGG_PROCESSING_AND_HATCHERY_ESTABLISHMENTS_Prepared_for_CONTENTS
- Amu O, Salami BA. Effect of common salt on some engineering properties of eggshell stabilized lateritic soil. ARPN-JEAS. 2010;5:64–73.
- Masuda Y. Hen’s egg shell calcium. Clin. Calcium. 2005;15:95–100.
- Brun LR, Lupo M, Delorenzi DA, et al. Chicken eggshell as suitable calcium source at home. Int. J. Food. Sci. Nutr. 2013;64:740–743.10.3109/09637486.2013.787399
- Andrews W. FAO Food and nutrition paper. Manual of food quality control. Food and Agriculture Organization of the United Nation; 1992 [cited 2015 Aug 13]. Available from: http://www.fao.org/docrep/014/T0610E/T0610E.pdf
- AOAC. Official methods of analysis. 19th ed. Washington (DC): Association of Official Analytical Chemists; 2012.
- Miller DD, Schricker BR, Rasmussen RR, et al. An in vitro method for estimation of iron availability from meals. Am. J. Clin. Nutr. 1981;34:2248–2256.
- Chang SO. A study on the calcium bioavailability of egg shell powder in the growing rats. Korean J. Nutr. 2003;36:684–690.
- Sittikulwitit S, Sirichakwal PP, Puwastien P, et al. In vitro bioavailability of calcium from chicken bone extracts powder and its fortified products. J. Food Comp. Anal. 2004;17:321–329.10.1016/j.jfca.2004.03.023
- Robert P, Hanzlic SC, Daniel HF. Relative bioavailability of calcium from calcium format, calcium citrate and calcium carbonate. J. Pharmacol. Exp. Ther. 2005;313:1217–1222.
- Harvey JA, Zobitz MM, Pak CY. Dose dependency of calcium absorption: a comparison of calcium carbonate and calcium citrate. J. Bone Miner. Res. 1988;1988:253–258.
- Heller HJ, Greer LG, Haynes SD, et al. Pharmacokinetic and pharmacodynamic comparison of two calcium supplement in postmenopausal women. J. Clinic. Pharmacol. 2000;40:1237–1244.
- Lindberg JS, Zobit MM, Poindexter JR, et al. Magnesium bioavailability from magnesium citrate and magnesium oxide. J. Am. Coll. Nutr. 1990;9:48–55.10.1080/07315724.1990.10720349
- World Health Organization/Food and Agriculture Organization of the United Nations. Vitamins and mineral requirement in human nutrition. 2nd ed. Geneva; 2004. p.252. [Online] [cited 2015 Aug 13]. Available from: http://apps.who.int/iris/bitstream/10665/42716/1/9241546123.pdf
- Teucher B, Olivares M, Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids. Int. J. Vitam. Nutr. Res. 2004;74:403–419.10.1024/0300-9831.74.6.403
- Conrad ME, Schade SG. Ascorbic acid chelates in iron absorption: a role for hydrochloric acid and bile. Gastroenterol. 1968;55:537–541.
- Vinson JA, Bose P. Comparative bioavailability to humans of ascorbic acid alone or in a citrus extract. Am. J. Clin. Nutr. 1988;48:601–604.
- Haro-Vicente JF, Martinez-Gracia C, Ros G. Optimization of in vitro measurement of available iron from different fortificants in citric fruit juices. Food Chem. 2006;98:639–648.10.1016/j.foodchem.2005.06.040