Abstract
Solubility of metal in contaminated soils is a key factor which controls the phytoavailability and toxic effects of metals on soil environment. The chemical equilibria of metal ions between soil solution and solid phases govern the solubility of metals in soil. Hence, an attempt was made to identify the probable solid phases (minerals), which govern the solubility of Zn2+ and Cd2+ in zinc smelter effluent-irrigated soils. Estimation of free ion activities of Zn2+ (pZn2+) and Cd2+ (pCd2+) by Baker soil test indicated that metal ion activities were higher in smelter effluent-irrigated soils as compared to that in tubewell water-irrigated soils. Identification of solid phases further reveals that free ion activity of Zn2+ and Cd2+ in soil highly contaminated with Zn and Cd due to long-term irrigation with zinc smelter effluent is limited by the solubility of willemite (Zn2SiO4) in equilibrium with quartz and octavite (CdCO3), respectively. However, in case of tubewell water-irrigated soil, franklinite (ZnFe2O4) in equilibrium with soil-Fe and exchangeable Cd are likely to govern the activity of Zn2+ and Cd2+ in soil solution, respectively. Formation of highly soluble minerals namely, willemite and octavite indicates the potential ecological risk of Zn and Cd, respectively in smelter effluent irrigated soil.
Introduction
Mining and smelting of metal ores are the important human activities responsible for the accumulation of heavy metals in environment.[Citation1] Elevated concentrations of various metals in smelter contaminated soils and their toxic effects to local ecosystems have been documented in surface soils near the metal smelters.[Citation2–5] Mining and metallurgical processes generate effluents such as reclaimed tailings water, acid mine drainage and seepage, and process acid streams.[Citation6] Depending on the type of ores and metallurgical processes, these effluents contain different proportions of heavy metals and other toxic compounds. These effluents are either discharged in the river stream or in the soil for irrigating the crops in the nearby vicinity of the smelter plants. The continuous application of such effluents results in the build up of toxic levels of heavy metals in the top soil and crop plants grown thereon.
Accumulation of elevated levels of heavy metals, particularly non-ferrous metals (Zn, Cd, Pb, etc.) in soils near base metal smelters is a matter of concern. Over the last few decades, smelting of ores particularly of zinc have produced highly contaminated sites all over the world.[Citation7–11] Besides the elevated level of Zn, heavy build up of Cd in soil was also reported in these studies. Cadmium occurs as a significant impurity (usually 0.2–0.4%) in the ore mineral sphalerite (ZnS), which is the important commercial source to produce high purity Zn through smelting.[Citation12]
Solubility vis-a-vis availability of metals in soil, to a great extent, depends on the intensity factor, i.e. metal concentrations or activity in soil solution.[Citation13] Solubility of metals in contaminated soils is a key factor which controls the phyto-availability and toxic effects of metals on soil flora and fauna. Various approaches have been employed to estimate the solubility of metals in contaminated soils over the years. These include operationally defined extraction of metals from soils by chemical extractants, viz. EDTA, diethylene triamine penta acetic acid (DTPA), CaCl2, etc.[Citation14–16] Further, there is considerable experimental evidence to suggest that response of plants and soil organisms to metal toxicity are explained by variation in free metal ion activity in soil pore water.[Citation13] But extraction of soil pore water, subsequent chemical analysis and speciation may be difficult to adopt on routine basis to assess eco-toxicity of metals in contaminated soils. Baker and Amacher [Citation17] developed the diagnostic soil testing programme to measure the intensity parameter of different ions in soil. In this procedure, soil is extracted with the Baker soil test solution and total concentration of metals, including pH, is determined in solution analytically. Free ionic activity of elements is calculated by successive iterations using computational algorithms as described by Baker and Amacher [Citation17]. However, effectiveness of this approach has not been studied extensively in characterizing the solubility of Zn and Cd in smelter effluent-irrigated soils. Similarly, identification of solid phases which govern the solubility of Zn and Cd would be worthwhile in characterizing the intensity of these metal ions in smelter effluent-irrigated soils. Study on the identification of probable solid phases of Zn and Cd would have much more practical relevance as far as solubility of such metals in soil is concerned.
A large area at Debari, Udaipur (Rajasthan) has been receiving effluents from zinc smelter plants for about five decades.[Citation8] Totawat et al. [Citation2] and Garg and Totawat [Citation8], from the same area, reported heavy build up of Zn and Cd in soil, ground water and plants. However, no attempt has been made to identify the probable solid phases governing the solubility of Zn and Cd in these smelter effluent-irrigated soils. The present study was undertaken to (i) assess the effect of long-term irrigation with zinc smelter effluent on Zn and Cd content and their activities in soil, and (ii) identify the probable solid phases governing the solubility of Zn2+ and Cd2+ in smelter effluent-irrigated soils.
Materials and methods
Study area and collection of soil/water samples
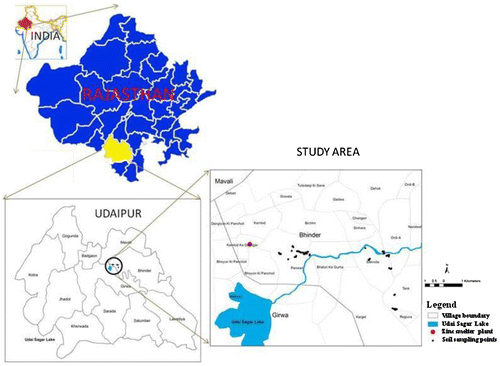
The study area is located at Debari of the Udaipur city, Rajasthan, India (Figure ). The zinc smelter plant (24°36′31.4″ N, 73°48′59.5″ E) at Debari was commissioned in 1968.[Citation2] Since its inception, the effluent from the plant has been discharged into a channel flowing about 3 km to the east to merge into a river connected to the Udai Sagar reservoir. The zinc smelter effluents flowing into the channel and river have been used for irrigation purposes in the surrounding agricultural fields since last five decades. The study area covers a distance of about 10 km in the east from effluent discharge point of zinc smelter plant at Debari. Composite surface soil samples (0–15 cm) were collected from both the farmers’ fields irrigated with zinc smelter effluent and tubewell water. In total, 59 geo-referenced (GPS coded) soil samples were collected from the study area (Figure ). Out of 59 samples, 51 and 8 soil samples were collected from smelter effluent and tubewell water-irrigated lands, respectively. The tubewell water-irrigated lands were found located away from the channel and/or river carrying the smelter effluent (Figure ). Water samples were also collected from both the channel and river carrying the zinc smelter effluent. In total, 20 water samples were collected from the channel and/or river randomly, irrespective of the distance from the effluent discharge point. Water samples from 10 different tubewells located in the tubewell water-irrigated area (non-contaminated area) were also collected.
Chemical analysis of soil and water samples
The collected soil samples were air-dried, ground and sieved to pass through a 2 mm sieve. Soil pH was determined using standard procedure.[Citation18] Organic carbon content in soil was determined by chromic acid oxidation method.[Citation19] For the determination of available Zn and Cd content, soil samples were extracted with 0.05 M EDTA-(Na)2 according to Quevauviller [Citation20] and metal contents in the extract were determined using inductively coupled plasma-mass spectrometer (ICP-MS). For analysis of total metal, soil samples were digested with aqua regia (HNO3 + 3HCl) on a hot plate and metal contents in the digest were determined as per the procedure of Quevauviller [Citation20] using ICP-MS. All the soil parameters were analyzed in triplicate. Water samples were analyzed for Zn and Cd contents using ICP-MS. The pH and EC of the water samples were determined using standard procedures.[Citation18]
Selection of soil sample for the estimation of free Zn2+ and Cd2+ ion activity
For the estimation of free ion activity and subsequent identification of solid phases governing the solubility of Zn and Cd, three soil samples were selected based on EDTA extractable and total metal content. Two samples (designated as contaminated-1 and contaminated-2) were selected out of 51 soil samples collected from zinc smelter effluent irrigated fields and one sample (designated as non-contaminated) was selected out of 8 samples collected from tubewell water irrigated fields. Out of 51 samples, the soil sample having the maximum concentration of EDTA extractable and total Zn was selected and designated as contaminated-1. The soil sample having Zn content nearest to the mean concentration of EDTA extractable and total Zn in effluent-irrigated soils was designated as contaminated-2 (Tables and ). The soil sample with metal contents nearest to the mean concentration of EDTA extractable and total metals (e.g. Zn and Cd) in tubewell water irrigated soils was selected and designated as non-contaminated (Tables and ). Soil texture in these soils was also determined following hydrometer method.[Citation21]
Table 1. Effect of long-term irrigation with zinc smelter effluent on important properties and metal content in soil.
Table 2. Important properties of the experimental soils used for identification of solid phases.
Estimation of free ion activity of Zn2+ (pZn2+) and Cd2+ (pCd2+) in soil
Intensity factor of Zn and Cd in the selected experimental soils was estimated following the procedure of Baker and Amacher [Citation17].
Baker soil test solution
1.5734 g of DTPA was dissolved in 300 mL of distilled water with gentle heating. The solution was transferred quantitatively to a 1 L volumetric flask; subsequently 10 mL of 0.25 M KCl, 10 mL of 1.0 M MgCl2, 25 mL of 2.0 M CaCl2·2H2O were added to the DTPA solution in a volumetric flask. The contents were made up to 1 L.
Blank preparation
A blank soil test solution was prepared by pipeting 40 mL of distilled water, 5 mL of soil test solution and 5 mL of 0.0275 M triethanol amine (TEA) into a polyethylene container and mixed. The pH of this solution was adjusted to 7.30 ± 0.05 by adjusting the amount of 0.0275 M TEA and distilled water in a final volume of 50 mL of the blank solution.
Procedure
Five grams of processed soil (air-dried and 2 mm sieved) was taken in a polyethylene container. Same amount of distilled water and 0.0275 M TEA was added, as determined in the blank preparation step to obtain a final volume of 50 mL at pH 7.30 ± 0.05. Then 5 mL of soil test solution was added. The contents were shaken for 1 h and then allowed to stand for additional 23 h. Small amounts of the blank and sample solutions were decanted for pH determination. The remaining solution was filtered and used for further analysis. Potassium (K) in the solution was determined by flame photometer; magnesium (Mg), calcium (Ca), manganese (Mn), iron (Fe), copper (Cu), cadmium (Cd), nickel (Ni), lead (Pb) and zinc (Zn) by atomic absorption spectrophotometer and aluminum (Al) by spectrophotometer with aluminon.[Citation22]
In Baker soil extract, at equilibrium, ions are distributed between soil and solution phases with no net movement of any ion from one phase to another. Some ions in solution remain chelated with DTPA (Baker solution) and other ions remain in free hydrated form. This distribution depends upon the amount of various ions in solution, strength of DTPA and stability constant for the formation of ion-DTPA complexes. The intensity factor of an ion is the relative partial free energy of an ion in the solution not chelated by DTPA. Based on this, a computer programme in FORTRAN-77 was developed, where based on thermodynamically characterized equilibrium constant, free metal ion activity could be estimated through successive iterations.[Citation23] Free ion activity of Zn2+ and Cd2+ was estimated by feeding the input data (Table ) as obtained from Baker soil test extract in this computer programme (FORTRAN-77). The inputs of computer programme included pH, total concentration of Ca, Mn, Ni, Cu, Zn, Cd, Pb, Mg, Al, Fe and K in Baker soil extract and total concentration of Cl− and DTPA in Baker solution. The concentrations of DTPA and Cl− in Baker solution were 0.0004 M and 3710 mg L−1, respectively. The free ion activity of Zn2+ and Cd2+ was expressed as negative logarithm of the metal ion activity (M L−1) (Table ).
Table 3. Input data on the concentration of metals (mg L-1) and pH in Baker soil extract used for estimating the free ion activity of Zn2+ and Cd2+ in different soils.
Table 4. Free ion activity of Zn (pZn2+) and Cd (pCd2+) in different soils as estimated by Baker’s soil test computer programme.
Development of solubility diagram and identification of solid phases
Solubility diagrams of Zn and Cd containing minerals were developed according to Lindsay [Citation24]. The solubility relationships of different Zn and Cd containing minerals with pH were developed from the thermodynamically characterized solubility equations of the minerals.[Citation24] The possibility of formation of Zn and Cd containing minerals not only depends on the activity of Zn and Cd, respectively, but also is affected by the activity of Fe and Si in soil solution. The solubility equations of different Zn and Cd containing minerals including those of various Fe and Si containing minerals in soil are presented in Table . The solubility relationships of several Zn and Cd containing minerals with pH were developed as follows:
Table 5. Solubility equations of some important minerals of Zn, Cd, Fe and Si in soil.
Zinc containing minerals
Soil-Zn provides very useful reference solubility for Zn2+ in soils. The solubility of soil-Zn can be expressed through the following reactions:(1)
(2)
Similarly, the solubility relationships of other Zn containing minerals namely, franklinite (ZnFe2O4) in equilibrium with soil-Fe, franklinite (ZnFe2O4) in equilibrium with maghemite (γ-Fe2O3), franklinite (ZnFe2O4) in equilibrium with goethite (α-FeOOH), willemite (Zn2SiO4) in equilibrium with quartz (SiO2) and smithsonite (ZnCO3) can be expressed through following reactions:(3)
(4)
(5)
(6)
(7)
Equation (7) indicates that the solubility of smithsonite is affected by the levels of partial pressure of CO2 (g). If CO2 (g) is 10−2.5 atm, the solubility of smithsonite can be expressed as(8)
From the above equations, log Zn2+ was computed at different pH of soil for a particular mineral and the values of log Zn2+ were plotted against pH values to get the solubility line of that mineral. In this way, the solubility lines of the above minerals were drawn to develop the solubility diagram of Zn containing minerals.
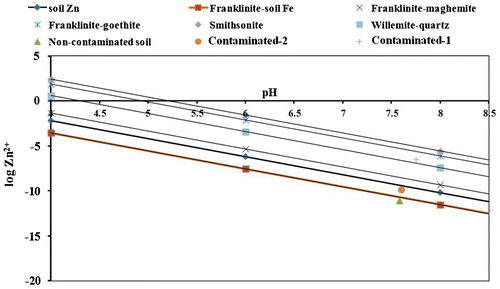
All the Zn-hydroxide minerals, zincite (ZnO) and smithsonite (ZnCO3) are too soluble to persist in normal soils. However, solubility of smithsonite (ZnCO3) largely depends on the partial pressure of CO2. Hence, in the solubility diagram of Zn containing minerals, the solubility lines of Zn-hydroxide minerals and zincite (ZnO) are not shown (Figure ).
Cadmium containing minerals
The solubility relationships of different Cd containing minerals namely, soil-Cd, octavite (CdCO3) and cadmium silicate (CdSiO3) with soil pH were developed [Citation24] as follows. The solubility of soil-Cd can be expressed through the following reactions:(9)
(10)
Equation (10) indicates that the activity of Cd2+ as governed by soil-Cd does not depend on soil pH. Hence, the solubility line of soil-Cd is parallel to the pH axis (Figure ). The solubility of other Cd minerals can be expressed as follows:(11)
(12)
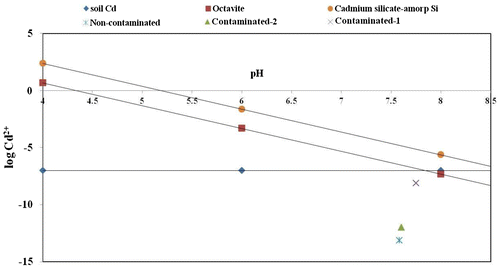
Solubility lines of the above minerals were developed according to their solubility equations to get the solubility diagram of Cd containing minerals. Since, CdO (monteponite) and ß-Cd(OH)2 (c) are too soluble to form in soil, the solubility lines of these minerals are not indicated in the solubility diagram of Cd containing minerals (Figure ).
For the identification of the most probable minerals (solid phases) which control the solubility of Zn and Cd in the experimental soils (contaminated-1, contaminated-2 and non-contaminated), the activities of Zn and Cd (as estimated by the computer programme based on Baker soil test) in those soils were superimposed on the solubility diagram of Zn and Cd containing minerals, respectively (Figures and ). By examining the proximity of the activity points to the solubility lines of Zn and Cd containing minerals, the probable solid phases supposed to control the solubility of Zn and Cd in different soils were identified.
Statistical analysis
The EDTA and aqua regia-extractable Zn and Cd content in smelter effluent- and tubewell water-irrigated soils was statistically compared and evaluated by applying t-test according to Snedecor and Cochran [Citation25]. The differences between the mean values of important soil properties namely, pH and organic carbon in effluent- and tubewell water-irrigated soils were also statistically evaluated by applying t-test.
Results
Characterization of water samples
The pH and EC values of the water samples collected from the channel and/or river ranged from 6.10 to 7.05 and 0.78 to 1.06 dS m−1, respectively; the corresponding values for the water samples collected from the tubewells located in the tubewell water-irrigated area (non-contaminated area) were 7.55 to 7.86 and 0.22 to 0.37 dS m−1 (data not shown). The concentration of Zn and Cd in channel and/or river water ranged from 1.47 to 2.82 and 0.02 to 0.08 mg L−1, respectively; the corresponding mean values were 2.19 and 0.04 mg L−1 (data not shown). The content of Zn in the water samples of the tubewells ranged from 0.08 to 0.21 mg L−1 with the mean value of 0.16 mg L−1. The Cd content in the tubewell water samples was below the detectable range (data not shown).
Effect of long-term irrigation with zinc smelter effluent on important properties and metal content in soil
Important soil properties namely, pH and organic carbon, and metal content as affected by long-term irrigation with zinc smelter effluent are presented in Table . Results indicated that soil pH was significantly lower in effluent-irrigated soils as compared to that in tubewell water irrigated soils. On an average, soil pH dropped by 0.31 units as a result of irrigation with zinc smelter effluent. Soil organic carbon (SOC) content was recorded to be significantly higher in effluent irrigated soils as compared to that in tubewell water irrigated soils. Organic carbon content varied from 0.28 to 1.81% (average 0.77%) in effluent-irrigated soils, the corresponding values for tubewell water-irrigated soils were 0.41–0.74% (average 0.58%). On an average, SOC content increased by 32% as a result of long-term effluent irrigation. The EDTA extractable and aqua regia digestible (total) Zn and Cd contents were significantly higher in effluent-irrigated soils as compared to that in tubewell water irrigated ones. On an average, long-term irrigation with zinc smelter effluent resulted into significant increase in EDTA extractable Zn and Cd content by 58.1 and 79.2 fold, respectively over tubewell water-irrigated soils. Long-term irrigation with smelter effluent also resulted into significant build-up of total Zn and Cd content by 27.0 and 167 fold, respectively.
Important properties of experimental soils used for the identification of solid phases of Zn and Cd
The important properties of the experimental soils are given in Table . The texture of contaminated-1, contaminated-2 and non-contaminated soils belonged to sandy loam, clay loam and sandy clay loam, respectively. All of the experimental soils were alkaline in nature with pH values of 7.76, 7.52 and 7.70 for contaminated-1, contaminated-2 and non-contaminated soils, respectively. The EDTA extractable and aqua regia digestible Zn and Cd contents were much higher in contaminated (effluent-irrigated) soils as compared to that in non-contaminated (tubewell water-irrigated) soil. Among the two contaminated soils, the EDTA extractable and aqua regia digestible Zn and Cd contents were higher in contaminated-1 in comparison to that in contaminated-2 soil.
Zn2+ and Cd2+ ion activity in soil
For the identification of the probable solid phases governing the solubility of Zn and Cd in the experimental soils, the free ion activities of the metals were estimated following the Baker’s soil test computer programme. The input data on the concentration of metals and pH in Baker soil extract fed into the computer programme for estimating pZn2+ and pCd2+ are presented in Table . The estimated free ion activity of Zn2+ and Cd2+ indicated that the intensities of the metals were far higher in the soil solution of contaminated-1 sample as compared to that in contaminated-2 (Table ). The lowest activities of Zn (pZn2+ = 11.072) and Cd (pCd2+ = 13.115) were found in the non-contaminated soil solution. Based on the decreasing order of metal ion activities in soil solution, the experimental soils can be arranged as contaminated-1 > contaminated-2 > non-contaminated soil. Results further indicated that the activities of Zn were much higher than that of Cd in the experimental soils.
Solid phases of zinc (Zn)
Solubility relationships of various Zn containing minerals (solid phases) namely, soil-Zn, franklinite (ZnFe2O4), willemite (Zn2SiO4) and smithsonite (ZnCO3) were developed with pH using the solubility equations (Table ) and presented through solubility lines in the solubility diagram (Figure ). Activities of Zn in the experimental soils as estimated by Baker’s soil test computer programme were superimposed on the solubility diagram of Zn containing minerals (Figure ). By examining the proximity of these points (of activities) to the solubility lines of various Zn containing minerals, the probable solid phases controlling the solubility of Zn in different soils were identified. Results indicated that the estimated free Zn-ion (Zn2+) activity for non-contaminated soil was very close to the solubility line of franklinite in equilibrium with soil-Fe. The point of free ion activity of Zn2+ for contaminated-2 soil was very close to the line of soil-Zn. However, in case of contaminated-1 soil, level of free Zn2+ ion activity was very close to the solubility line of willemite (Zn2SiO4) in equilibrium with quartz (SiO2). Hence, it appears that in case of non-contaminated soil, Zn ion activity in solution is likely to be governed by franklinite-soil Fe; whereas, soil-Zn governed the solubility of Zn in contaminated-2 soil. In case of contaminated-1soil, it is probably willemite-quartz which can govern the solubility of Zn in soil solution.
Solid phases of cadmium (Cd)
The free Cd2+ ion activities in the experimental soils as estimated by Baker’s soil test based computer programme were superimposed on the solubility diagram of Cd containing minerals (Figure ). Results indicated that estimated free Cd2+ ion activity for contaminated-1 soil was close to the solubility lines of soil-Cd and octavite (CdCO3). The points of free ion activities of Cd2+ for contaminated-2 and non-contaminated soils lie much below the solubility lines of CdCO3 and soil-Cd. Hence, it appears that in contaminated-1 soil, Cd2+ ion activity is likely to be governed by soil-Cd or CdCO3. Whereas, in case of contaminated-2 and non-contaminated soils, free Cd2+ ion activity in solution may probably be buffered by exchangeable Cd.
Discussion
Soil properties and metal content as affected by long-term irrigation with zinc smelter effluent
In the present investigation, lower soil pH and higher organic carbon content were recorded in the smelter effluent irrigated soils as compared to that in tubewell water irrigated ones. The present findings derive support from the findings of Bansal et al. [Citation26], where they reported that soils irrigated with industrial waste water had lower pH and higher organic carbon as compared to those which received tubewell water as the source of irrigation. Wang et al. [Citation27] reported that the pH of soils near to a copper smelter was significantly lower than that of soils situated away from the smelter. Higher content of soil organic carbon (SOC) was also recorded in soils near to a copper smelter in China and SOC content was found to decrease with increasing distance from the smelter.[Citation28] The build up of organic carbon in smelter effluent-irrigated soil may be attributed to the high cyanide (CN−) content in the smelter effluent.[Citation6] Depending on the type of ores and metallurgical processes, the smelter effluents may contain different toxic compounds and cyanide is one of the substances dominantly present in these effluents. Higher build up of EDTA extractable and aqua regia digestible Zn and Cd contents were recorded in smelter effluent irrigated soils in comparison to that in tubewell water irrigated soils. This may be attributed to the long-term irrigation with zinc smelter effluent in the agricultural fields.[Citation8] Higher concentrations of Zn and Cd in the water samples collected from the channel and/or river carrying the smelter effluent in comparison to the water collected from the tubewells also provide support to the present findings. However, high metal accumulation in soil near to the smelter plant may also be attributed to high atmospheric deposition.[Citation27,29–31] Based on the EDTA extractable and aqua regia digestible (total) Zn and Cd contents, contaminated-1 soil could be rated as highly contaminated soil and the level of metal contamination in this soil was higher than that in contaminated-2 soil. However, the total Zn and Cd contents in both contaminated-1 and contaminated-2 soils exceeded the maximum allowable limits of 300 and 3 mg kg−1, respectively as followed in Great Britain.[Citation32] Total Zn and Cd contents in non-contaminated soil were far below the maximum allowable limits. As expected, the experimental soils followed the same order in respect of free ion activities of Zn2+ and Cd2+ as in case of EDTA extractable and total Zn and Cd contents.
Solid phases governing the solubility of Zn and Cd in Zn smelter effluent irrigated soils
Soil-Zn provides very useful reference solubility of Zn2+ in soil. Soil-Zn as such is not any mineral. This is a solid phase and its solubility is usually very close to the solubility line of franklinite (ZnFe2O4) in equilibrium with soil-Fe, because in normal soil, soil-Fe governs the solubility of Fe.[Citation33,34] In normal soil, soil-Zn is considered as solid phase which is indeed franklinite in equilibrium with soil-Fe. In the present study, both in non-contaminated and contaminated-2 soils, there is possibility that franklinite in equilibrium with soil-Fe governs the solubility of Zn.
All the Zn-hydroxide minerals, zincite (ZnO) and smithsonite (ZnCO3) are too soluble to persist in normal soils.[Citation24,35] Willemite is of intermediate solubility but too soluble to account for the level of soil Zn as occurring in most of the soil. In the present case, the level of free ion activity of Zn2+ in contaminated-1 (highly contaminated) soil is close to the solubility line of willemite (Zn2SiO4) in equilibrium with quartz. The EDTA extractable Zn in this soil is unusually very high (2520 mg kg−1). In such soil, it is quite natural that ionic product of willemite might have exceeded the solubility product of this mineral leading to the formation or precipitation of Zn as willemite.
Soil-Cd provides very useful reference solubility of Cd2+ in soil. Plotting of values of free Cd2+ ion activity of different soils in solubility diagram (Figure ) indicated that Cd2+ ion activity of only one soil (contaminated-1) was very close to the solubility lines of soil-Cd as well as octavite (CdCO3). Free ion activities of Cd2+ in other two soils were far below the solubility lines of soil-Cd and octavite. The mineral cadmium silicate (CdSiO3) is usually more soluble than octavite and it is not expected to form in soil.[Citation24] Other minerals like monteponite (CdO) and ß-Cd(OH)2 (c) are too soluble to persist in soil. Usually Cd2+ activity in soil is limited by the formation of octavite at pH value above 7.5 depending on the partial pressure of CO2. From such results it can be inferred that activity of Cd2+ is controlled by octavite (CdCO3) in highly cadmium contaminated soil (contaminated-1), where ionic product of this mineral exceeded the solubility product. In case of other two soils (non-contaminated and contaminated-2), it appears that none of the known minerals of Cd governs the activity of Cd2+. In such case, the level of free ion activity of Cd2+ in soil solution might have been buffered by exchangeable Cd. In limited number of studies, it has been reported that octavite controls the Cd2+ solubility in smelter contaminated soil.[Citation36,37]
Conclusions
Long-term irrigation with zinc smelter effluent resulted into a significant build up of Zn and Cd in both available and total pools. Decline in soil pH and build up of organic carbon are also associated with smelter effluent irrigation. Higher free ion activities of Zn2+ and Cd2+ in smelter effluent irrigated soils indicate that appropriate remediation measures are to be taken up for reclamation of such soils. It can also be concluded that free ion activity of Zn2+ and Cd2+ in highly contaminated soil is limited by the solubility of willemite (Zn2SiO4) in equilibrium with quartz and octavite (CdCO3), respectively. In case of other soils (contaminated-2 and non-contaminated), franklinite (ZnFe2O4) in equilibrium with soil-Fe and exchangeable Cd are supposed to govern the activity of Zn2+ and Cd2+ in soil solution, respectively. Such information may be useful in devising reclamation strategy to reduce Zn and Cd toxicity in zinc smelter effluent-irrigated soils.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgement
Authors would like to place on record their sincere thanks to the Department of Science and Technology (DST), Government of India and Indian Council of Agricultural Research (ICAR), New Delhi, India for funding and providing laboratory facilities, respectively to carry out the study.
References
- Zhuang P, Zou B, Li NY, et al. Heavy metal contamination in soils and food crops around Dabaoshan mine in Guangdong, China: implication for human health. Environ Geochem Health. 2009;31:707–715.10.1007/s10653-009-9248-3
- Totawat KL, Upadhyay RN, Chauhan SC. Effects of zinc smelter effluent on water, land and vegetation. Indian J Environ Health. 1994;36:237–247.
- Kabala C, Singh BR. Fractionation and mobility of copper, lead and zinc in soil profiles in the vicinity of a copper smelter. J Environ Qual. 2001;30:485–492.10.2134/jeq2001.302485x
- Kashem MA, Singh BR, Huq SMI, et al. Fractionation and mobility of cadmium, lead and zinc in some contaminated and non-contaminated soils of Japan. J Soil Sci Environ Manage. 2011;3:241–249.
- Zhou M, Liao B, Shu W, et al. Pollution assessment and potential sources of heavy metals in agricultural soils around four Pb/Zn mines of Shaoguan city, China. Soil Sediment Contam. 2015;24:76–89.10.1080/15320383.2014.914152
- Kuyucak N. State-of-the-art technologies for the treatment of acid mine drainage and mining effluents. International Mining and Environment Congress; 2001 September; Peru, Lima.
- Ketterer ME, Lowry JH, Simon J Jr., et al. Lead isotopic and chalcophile element compositions in the environment near a zinc smelting-secondary zinc recovery facility, Palmerton, Pennsylvania, USA. Appl Geochem. 2001;16:207–229.10.1016/S0883-2927(00)00029-9
- Garg VK, Totawat KL. Heavy metal accumulation, movement and distribution in soil profiles near zinc smelter effluent stream. J Indian Soc Soil Sci. 2005;53:141–144.
- Cabala J, Teper L. Metalliferous constituents of rhizosphere soils contaminated by Zn–Pb mining in southern Poland. Water Air Soil Pollut. 2007;178:351–362.10.1007/s11270-006-9203-1
- Douay F, Pruvot C, Roussel H, et al. Contamination of urban soils in an area of northern France polluted by dust emissions of two smelters. Water Air Soil Pollut. 2008;188:247–260.10.1007/s11270-007-9541-7
- Yang Y, Li S, Bi X, et al. Lead, Zn, and Cd in slags, stream sediments, and soils in an abandoned Zn smelting region, southwest of China, and Pb and S isotopes as source tracers. J Soils Sediments. 2010;10:1527–1539.10.1007/s11368-010-0253-z
- Greenwood NN, Earnshaw A. Chemistry of the elements. Oxford: Butterworth Heinemann; 2001.
- Datta SP, Young SD. Predicting metal uptake and risk to the human food chain from leaf vegetables grown on soils amended by long-term application of sewage sludge. Water Air Soil Pollut. 2005;163:119–136.10.1007/s11270-005-0006-6
- Hooda PS, Alloway BJ. Effects of time and temperature on the bioavailability of Cd and Pb from sludge-amended soils. J Soil Sci. 1993;44:97–110.10.1111/ejs.1993.44.issue-1
- Wang JJ, Harrell DL, Henderson RE, et al. Comparison of soil test extractants for phosphorus, potassium, calcium, magnesium, sodium, zinc, copper, manganese, and iron in louisiana soils. Commun Soil Sci Plant Anal. 2004;35:145–160.10.1081/CSS-120027640
- Gupta AK, Sinha S. Chemical fractionation and heavy metal accumulation in the plant of Sesamum indicum (L.) var. T55 grown on soil amended with tannery sludge: selection of single extractants. Chemosphere. 2006;64:161–173.10.1016/j.chemosphere.2005.10.016
- Baker DE, Amacher MC. The development and interpretation of a diagnostic soil-testing programme. University Park: Pennsylvania State University, Agricultural Experiment Station; 1981. ( Bulletin No. 826).
- Jackson ML. Soil chemical analysis. New Delhi: Prentice Hall of India Private Limited; 1973.
- Walkley A, Black IA. An examination of the degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37:29–38.10.1097/00010694-193401000-00003
- Quevauviller PH. Operationally defined extraction procedures for soil and sediment analysis I. Standardization. TrAC Trends Anal Chem. 1998;17:289–298.10.1016/S0165-9936(97)00119-2
- Bouyoucos GJ. Hydrometer method improved for making particle size analysis of soils. Agron J. 1962;54:464–465.10.2134/agronj1962.00021962005400050028x
- Page AL. Methods of soil analysis: chemical and microbiological properties. Vol. 9. Madison (WI): Soil Science Society of America; 1982.
- Datta SP, Meena BL, Rattan RK. Development of a computer programme for calculating metal ion activity using Baker soil test. J Indian Soc Soil Sci. 2013;61:47–50.
- Lindsay WL. Chemical equilibria in soils. New York: Willey inter sciences; 1979.
- Snedecor GW, Cochran W. Statistical methods. 6th ed. Lowa: Lowa State University Press; 1967.
- Bansal RL, Nayyar VK, Takkar PN. Accumulation and bioavailability of Zn, Cu, Mn and Fe in soils polluted with industrial waste water. J Indian Soc Soil Sci. 1992;40:796–799.
- Wang YP, Shi JY, Wang H, et al. The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol Environ Saf. 2007;67:75–81.10.1016/j.ecoenv.2006.03.007
- Liu L, Wu L, Luo Y, et al. The impact of a copper smelter on adjacent soil zinc and cadmium fractions and soil organic carbon. J Soils Sediments. 2010;10:808–817.10.1007/s11368-010-0227-1
- Cui YJ, Zhu YG, Zhai RH, et al. Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China. Environ Int. 2004;30:785–791.10.1016/j.envint.2004.01.003
- Svendsen M, Steinnes E, Blom HA. Vertical and horizontal distributions of Zn, Cd, Pb, Cu, and Hg in uncultivated soil in the vicinity of a zinc smelter at Odda, Norway. Soil Sediment Contam. 2007;16:585–603.
- Yang YG, Jin ZS, BI XY, et al. Atmospheric deposition-carried Pd, Zn and Cd from a zinc smelter and their effect on soil microorganisms. Pedosphere. 2009;19:422–433.10.1016/S1002-0160(09)60135-1
- Rattan RK, Datta SP, Chandra S, et al. Heavy metals and environmental quality-Indian scenario. Fertiliser News. 2002;47:21–40.
- Adhikari T, Datta SP, Rattan RK. Evaluation of soil fertility status by a diagnostic soil testing programme. Int J Trop Agric. 2000;18:319–326.
- Barman M, Datta SP, Rattan RK. Identification of the solid phase in relation to the solubility of nickel in alluvial soils. J Environ Biol. 2014;35:901–906.
- Deb DL, Sakal R, Datta SP. Micronutrients. In: Fundamentals of soil science. 2nd ed. New Delhi: Indian Society of Soil Science; 2009;461–490.
- McGowen SL, Basta NT, Brown GO. Use of diammonium phosphate to reduce heavy metal solubility and transport in smelter-contaminated soil. J Environ Qual. 2001;30:493–500.10.2134/jeq2001.302493x
- Basta NT, McGowen SL. Evaluation of chemical immobilization treatments for reducing heavy metal transport in a smelter-contaminated soil. Environ Pollut. 2004;127:73–82.10.1016/S0269-7491(03)00250-1