Abstract
We investigated the distribution and chemical speciation of Cd, Cr, Cu, Pb and Zn in the water level-fluctuating (WLF) zone of the main stream (MS) and tributaries (ZX and MX) of the Three Gorges Reservoir. We evaluated the ecological risk and pollution level from heavy metals based on the Potential Ecological Risk Index (RI), Risk Assessment Code (RAC), and Ratio of Secondary Phase and Primary Phase (RSP). Our results indicated that the total and bio-available heavy metal contents were higher in the tributaries than in the MS. Moderate pollution from Cd and light pollution from Pb were observed both at the MS and ZX sites, whereas the MX site exhibited a pattern of heavy Cd pollution and light Cr and Pb pollution. In our study area, the results indicated that Cd exhibited a higher ecological risk than did the other heavy metals. Finally, the pH and nitrogen content of sediments may play a key role in controlling the amount of heavy metal bioavailability, further inducing a higher potential ecological risk.
1. Introduction
Heavy metals are non-biodegradable and bioaccumulative and have been closely studied in recent decades [Citation1,2]. The WLF zone of reservoirs plays a key role as an interface between terrestrial and aquatic ecosystems. The WLF zone of the Three Gorges Reservoir Area (TGRA) covers more than 400 km2 [Citation3]. Due to periodic water fluctuation in the riparian zone, fast turnover rates of nutrients and heavy metals have been observed [Citation4]. Following the manipulation of the water storage regimen, the WLF zone of the TGRA commonly exhibits a dry period followed by a period of flooding from October to April of the following year [Citation5]. Therefore, when the riparian zone was used as agricultural land during the dry period, the period of flooding that followed resulted in a potential risk from the use of improper fertilizer rates during the dry period [Citation6]. The heavy metals associated with fertilizer use accumulate in the sediment of the riparian zone during the dry periods and are released into the water during the flooding period. This periodic fertilizer-based heavy metal loading in the sediment has been observed since 2003 in fluctuating water levels between 145 and 175 m [Citation7]. Thus, there is a potential risk of heavy metals in the TGRA riparian zone that could detrimentally affect the water quality.
Currently, numerical methods to estimate the potential risk from heavy metals in the sediment of the riparian zone have been reported, such as the Enrichment Factor [Citation7], the Index of Geo-accumulation [Citation8] and RI [Citation9]. However, these risk assessment methods have been based only on the total heavy metal content. Thus, including the available content of individual heavy metals into ecological risk assessments is necessary. As previous studies have shown, the potential ecological risk from heavy metals is positively correlated with their effective chemical speciation [Citation10]. Thus, evaluating the contribution of various chemical fractions from heavy metals requires a reliable ecological risk assessment approach. Therefore, available heavy metal-based methods have been developed, e.g. the RAC and RSP [Citation11–13]. These assessment methods relate the free ion [Citation12] and sediment surface to the weakly bound phase of heavy metals [Citation13–15].
Therefore, the aim of the current study was to estimate the ecological risk from heavy metals in the riparian zone sediment in the TGRA. The chemical speciation and bioavailability of various heavy metals were determined and their potential risk of leaching into the water was also evaluated. To assess the ecological risk from heavy metals, an effective heavy-metal content-based method was applied using RAC and RSP assessment. Furthermore, the study aimed to elucidate the factors controlling heavy metal transformation in the WLF zone sediment following periodic water fluctuations and the subsequent potential risks to the water quality of the TGRA.
2. Materials and methods
2.1. Study sites and sampling
The present study was carried out on the Yangtze River’s Wanzhou Section (MS) and its two tributaries, called ZX and MX (N30.85372° – 30.802821°, E108.335172° – 108.440726°) in the northeastern Chongqing municipality in China (Figure ). The WLF zone typically comprises bench terraced valley slopes that were previously used as farmland. The region is characterized by a north subtropical humid monsoon climate with an average annual temperature of 18.2 °C, average annual precipitation of 1053.15 mm, average annual effective solar radiation of between 3600 and 3700 MJ/m2, and average annual sunshine duration of approximately 1400 h. Surface (0–10 cm) sediments were collected from the WLF zone during the dry season (Figure ).
2.2. Pre-treatment and analytical procedures
The soil samples were sieved through a 1-mm mesh filter after removing plant residues and were then air-dried at room temperature and stored in a brown glass bottle. Then, 0.5 g samples were digested in a microwave oven (Mars-5, CEM Co., Matthews, NC, USA) with an acid mixture (9 ml of 14.0 M HNO3, 3 ml of 11.7 M HCl, 2 ml of 23.0 M HF and 2.5 ml of 8.8 M H2O2) and then condensed to 1–2 ml for total metal analysis on an inductively coupled plasma mass spectrometer (Agilent Technologies Co. Ltd., USA). The speciation of metals, including acid-soluble (F1), reducible (F2), oxidisable (F3) and residual (F4) metals, was conducted with a modified BCR [Citation13]. Quality control was assured by using a reference material (GSS-14, from Chinese Environmental Monitoring Center) that was analysed in the same way as the samples in both duplicate and parallel. The duplicates exhibited a difference of <9%, and satisfactory recoveries were obtained for Cd (94~105%), Cu (96~106%), Pb (98~104%), Cr (95~99%) and Zn (95~102%).
Organic matter (OM) was determined by using the modified Walkley–Black procedure [Citation16]. The pH was analysed in deionized water extracts (1:2.5 w/w). The cation exchange capacity (CEC) was determined using the barium chloride method adjusted to the pH [Citation17]. The total N was determined using the Kjeldahl method [Citation18].
2.3. Risk assessment
The RSP, RAC and RI methods were used to assess the potential ecological risk from, and bioavailability of, heavy metals [Citation11–13].
2.4. Statistical analysis
A two-way ANOVA was used to determine the differences in the heavy metal contents and their risk among the three study areas. All of the statistical analyses and the correlations between heavy metals were performed by using IMB SPSS Statistics for Windows version 20.0 at a significance level of 0.05.
3. Results and discussion
3.1. Edaphic factors and the total heavy metal content
Edaphic factors are important for the bioavailability of heavy metals. As shown in Table , the physicochemical characteristics in MS, ZX and MX were as follows: the SOM content was 64.24 ± 6.01, 65.04 ± 4.08 and 68.11 ± 6.25 g·kg−1; the TN content was 0.28 ± 0.06, 0.41 ± 0.09 and 0.40 ± 0.05 g·kg−1; the pH was 8.32 ± 0.22, 8.21 ± 0.46 and 7.45 ± 0.91; the CEC content was 48.4 ± 41.7, 37.1 ± 8.3, and 19.6 ± 4.4 cmol·kg−1; and the TP was 0.74 ± 0.33, 0.70 ± 0.07, and 0.84 ± 0.18 g·kg−1, respectively. Compared with the two tributaries, the MS had low SOM and TN, with a higher pH and CEC. The sediment particle size composition indicated that clay < silt < sand in the three study areas, and the proportion of sand was higher in the tributaries than in MS. The higher TN in MX and ZX might result from the accumulation of aquatic organisms and terrestrial plant residues on a gentler sloped floodplain in the WLF zone of the tributaries [Citation19]. The lower CEC in the tributaries might result from the higher proportion of sand in the tributaries [Citation20].
Table 1. Physicochemical characteristics of the WLFZ sediments.
The descriptive statistics of the heavy metals in the WLF zone of the MS and its tributaries (ZX and MX) exhibited a distinct change (Table ). The Cd, Cr, Cu, Pb and Zn contents varied between 4.58 and 6.47, 91.23 and 137.93, 18.38 and 18.50, 125.96 and 137.36, 85.68 and 91.93 mg/kg, respectively, and their average contents were 5.85, 114.76, 18.43, 133.34 and 89.66 mg/kg, respectively. The heavy-metal background values from Sichuan were used as reference values [Citation21]. The contents of Zn, Cr, Pb and Cd were 1.04, 1.45, 4.32 and 73.13-fold higher than the background values, respectively. The pattern of increase in the total Cd, Cr, Zn and Pb contents across the three study sites was MX > ZX > MS, whereas, for total Cu, it was MS > ZX > MX. The average contents of Cd, Cr, Cu, Pb and Zn in the WLFZ of MX were 6.47, 137.93, 18.38, 137.36 and 91.93 mg/kg, respectively, which were higher than those in the other two regions. The gently sloping tributaries had more farming activities than MS [Citation22]. The water flow in the tributaries was also much slower, which could lead to heavy metal accumulation [Citation23].
Table 2. Total contents of metals in the WLFZ sediments (mg·kg−1).
3.2. Heavy metal fractions in the WLFZ sediments
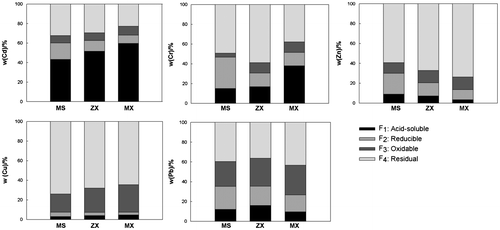
As shown in Figure , both Cu and Zn were predominantly associated with the residual fraction (F4), whereas a relatively higher proportion of Cu bound to F4 occurred in the MS than in its tributaries. By contrast, a higher proportion of Zn bound to F4 occurred in the tributaries than in the MS. Pb was mainly bound to both the oxidisable (F3) and residual fraction (F4), which exhibited no significant difference between MS and its tributaries. By contrast, a higher proportion of Cd existed in the acid-soluble fraction (F1), which was more prevalent in the tributaries than in the MS. The complexation of the heavy metals with the dissolved organic matter [Citation24,Citation25] and redox conditions influenced by water fluctuations might be the main factors controlling the transportation and transformation of the metal fractions [Citation26–30].
3.3. The factors influencing the distribution of heavy metal fractions
The relationships between heavy metal contents and physicochemical characteristics (pH, SOM, TN, TP, and CEC) in the WLF zone are shown in Table . For Cd, F1, F2, F3 and the total content were significantly correlated with pH and TN (p < 0.05). The F2 fraction was negatively correlated with OM (p < 0.05), whereas the F4 fraction was positively correlated with CEC (p < 0.05). The F1 fraction was the major speciation of Cd. Thus, Cd transformation was mainly controlled by pH and TN.
Table 3. Relationship between the heavy metal fractions and physicochemical characteristics.
For Cr, F1 and total content were negatively correlated with pH and CEC (p < 0.05). The F2 and F3 fractions were negatively correlated with TN and pH (p < 0.05). The F4 fraction was positively correlated with TN (p < 0.05). Thus, the controlling factor for Cr transformation was TN.
For Cu, the F1 and F3 fractions were negatively correlated with pH (p < 0.01), whereas the F2 and F3 were positively correlated with TN (p < 0.01). Furthermore, the total Cu content was negatively correlated with TP (p < 0.05). Therefore, TP has a strong impact on Cu transformation.
For Pb, the F2 fraction was correlated with pH and TN, whereas the F3 fraction was correlated with pH, organic matter and TN (p < 0.05). F4 was negatively related with pH, and the total Pb was positively correlated with TN (p < 0.05). Given that the main fractions of Pb were F3 and F4, pH, organic matter and TN mainly affected the transformation of Pb.
The F1 and F2 fractions of Zn were negative correlated with TN, whereas F3 was positively correlated with both organic matter and TN (p < 0.05). The F4 fraction of Zn had no correlation with any of the above-mentioned factors.
We found that the heavy metal fractions and their release were controlled by the pH and TN content. The pH is believed to be the key factor affecting the speciation of heavy metals [Citation31]. Laboratory simulation has shown that exotic heavy metals are mostly bonded to oxides under alkaline conditions and onto organic matter in acidic conditions in soil [Citation32]. The pH is positively correlated with the reducible fraction (F2) of heavy metals [Citation32]. Wang et al. found that, with increasing pH, the speciation of heavy metals in sludge was transformed to oxidisable and residual fractions [Citation33]. However, the negative correlation between pH and the oxidisable fraction reported in the current study are contrary to those of Wang et al. This was probably caused by microbial processes that occur in organic matter that was deposited in the lower water table in the WLF zone. He et al. found that the abundance of bacteria and actinomycetes was significantly correlated (at the 0.05 level) with the mobility factor of heavy metals and the pH conditions of the soil surrounding a coal gangue dump [Citation34]. Furthermore, Zeng et al. found that the content of heavy metals absorbed by plants was positively correlated with the soil organic matter content [Citation35]. Meanwhile, the edaphic factors in the current study indicated that the pH of the tributaries was lower than that of the MS, and the organic matter content was higher than that of the MS in the WLF zone of the TGRA (Table ). Therefore, this means that the pH of the soil decreased with the microbe humification process in the WLF zone. Meanwhile, the reducible fraction of heavy metals can be dissolved and transformed into acid soluble fractions, resulting in an increase in the mobility and bioavailability of heavy metals [Citation36,37]; In addition, the oxidisable and residual fractions of heavy metals can from the chelation, complexation, adsorption, and ion exchange of all the humification products during the microbial processes of the organic carbon and nitrogen cycles [Citation38–40]. In addition to pH, TN is also a controlling factor of the heavy metal fraction and mobility. The nitrogen fixation by microbes and plants during the flooding period [Citation19] might affect the pH, the combination of soil particles and heavy metal fractions, and the mobility and speciation of heavy metals [Citation41].
3.4. Potential ecological risk
The RI was assessed to illustrate the potential risk caused by heavy metal pollution in sediment and the sensitivity of various organisms to a toxic substrate. Cd seriously polluted the sediments and had a much greater potential ecological risk than the other heavy metals (Table ). The RI values of the study sites were ranked as follows: MX > ZX > MS. A similar pattern was also found by Lin [Citation8].
Table 4. Hakanson’s potential ecological risk index of heavy metals.
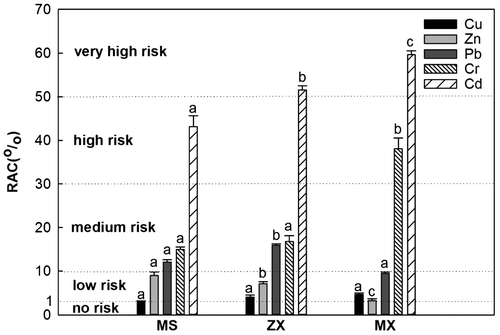
The RAC results indicated that the percentages of the exchangeable and carbonate fractions of Cu and Zn were less than 10% for all of the study areas. Thus, both Cu and Zn had low ecological risk over the whole study area (Figure ). Pb and Cr had RACs between 10 and 30% and, thus, exhibited medium ecological risk in ZX and MS. By contrast, in MX, the RAC value of Pb was less than 10%, which represents a low risk, whereas the value of Cr was between 30 and 50%, representing a high risk. Moreover, the RAC values of Cd in ZX and MX were higher than 50%, representing an extremely high risk, as did the Cd RAC of 42% in MS.
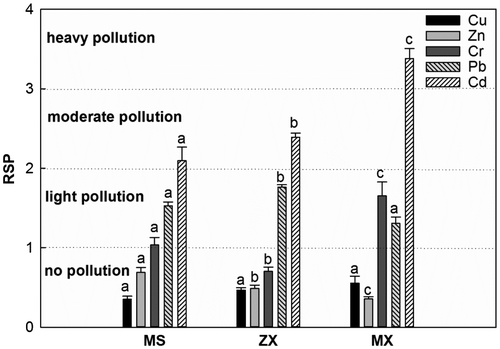
In general, the calculated mean KRSP values of the heavy metals were ranked as follows: Cd > Pb > Cr > Zn > Cu, where Cd had the highest KRSP value of 2.8, which suggests that the sediments studied were seriously polluted by Cd (Figure ). The average values of KRSP for Pb and Cr were 1.5 and 1.3, respectively, representing a medium degree of pollution. However, the mean KRSP values of Zn and Cu were less than 1, indicating that Zn and Cu were not polluting. Furthermore, the mean KRSP values from Cd in the study areas were ranked as follows: MX > ZX > MS. The mean KRSP value from Cr in MX was higher than in MS and ZX. Meanwhile, the KRSP value of Pb in ZX was the highest among the three study areas.
The MX is an important tributary of the Yangtze River’s Wanzhou sections and covers a basin area of 4.71 km2. The population of this area has gradually increased to 0.05 million, and the river passes through the city centre. Thus, a huge volume of wastewater from increasing industrial activities drains into the river in MX [Citation42]. Furthermore, the slow water flow may also be decreasing the diffusion of heavy metals [Citation43,44]. Therefore, the geographical distribution of heavy metals around the river indicated that the anthropogenic sources of heavy metals contributed a higher fraction of the heavy metal pollution in sediment around the WLFZ. The Cr of the F1 fraction also exhibited an increasing trend in MX. This indicated that the F1 fraction of both Cd and Cr in the tributaries exhibited a higher potential risk of transforming from a solid phase into a liquid phase. A similar phenomenon was also reported in a recent study by Chen [Citation45]. The reducible fraction (F2) of Cd and Cr could be transformed to an acid soluble fraction (F1) due to submerged conditions and higher organic matter inputs into the tributaries [Citation28,46]. The organic matter could be gradually degraded into small-molecule organic acids, resulting in the dissociation of a reducible fraction of heavy metals [Citation47].
4. Conclusions
In summary, the total contents of Cd, Cr, and Pb were ranked in the following decreasing order: MX > ZX > MS. The pollution levels from heavy metals followed a ranking of Cd > Pb > Cr > Zn > Cu. Among these heavy metals, Cd, Cr, and Pb were considered to pose a serious threat to residents’ health and to the aquatic ecosystem because they accounted for a significant proportion of the non-residual fractions. The pollution from Cd was higher, and Cr and Pb were relatively low in MX, whereas the MS and ZX only received moderate pollution from Cd, and light pollution from Cr. The pH and TN in the sediments were the core factors affecting the transformation of these heavy metals. Furthermore, RI and RAC revealed that Cd was the primary pollutant and determined that it had the highest risk among the heavy metals evaluated. Overall, the risk levels in the study areas from heavy metals were ranked as follows: MX > ZX > MS. Therefore, due to wastewater inputs and the slow water flow, accumulation of heavy metals in the surface sediments may occur. Thus, the potential ecological risk from the fluctuating water level zone should play a key role in the water level management of the Yangtze River tributaries in the Wanzhou section.
Funding
This work was supported by the Scientific and Technological Research Program of Chongqing Municipal Education Commission [grant number KJ1601016, KJ1710260]; Chunhui Project from Education Ministry of China [grant number Z2015133]; National Natural Science Foundation of China [grant number 31670467, 41601090, 31270451, 41371222]; Natural Science Foundation of Jiangsu Province [grant number BK20160950]; Chongqing Municipal Key Laboratory of Institutions of Higher Education [grant number WEPKL2016ZD-01, WEPKL2016ZZ-01, WEPKL2016LL-03]; Key Laboratory of Reservoir Aquatic Environment, Chongqing Institute of Green and Intelligent Technology, Chinese Academy of Science [grant number RAE2014BA01B]; and Youth Project Fund, Chongqing Three Gorges University [grant number 12QN18].
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
The authors are grateful to Lu Ye, Liang Sha and Xiaojun Xiao. We would like to express our appreciation to the anonymous reviewers for their thoughtful comments.
References
- Salazar MJ, Rodriguez JH, Nieto GL, et al. Effects of heavy metal concentrations (Cd, Zn and Pb) in agricultural soils near different emission sources on quality, accumulation and food safety in soybean [Glycine max (L) Merrill]. J Hazard Mater. 2012;233:244–253.10.1016/j.jhazmat.2012.07.026
- Rocio M, Elvira E, Pilar Z, et al. Could an abandoned mercury mine area be cropped? Environ Res. 2013;125:150–159.10.1016/j.envres.2012.12.012
- Wang Y, Huang P, Ye F, et al. Nitrite-dependent anaerobic methane oxidizing bacteria along the water level fluctuation zone of the Three Gorges Reservoir. Applied microbiology and biotechnology. 2016;100:1977–1986.
- Liu J, Jiang T, Huang R, et al. A simulation study of inorganic sulfur cycling in the water level fluctuation zone of the Three Gorges Reservoir, China and the implications for mercury methylation. Chemosphere. 2017;166:31–40.
- Bao Y, Gao P, He X. The water-level fluctuation zone of Three Gorges Reservoir – a unique geomorphological unit. Earth Sci Rev. 2015;150:14–24.10.1016/j.earscirev.2015.07.005
- Ye C, Li S, Zhang Y, et al. Assessing heavy metal pollution in the water level fluctuation zone of China’s Three Gorges Reservoir using geochemical and soil microbial approaches. Environ Monit Assess. 2013;185:231–240.10.1007/s10661-012-2547-7
- Ye C, Li S, Zhang Y, et al. Assessing soil heavy metal pollution in the water-level-fluctuation zone of the Three Gorges Reservoir, China. J Hazard Mater. 2011;191:366–372.10.1016/j.jhazmat.2011.04.090
- Lin J, Fu C, Zhang X, et al. Heavy metal contamination in the water-level fluctuating zone of the Yangtze River within Wanzhou Section, China. Biol Trace Elem Res. 2012;145:268–272.10.1007/s12011-011-9179-6
- Zhang W, Feng H, Chang J. Heavy metal contamination in surface sediments of Yangtze River intertidal zone: an assessment from different indexes. Environ Pollut. 2009;157:1533–1543.10.1016/j.envpol.2009.01.007
- Mossop KF, Davidson CM. Comparison of original and modified BCR sequential extraction procedures for the fractionation of copper, iron, lead, manganese and zinc in soils and sediments. Anal Chim Acta. 2003;478:111–118.10.1016/S0003-2670(02)01485-X
- Singh KP, Mohan D, Singh VK, et al. Studies on distribution and fractionation of heavy metals in Gomti river sediments-a tributary of the Ganges, India. J Hydrol. 2005;312:14–27.10.1016/j.jhydrol.2005.01.021
- Liu G, Zhou C, Kang Y, et al. Mobility, binding behavior and potential risks of trace metals in the sediments of the fifth largest freshwater lake, China. Water Sci Technol. 2013;67:2503–2510.10.2166/wst.2013.099
- Matong JM, Nyaba L, Nomngongo PN. Fractionation of trace elements in agricultural soils using ultrasound assisted sequential extraction prior to inductively coupled plasma mass spectrometric determination. Chemosphere. 2016;154:249–251.10.1016/j.chemosphere.2016.03.123
- Kalhori A, Jafari H, Yavari A, et al. Evaluation of anthropogenic impacts on soil and regolith materials based on BCR sequential extraction analysis, Assaluyeh. Int J Environ Res. 2012;6:185–194.
- Hakanson L. An ecological risk index for aquatic pollution control a sedimentological approach. Water Res. 1980;14:975–1001.10.1016/0043-1354(80)90143-8
- Nelson DW, Sommers LE. Total carbon, organic carbon and organic matter [part 2: methods of soil analysis]. Methods Soil Anal Part 3-Chem Methods. 1996;21:961–1010.
- Rhoades JD. Cation exchange capacity. Methods Soil Anal Part 3-Chem Methods. 1982;32:149–157.
- Tamburini E, Ferrari G, Marchetti MG, et al Development of FT-NIR models for the simultaneous estimation of chlorophyll and nitrogen content in fresh apple (Malus Domestica) leaves. Sensors. 2015;15:2662–2679.10.3390/s150202662
- Tang Q, Bao Y, He X. Sedimentation and associated trace metal enrichment in the riparian zone of the Three Gorges Reservoir, China. Sci Total Environ. 2014;479:258–266.10.1016/j.scitotenv.2014.01.122
- Ersahin S, Gunal H, Kutlu T, et al. Estimating specific surface area and cation exchange capacity in soils using fractal dimension of particle-size distribution. Geoderma. 2006;136(3):588–597.10.1016/j.geoderma.2006.04.014
- Zhu ZM, Lu LN, Yao XN, et al. Elemental backgrorund values of soils in China. Beijing: Environment Science Press of China; 1990.
- Tu JJ, Chen ZJ, Chen GJ, et al. A study on land consolidation and utilization of the water-level-fluctuating zone in the Three Gorges Reservoir – a case study of Kaixian county, Chongqing city. J Mountain Res. 2002;6:011.
- Lian J, Yao Y, Ma C, et al. Reservoir operation rules for controlling algal blooms in a tributary to the impoundment of Three Gorges dam. Water. 2014;6(10):3200–3223.10.3390/w6103200
- Tranvik LJ, Downing JA, Cotner JB. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol Oceanogr. 2009;54:2298–2314.10.4319/lo.2009.54.6_part_2.2298
- Weng L, Temminghoff EJM, Lofts S. Complexation with dissolved organic matter and solubility control of heavy metals in a sandy soil. Environ Sci Technol. 2002;36:4804–4810.10.1021/es0200084
- Reeder RJ. Crystal chemistry of the rhombohedral carbonates. Rev Mineral Geochem. 1983;11:1–47.
- Rodríguez L, Ruiz E, Alonso-Azcárate J. Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J Environ Manage. 2009;90:1106–1116.10.1016/j.jenvman.2008.04.007
- Florido MC, Madrid F, Ajmone-Marsan F. Variations of metal availability and bio-accessibility in water-logged soils with various metal contents: in vitro experiments. Water Air Soil Pollut. 2011;217:149–156.10.1007/s11270-010-0575-x
- Guo SH, Liu ZL, Li QS. Leaching heavy metals from the surface soil of reclaimed tidal flat by alternating seawater inundation and air drying. Chemosphere. 2016;157:262–270.10.1016/j.chemosphere.2016.05.019
- Wang TJ, Yang QW, Jin P. Chemical fraction composition characteristics of heavy metals in sediments of water-level-fluctuating zone of Three Gorges Reservoir area. J Environ Health. 2012;29:905–909.
- Huang JH, Fang Y, Zeng GM, et al. Influence of pH on heavy metal speciation and removal from wastewater using micellar enhanced ultrafiltration. Chemosphere. 2016;173:199–206.
- Azouzi R, Charef A, Hamzaoui AH. Assessment of effect of pH, temperature and organic matter on zinc mobility in a hydromorphic soil. Environ Earth Sci. 2015;74:1–14.
- WangY X, Chen ML, Ding JW. Experiment research on the relationship of availability and fraction transformation of copper and zinc with pH. Sci Technol Eng. 2014;18:14–19.
- He H, Hong FF, Tao XX, et al. A study on soil basic characteristics, main microbial flora and typical metal fraction surrounding coal gangue dump in Xiangtan Hunan Province, south of China. Environ Earth Sci. 2016;75:1–9.
- Zeng F, Ali S, Zhang H, et al. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ Pollut. 2011;159:84–91.10.1016/j.envpol.2010.09.019
- Calmano W, Hong J, Förstner U. Binding and mobilization of heavy metals in contaminated sediments affected by pH and redox potential. Water Sci Technol. 1993;28:223–235.
- Hernandez-Soriano MC, Jimenez-Lopez JC. Effects of soil water content and organic matter addition on the speciation and bioavailability of heavy metals. Sci Total Environ. 2012;423:55–58.10.1016/j.scitotenv.2012.02.033
- Chaturvedi PK, Seth CS, Misra V. Sorption kinetics and leachability of heavy metal from the contaminated soil amended with immobilizing agent (humus soil and hydroxyapatite). Chemosphere. 2006;64:1109–1114.10.1016/j.chemosphere.2005.11.077
- García C, Moreno JL, Hernández T, et al. Effect of composting on sewage sludges contaminated with heavy metals. Biores Technol. 1995;53:13–19.10.1016/0960-8524(95)00040-L
- Peruzzi E, Masciandaro G, Macci C. Heavy metal fractionation and organic matter stabilization in sewage sludge treatment wetlands. Ecol Eng. 2011;37:771–778.10.1016/j.ecoleng.2010.05.009
- Bo Li, Qing CL, Zhou ZB, et al. Effects of nitrogen, phosphorus and organic matter on heavy metal behavior in soils and its application of controlling pollution. Agro-Environ Prot. 2000;6:34–39.
- Holbach A, Norra S, Wang L, et al. Three Gorges Reservoir: density pump amplification of pollutant transport into tributaries. Environ Sci Technol. 2014;48:7798–7806.10.1021/es501132k
- Zhu K, Bi Y, Hu Z. Responses of phytoplankton functional groups to the hydrologic regime in the Daning River, a tributary of Three Gorges Reservoir, China. Sci Total Environ. 2013;450:169–177.10.1016/j.scitotenv.2013.01.101
- Lin JJ, Yu ZG. Ecological risk caused in soil by heavy metals in the water-level-fluctuating zone of a Yangtze River tributary. Environ Eng Manage J. 2014;13:923–928.
- Chen CX, Xia J, Zhan YZ, et al. Speciation distribution and potential ecological risk assessment of heavy metals in sediments of Taihu Lake, China. Environ Sci. 2011;31:1842–1848.
- Kashem M, Singh B. Transformations in solid phase species of metals as affected by flooding and organic matter. Commun Soil Sci Plant Anal. 2004;35:1435–1456.10.1081/CSS-120037556
- Mohamed I, Ahamadou B, Li M, et al. Fractionation of copper and cadmium and their binding with soil organic matter in a contaminated soil amended with organic materials. J Soils Sediments. 2010;10:973–982.10.1007/s11368-010-0199-1