Abstract
Background: To date, autologous punch grafting appears to be the easiest and least expensive surgical technique for stable vitiligo and piebaldism. Punch grafting is available worldwide, with no need for specialised instruments. However, no reliable data on efficacy and safety of different punch depths and punch sizes are available.
Objective/methods: To compare the efficacy and safety of different punch depths and punch sizes in autologous punch grafting, a randomised controlled trial was performed in 33 patients with vitiligo or piebaldism. In each patient, four depigmented regions were allocated to: 1.5 mm deep grafts, 1.5 mm superficial grafts, 1.0 mm deep grafts, and 1.0 mm superficial grafts. Primary outcome was the total pigmented surface area. Secondary outcomes were Patients’ Global Assessment (PGA) and side effects.
Results: Six months after grafting, 1.5 mm grafts showed a significantly larger pigmented surface area compared to 1.0-mm grafts (p < 0.001), though more side effects as well. No significant differences in the total pigmented surface between different punch depths were found. Deep grafts showed more erythema compared to superficial grafts.
Conclusion: We recommend 1.5 mm superficial grafts in autologous punch grafting for trunk and proximal extremities in patients with stable vitiligo and piebaldism.
Introduction
In piebaldism and stable vitiligo unresponsive to other therapies, autologous melanocyte transplantation is the treatment of choice (Citation1,Citation2). Available techniques are split-thickness grafting, epidermal blister grafting, cultured or non-cultured melanocyte (epidermal suspension) grafting and punch grafting (Citation3,Citation4). The punch grafting technique is a safe, simple and cheap technique, and therefore widely available and much used (Citation3–7). In punch grafting, grafts are punched out from normally pigmented donor sites and are transplanted into depigmented recipient sites from which similar punch grafts have been removed previously (Citation8). Large grafts (>2 mm) have high risks on side effects like cobblestone formation (Citation3,Citation4,Citation7,Citation9). Hence, the current technique involves 1- to 1.5-mm superficial punch grafts, depending on anatomical location (Citation10). Superficial punch grafts are the method of choice, since it is suggested that in thinner grafts, the superficial capillary network allows an earlier process of vascularisation, preventing side effects and the loss of grafts (Citation11,Citation12).
The current technique of grafting is poorly standardised and varies based on personal experience (Citation4–6). In the past decade, clinical trials focused mainly on complex and expensive techniques, while punch grafting was neglected.
Consequently, little reliable data on the efficacy and safety of different punch depths and sizes are available. In this randomised controlled trial, we explored the differences between the currently used methods for punch grafting in vitiligo and piebaldism. The aim of this study was to compare the efficacy as well as safety of two different punch depths and sizes. The outcome of these comparisons refines this technique and enables clinicians to choose the most effective punch size and depth.
Patients and methods
Patients
From June 2011 to January 2012, consecutive patients with segmental vitiligo, non-segmental stable vitiligo or piebaldism were evaluated for study eligibility and recruited from the outpatient clinic of the Department of Dermatology and the Netherlands Institute for Pigment Disorders of the Academic Medical Centre (AMC) Amsterdam, The Netherlands.
Eligible for study participation were patients aged between 18 and 60, Fitzpatrick skin types II–VI, patients with segmental and non-segmental stable vitiligo or piebaldism with lesions on the trunk or proximal extremities larger than 5 × 5 cm and willingness and ability to comply with the study protocol. Stable vitiligo was defined by the absence of new lesions and absence of progression of existing lesions [Vitiligo Disease Activity (VIDA) score 0 and −1] for at least 12 months (Citation13). Additionally, stability was confirmed by a punch grafting test in non-segmental vitiligo (Citation14). We excluded patients who received UV-therapy or systemic immunosuppressive treatment during the last 12 months or topical treatment during the last 6 months, lesions with follicular or non-follicular repigmentations in the treatment area, patients with a history of hypertrophic scars and/or keloid, hypersensitivity to (UV) light, allergy to local anaesthetics, atypical nevi and/or melanoma skin cancer. Prior to study start, verbal and written information regarding risks and benefits was given, and written informed consent was obtained from all study participants. The study was approved by the Medical Ethical Committee of the AMC, University of Amsterdam (METC 2011_091#C2011105) and registered on http://clinicaltrials.gov (NCT01377077).
Intervention
The study was a prospective randomised controlled trial. In each patient, four depigmented lesions on the trunk or proximal extremities were randomly allocated to receive four punch grafts per lesion according to the following treatment regimens: (1) 1.5 mm deep punch grafts, (2) 1.5 mm superficial punch grafts, (3)1 mm deep punch grafts and (4) 1 mm superficial punch grafts. Follow-up was 6 months after treatment.
Deep grafts were taken up to the subcutis, and superficial grafts were taken as superficial as possible in the upper dermis.
Before treatment, the skin was prepared with chlorhexidine solution and anaesthetised by infiltration. In each treatment region of the recipient site, four punch grafts were punched out. Matched punch grafts of the donor site localised on the hip were taken and directly placed into the prepared recipient site. The punch grafts at the donor site were taken as close to each other as possible in order to reduce the size of an impending scar. After grafting, steri-strips™ (3M Health Care, St. Paul, MN) and Tegaderm™ (3M Health Care, St. Paul, MN) dressings were applied on the recipient site for five days. The donor site received a wound regimen of silversulfadiazine cream, sterile gauzes and Tegaderm™ dressings. All grafting procedures were executed by the same two physicians (C.V, L.K).
Five days after the transplantation, UV-treatment (UVA facial tanner, 924T, Eurosolar, the Netherlands) was started at home, two times a week, and continued until 3 months after the procedure. According to previous studies, the exposure-time increased from 4–8 to 12 minutes in the first three sessions (Citation15). From the fourth session onwards, exposure-time remained 14 minutes (Citation15). If patients possessed another facial tanner or NB-UVB device, these were used because of practical and financial reasons.
Outcomes
Total pigmented surface area
Six months after grafting, the diameter of each graft was measured with a ruler by a physician blinded for treatment allocation. Subsequently, the pigmented surface area was calculated using the following formula: pigmented surface area = π*0.5 diameter ×*0.5 diameter Y (X = horizontal diameter, Y = vertical diameter). The total pigmented surface area of one treatment regimen was calculated by the sum of the pigmented surface areas of the four punch grafts in that treatment regimen. The grafts that had not shown pigment outgrowth were taken into account as zero.
Patient’s global assessment
Patients were blinded for treatment allocation and asked to indicate the general outcome (satisfaction) per treatment regimen for both recipient and donor site on a categorical scale (very poor, poor, neutral, good and very good) 6 months after grafting.
Side effects
Assessment of the side effects was performed per treatment regimen using a categorical scale of 0–3 (0 = absent, 1 = mild, 2 = moderate and 3 = severe). The recipient site was scored for hyperpigmentation, hypopigmentation, scarring and cobblestone effect, and the donor site was scored for hyperpigmentation, hypopigmentation, scarring, texture and erythema.
Randomisation and concealment of allocation
For randomisation, we used a digitally created random list generated by an independent person (Graph Pad Software Inc. La Jolla, CA). Opaque-sealed envelopes containing cards indicating the allocation were opened in ascending order at inclusion. The allocation was concealed from the assessor throughout the study and only revealed to the physicians who performed the grafting treatments (CV, LK).
Statistical methods
Statistical analyses were performed using IBM SPSS Statistics 20 software (Armonk, NY). A standard deviation of 0.4 (40% differences of repigmentation) was estimated with an estimated true difference in the mean response of 0.4 (40%) for punch depth and 0.2 (20%) for punch size. With a probability (power) of 0.8 and α of 0.05, the analysis showed that 33 subjects were needed. As the data did not fit a normal distribution, non-parametric analyses were performed. The Friedman test and the Wilcoxon signed-rank test were used to compare the pigmented surface areas of the different treatment regions. The Bonferroni method was used to adjust the significance level for multiple testing. The significance level was set at p < 0.05. Side effects were described per complaint. To test for differences in side effects, data were analysed by a Wilcoxon test to compare test regions in pairs.
Results
Participants
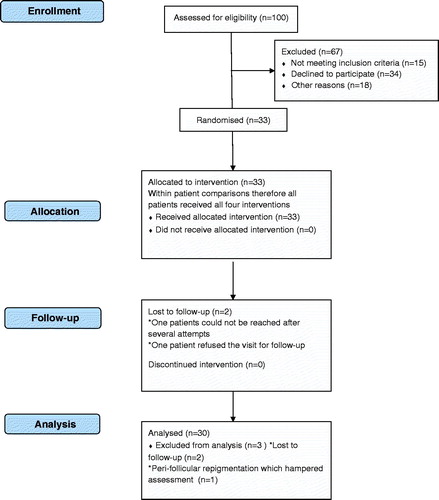
Between June 2011 and January 2012, 33 consecutive patients with stable non-segmental and segmental vitiligo, or piebaldism were included in this study. Study close out took place in July 2012, when the last patient was assessed after 6 months follow-up. Three non-segmental vitiligo patients were excluded from the study analysis (. Two were lost to follow-up after the grafting procedure and one was excluded because of perifollicular repigmentation, which hampered retrieving and measuring the grafts.
Thirty patients were analysed for primary and secondary outcomes. An exception was the PGA that was analysed for 25 patients, since 5 patients were not able to assess their own skin. For the donor site, the PGA was analysed in 28 patients.
Baseline characteristics of the included patients are shown in . The study population had a mean age of 35.8 years with skin type II–VI and equally distributed sexes. Almost half of the population had piebaldism (46%).
Table 1. Baseline characteristics of study population.
Outcomes
Total pigmented surface area
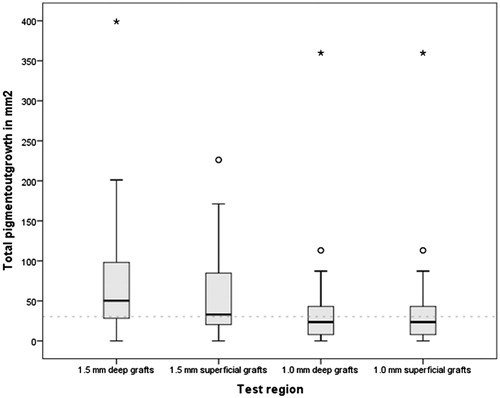
The median total pigmented surface area of 4 grafts per test region was 50.3 mm2, 33.0 mm2, 29.1 mm2 and 23.6 mm2 in 1.5 mm deep, 1.5-mm superficial, 1 mm superficial and 1 mm deep grafts, respectively (. An overall significant difference between the different test regions was found (p < 0.001). Between punch sizes, significant differences were found between the 1.5 mm deep and both 1.0 mm deep (p < 0.001) and 1.0 mm superficial grafts (p < 0.001) and between the 1.5 mm superficial and both 1.0 mm superficial (p < 0.02) and 1.0 mm deep grafts (p < 0.03). No significant differences between punch depths were found, i.e. 1.5 mm deep versus 1.5 mm superficial grafts, and 1.0 mm deep versus 1.0 mm superficial grafts showed no significant differences. More grafts were lost in the group of 1.0 mm [6.9% (deep), 5.8% (superficial)] compared to 1.5 mm grafts [3.3% (deep) and 4.2% (superficial)].
Figure 2. Total pigmented surface area of 4 grafts per treatment region. Data represent median; IQR (50.3 mm2, 33.0 mm2, 29.1 mm2 and 23.6 mm2 in respectively 1.5 mm deep grafts, 1.5 mm superficial grafts, 1 mm superficial grafts and 1 mm deep grafts.) p < 0.001, calculated with the related samples of Friedman’s two-way analysis of variance by ranks. N = 30.
The different treatment indications showed a median total pigment surface area for the 1.5 mm deep grafts of 22.76 mm2, 84.82 mm2 and 50.27 mm2 in non-segmental vitiligo, segmental vitiligo and piebaldism patients, respectively.
Patient’s global assessment
shows the general outcome (satisfaction) of both donor and recipient site assessed by the patient on a categorical scale per test region. For the donor site, the majority [22–26 patients (78.1–92.9%)] of the patients reported a good or very good outcome for all test regions, a poor outcome was only reported for the 1.5 mm deep grafts by one patient (3.6%), and no very poor outcome was scored. For the recipient site, the majority also reported good or very good outcomes. A very poor outcome was scored by three patients (12%) for all treatment regimens.
Table 2. PGA of the general outcome of both donor and recipient sites 6 months after grafting.
Side effects
shows the side effects in both donor and recipient sites. For the donor site, the 1.5 mm deep grafts showed significantly more hypopigmentation compared to 1.0 mm superficial grafts (p = 0.01) and more erythema compared to 1.5 mm superficial, 1.0 mm deep and 1.0 mm superficial grafts (p < 0.01; p = 0.01; p < 0.01, respectively). At the recipient site, the 1.5 mm deep grafts showed significantly more cobblestone formation compared to the 1.0 mm superficial grafts (p = 0.03). The 1.5 mm superficial grafts showed significantly more hypopigmentation at the donor site (p = 0.01) and more cobblestone formation at the recipient site (p = 0.05) compared to the 1.0 mm superficial grafts. No significant differences were found between 1.5 mm superficial and 1.0 mm deep grafts. The physicians experienced that the 1.5 mm superficial grafts were easier to harvest and to transplant than the 1.0 mm and deep grafts.
Table 3. Side effects in the donor and recipient sites.
Discussion
Punch grafting transplantation is a worldwide available, less expensive and easier-to-use technique than other autologous grafting techniques in vitiligo. Moreover, it requires no specific devices. In the last decade, more elaborate techniques have dominated the literature. However, a recent comparative study of the mini-punch grafting and hair follicle transplantation by Mapar and colleagues concluded that punch grafting shows the same treatment results and is much easier than follicular transplantation (Citation16). Therefore, punch grafting was recommended for drug-resistant vitiligo. However, there is a lack of evidence on the punch sizes and depths that should be used. Our study shows significant differences in both efficacy and safety for different punch sizes but not for different punch depths. Treatment regimens with 1.5 mm grafts showed to have not only significantly larger pigmented surfaces compared to 1.0 mm grafts, but also more side effects for both donor and recipient sites. Patients reported a good or very good general outcome for all treatment regimens.
The 1.5 mm deep grafts induced the largest pigmented surface area. A possible explanation could be that a larger circumference and thus a larger border promote horizontal migration of melanocytes and transfer of melanin to the surrounding keratinocytes (Citation17). Moreover, fewer grafts were lost in the 1.5-mm regimens, probably because they attached better at the recipient site and were easier to transplant compared to 1.0 mm grafts.
Large grafts showed to have more side effects compared to smaller grafts. Graft depth had less influence. Although a direct comparison has not been performed before, our results are in line with previous studies (Citation3). Cobblestone formation is more often seen in large grafts (Citation17,Citation18). Good matching of grafts and recipient sites was suggested to reduce the risk of side effects like cobblestone formation (Citation6,Citation7).
Earlier published punch graft studies reported a maximum diameter of pigment spread of 6.5 mm which is markedly larger than in our study (Citation12,Citation16). Explanations could be a longer follow-up period (up to 18 months), a longer period of UV-treatment, a different population with darker skin types, younger age and different anatomical locations of the recipient sites (i.e. face) (Citation7). For the different treatment indications, we found the poorest repigmentation in non-segmental vitiligo patients and the best repigmentation in segmental vitiligo patients. It is a remarkable observation that despite the positive punch grafting test and despite survival of pigmented cell in the grafts in non-segmental vitiligo, the outgrowth is less than in segmental vitiligo and piebaldism. The patients with non-segmental vitiligo probably still have (sub)clinical disease activity that inhibits melanocyte proliferation and/or migration.
Limitations of the study
First, the non-standardised way of harvesting the grafts, although performed by the same two physicians in all patients, may have introduced unwanted variation. There was also a lack of standardisation of UV irradiation, and different types of vitiligo and piebaldism patients were enroled. On the other hand, the included indications and the UV irradiation are according to good clinical practice and therefore reflects real practice. Furthermore, because the comparisons were within patients, the risk of influencing the outcome analyses is low. Another limitation which could have influenced the repigmentation was the follow-up of six months. This follow-up is commonly used in repigmentation studies; however, longer follow-up may yield more repigmentation.
Generalisability
As the grafting was performed in both sexes, different ages, different skin types and both stable vitiligo and piebaldism, the results indicate that a broad range of patients with small non-progressive depigmentation benefit from autologous grafting with 1.5 mm grafts. By enroling both (stable) vitiligo, segmental vitiligo and piebaldism patients we covered a broad spectrum of depigmented skin diseases. However, to minimise sun exposure as confounder we only included trunk and proximal extremities. Strictly speaking, our conclusions apply to these regions only. More sensitive regions like neck and chest may show more side effects, while the face may show better repigmentation. Especially the neck is known to be prone for cobblestone formation which urges for a technique with low risk of side effects, like small and superficial grafts. Note that punch grafting is most suitable for small depigmented lesions. For larger depigmented areas, other techniques like autologous epidermal suspension grafting seem to be more suitable.
Conclusion and implications
Larger grafts give more absolute pigment outgrowth, though more risk for hypopigmentation and cobblestone formation. Larger grafts are easier and faster to transplant and show less loss of grafts. Superficial grafts show less erythema and are less invasive compared to deep grafts. Therefore, we recommend 1.5 mm superficial grafts in autologous punch grafting for trunk and proximal extremities in stable vitiligo and piebaldism patients.
Acknowledgements
We thank Emma Westerduin for her help in the randomisation
The study was approved by the Medical Ethical Committee of the AMC, University of Amsterdam (METC 2011_091#C2011105) and registered on http://clinicaltrials.gov (NCT01377077)
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473–91.
- Falabella R, Barona M, Escobar C, et al. Surgical combination therapy for vitiligo and piebaldism. Dermatol Surg. 1995;21:852–7.
- Njoo MD, Westerhof W, Bos JD, et al. A systematic review of autologous transplantation methods in vitiligo. Arch Dermatol. 1998;134:1543–9.
- Mulekar SV, Isedeh P. Surgical interventions for vitiligo: an evidence-based review. Br J Dermatol. 2013;169:57–66.
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;CD003263. DOI: 10.1002/14651858.CD003263.pub4.
- Van Geel N, Ongenae K, Naeyaert JM. Surgical techniques for vitiligo: a review. Dermatology 2001;202:162–6.
- Fongers A, Wolkerstorfer A, Nieuweboer-Krobotova L, et al. Long-term results of 2-mm punch grafting in patients with vitiligo vulgaris and segmental vitiligo: effect of disease activity. Br J Dermatol. 2009;161:1105–11.
- Falabella R. Repigmentation of stable leukoderma by autologous minigrafting. J Dermatol Surg Oncol. 1986;12:172–9.
- Falabella R. Surgical treatment of vitiligo: why, when and how. J Eur Acad Dermatol Venereol. 2003;17:518–20.
- Falabella R, Barona MI. Update on skin repigmentation therapies in vitiligo. Pigment Cell Melanoma Res. 2009;22:42–65.
- Lahiri K. Evolution and evaluation of autologous mini punch grafting in vitiligo. Indian J Dermatol. 2009;54:159–67.
- Lahiri K, Malakar S, Sarma N, et al. Repigmentation of vitiligo with punch grafting and narrow-band UV-B (311 nm)-a prospective study . Int J Dermatol. 2006;45:649–55.
- Njoo MD, Das PK, Bos JD, et al. Association of the Köbner phenomenon with disease activity and therapeutic responsiveness in vitiligo vulgaris. Arch Dermatol. 1999;135:407–13.
- Falabella R. The minigrafting test for vitiligo: validation of a predicting tool. J Am Acad Dermatol. 2004;51:672–3.
- Wind BS, Meesters AA, Kroon MW, et al. Punchgraft testing in vitiligo; effects of UVA, NB-UVB and 632.8 nm Helium-Neon laser on the outcome. J Eur Acad Dermatol Venereol. 2011;25:1236–7.
- Mapar MA, Safarpour M, Mapar M, et al. A comparative study of the mini-punch grafting and hair follicle transplantation in the treatment of refractory and stable vitiligo. J Am Acad Dermatol. 2014;70:743–7.
- Kovacs D, Abdel-Raouf H, Al-Khayyat M, et al. Vitiligo: characterization of melanocytes in repigmented skin after punch grafting. J Eur Acad Dermatol Venereol. 2015;29:581–90.
- Majid I. Grafting in vitiligo: how to get better results and how to avoid complications. J Cutan Aesthet Surg. 2013;6:83–9.