Abstract
Atopic dermatitis (AD) is a very common chronic inflammatory skin disease requiring long-term treatment. Mycophenolic acid (MPA) is used off-label in treatment of patients with severe AD failing Cyclosporin A (CsA) treatment, however clinical efficacy is observed in only half of the AD patients. In blood, MPA levels are known to have a large interindividual variability. Low MPA exposure and increased enzyme activity correlates with the presence of UGT1A9 polymorphisms. In this retrospective study, 65 adult AD patients treated with MPA were classified as responder or non-responder to MPA treatment. UGT1A9 polymorphisms were determined using PCR. A significantly higher number of UGT1A9 polymorphisms was found in the group that did not respond to MPA treatment. Of the patients that carried a UGT1A9 polymorphism, 85.7% were non-responsive to MPA treatment. This implies that non-responsiveness in AD patients is more likely to occur in carriers of a UGT1A9 polymorphism. In a binary logistic regression analysis the odds ratio (OR) was 8.65 (95% confidence interval: 0.93–80.17). Our results show that UGT1A9 polymorphisms can be used to identify patients with non-responsiveness to MPA. Patients with UGT1A9 polymorphisms might benefit from higher MPA dosage.
Introduction
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases worldwide. The lifetime prevalence of AD is estimated between 15–30% in children and 2–10% in adults (Citation1,Citation2). AD is characterized by intense itching and follows a relapsing and remitting course (Citation3). The pathogenesis of AD is multifactorial and involves genetic, immunologic and environmental factors (Citation4). In the management of AD, stabilization of the disease by prevention of exacerbations is the major treatment goal; therefore long-term treatment is often indicated.
Although the majority of the AD patients can be adequately treated with topical treatment and/or UV-light therapy, there is a large group of patients in whom oral immunosuppressive drugs are indicated. Various immunosuppressive drugs are used in AD, including Cyclosporin A (CsA), mycophenolic acid (MPA), methotrexate, azathioprine and oral corticosteroids (Citation5). In many countries, CsA is the only registered oral immunosuppressive drug for AD and therefore often first choice of treatment in severe AD (Citation5).
MPA is used off-label in patients with severe AD who have failed CsA treatment (Citation6). MPA inhibits the de novo purine synthesis by arresting the cell cycle at the G0/G1 to the S transition phase (Citation7,Citation8), which results in selective inhibition of cell proliferation of B- and T cells (Citation6). Since MPA does not influence the survival of activated B and T cells, it has a delayed clinical response of approximately two to three months (Citation9). The efficacy of MPA in AD has been proven in clinical studies (Citation6,Citation9). However, in clinical practice, MPA is ineffective in nearly half of the AD patients (Citation10,Citation11). To date, response to treatment with MPA has been very difficult to predict. Delayed clinical response and the difficulties to adequately predict MPA response may result in the fact that some patients are treated with MPA for several months without any clinical benefit.
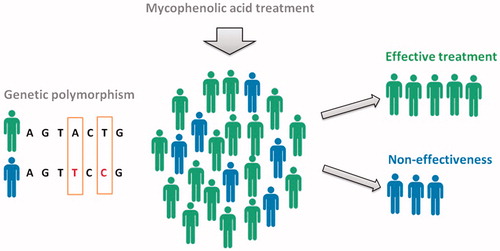
In blood, MPA levels are known to have a large interindividual variability. This has been observed in kidney transplant recipients, in whom a lower level of MPA exposure is closely associated with lower efficacy of drug therapy and acute rejection of the transplanted organ (Citation12,Citation13). Low MPA exposure and increased enzyme activity of the metabolizing enzyme uridine diphosphate-glucuronosyltransferase 1A9 (UGT1A9) correlate to the presence of single nucleotide polymorphisms (SNPs) in the gene promotor region (275T > A and/or 2152C > T) (Citation12,Citation13). MPA is predominantly metabolized by the enzyme UGT1A9 to mainly the inactive phenolic-glucuronide metabolite. Therefore, increased UGT1A9 activity due to SNPs would result in lower MPA exposure in serum. We hypothesized that also for AD patients, low MPA exposure due to the presence of UGT1A9 polymorphisms might contribute to the inefficacy during MPA treatment. This would enable prediction of non-response based on UGT1A9 polymorphisms in the future ().
Figure 1. Pharmacogenomics in AD patients treated with MPA. Low MPA exposure and increased enzyme activity correlate to the presence of UGT1A9 polymorphisms. It is likely that these UGT1A9 polymorphisms occur in AD patients treated with MPA. We hypothesized that low MPA exposure due to the presence of UGT1A9 polymorphisms might contribute to the inefficacy during MPA treatment. This would enable prediction of non-response based on UGT1A9 polymorphisms in the future.
In this study, we evaluated the difference in frequency of UGT1A9 polymorphisms between responders and non-responders in AD patients treated with MPA, and found a positive association between the presence of the polymorphisms and non-responsiveness.
Materials and methods
Study population
In a retrospective cohort study, 65 patients with severe AD treated with MPA 1440 mg/day at the University Medical Center Utrecht between January 1st 2004 and January 31st 2016 were included. Data were collected on March 15th 2016. Patients were diagnosed with AD according to the criteria of Hanifin and Rajka (Citation14).
The response to MPA treatment was assessed based on a 6-point Investigators’ Global Assessment (IGA) (Citation15). Patients were classified as responders in case the IGA decreased at least two points after a minimum of three months of treatment, and as non-responder in case the decrease in IGA was less than two points.
The study was approved by the Institutional Review Board of the University Medical Center Utrecht, adhering to the Declaration of Helsinki Principles.
Genotyping
The MagNA Pure LC system (Roche Diagnostics, Mannheim, Germany) was used to isolate genomic DNA was isolated from 1 ml EDTA serum. UGT1A9 genotyping was performed using Taqman allelic discrimination assays on an ABI prism 7000 sequence detection system (Applied Biosystems, Nieuwerkerk a/d IJssel, The Netherlands). For UGT1A9-275T > A polymorphism, PCR was performed in a volume of 12.5 μl, containing assay-specific primers, allele specific probes, TaqMan Universal PCR Master Mix, and genomic DNA (12.5 ng). Genotypes were scored by measuring allelic-specific fluorescence using SDS 1.2.3 software (Applied Biosystems, Nieuwerkerk a/d IJssel, The Netherlands). For UGT1A9–2152C > T polymorphism, genomic DNA (12.5 ng) was amplified in a volume of 50 μl, PCR buffer II (Applied Biosystems, Nieuwerkerk a/d IJssel, The Netherlands), 1.75 mmol/l MgCl2, 0.2 mmol/l deoxynucleotide triphosphates (Roche Diagnostics, Mannheim, Germany), 1.25U AmpliTaq Gold (PerkinElmer, Waltham, MA, US), and 40 pmol of each primer. PCR products were incubated with 10 U TruI for two hours. Fragments were separated by electrophoresis on a 3% agarose gel with ethidium bromide staining.
Statistical analysis
Statistical analysis was performed using SPSS (for Windows, version 21.0, SPSS Inc., Chicago, IL, US). Pearson’s Chi square test or Fisher exact test was used to compare categorical data between groups (e.g. responder and non-responder to MPA treatment). The strength of the association between UGT1A9–275/−2152 genotype and response to MPA treatment was calculated using binary logistic regression. Odds ratios (OR) were stated with 95% confidence intervals (95% CI). Probability levels of 0.05 and below were considered to indicate statistical significance.
Results
Out of 65 patients, 33 were classified as MPA responder and 32 were classified as MPA non-responder. Patient characteristics are shown in . The UGT1A9–275T > A and UGT1A9–2152C > T SNPs were found in seven patients as heterozygous, with all seven heterozygous patients having both SNPs. This prevalence is similar to those previously described in the Caucasian population (Citation12). The presence of UGT1A9 polymorphisms was significantly higher in the patient group that did not respond to MPA treatment compared to the group that did respond to MPA treatment (). One out of seven (14.28%) UGT1A9 polymorphism carriers was a responder, and six out of seven (85.71%) patients were non-responders to MPA treatment. This implies that non-responsiveness in AD patients is more likely to occur in carriers of a UGT1A9 polymorphism. In a binary logistic regression analysis, adjusting for age and gender, the OR was 8.65 (95% confidence interval: 0.93–80.17) (). Significance was not reached, probably due to the small number of patients.
Table 1. Baseline table of patient characteristics.
Table 2. Binary logistic regression analysis of non-response to MPA treatment, adjusting for age and gender.
Discussion
Pharmacogenomics are increasingly used in the in the management of transplantation patients, however data in chronic inflammatory diseases, such as AD are scarce.
To the best of our knowledge, this is the first study to evaluate the effect of UGT1A9 polymorphisms on MPA therapy responsiveness in AD. A significantly higher number of UGT1A9 polymorphisms was found in patients who did not respond to MPA treatment compared to patients who did respond to MPA treatment.
Since previous studies reported an association between the presence of UGT1A9 polymorphisms and low MPA exposure, the non-responsiveness to MPA in patients with UGT1A9 polymorphism is presumably due to low MPA exposure (Citation13). The therapeutic strategy in non-responsive UGT1A9 polymorphism carriers would have been to prescribe a higher dosage of MPA and thereby increasing MPA exposure.
Our findings are consistent with previous studies investigating UGT1A9 polymorphisms in renal transplant patients, showing a statistically significantly higher rate of acute rejection of the graft in UGT1A9 heterozygous renal transplant recipients (Citation13,Citation16).
Remarkably, one out of seven patients with UGT1A9 polymorphisms, did respond adequately to MPA treatment. A possible explanation is that this patient has an extra genetic polymorphism in UGT1A9, resulting in an overall decrease of UGT1A9 activity, which would increase MPA exposure in contrary to expectations based on the −275 SNP, as this is described before in other studies (Citation17,Citation18).
Treatment options in patients with severe AD are very limited at this moment. CsA is often the drug of first choice in these patients, however nearly half of the patients have to discontinue treatment due to side effects and/or inefficacy (Citation19). MPA is an interesting second choice treatment option in severe AD patients, as the side effect profile seems to be better than most other oral immunosuppressive drugs used in AD (Citation11,Citation20). However, previous studies have shown that around half of the AD patients do not respond to MPA (Citation10,Citation11). Prediction of treatment response to MPA in AD is very valuable, since treatment response can only be determined after three to four months of treatment.
In conclusion, this study found an association between UGT1A9 polymorphisms and non-responsiveness to MPA treatment in severe AD patients. In the non-responder group, six out of 32 patients carried a UGT1A9 polymorphism. This means that pretreatment screening for UGT1A9 polymorphisms could have identified 19% of the patients with non-responsiveness to MPA treatment. Although this is a relatively small percentage, it does show the potential benefits of pharmacogenetic screening for UGT1A9 in AD. Future research is needed to identify other SNPs and further optimize MPA treatment to ensure maximal efficacy with minimal side effects. Although pharmacogenomics are scarcely used in dermatological research, it enables “personalized medicine” by prescribing drugs based on the genetic makeup of an individual.
Disclosure statement
The authors declare no conflict of interest.
References
- Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–37.
- Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43.
- Garmhausen D, Hagemann T, Bieber T, et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy. 2013;68:498–506.
- Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974–82.
- Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol. 2012;26:1176–93.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327–49.
- Cohn RG, Mirkovich A, Dunlap B, et al. Mycophenolic acid increases apoptosis, lysosomes and lipid droplets in human lymphoid and monocytic cell lines. Transplantation. 1999;68:411–8.
- Laliberte J, Yee A, Xiong Y, Mitchell BS. Effects of guanine nucleotide depletion on cell cycle progression in human T lymphocytes. Blood. 1998;91:2896–904.
- Haeck IM, Knol MJ, Ten Berge O, et al. Enteric-coated mycophenolate sodium versus cyclosporin A as long-term treatment in adult patients with severe atopic dermatitis: a randomized controlled trial. J Am Acad Dermatol. 2011;64:1074–84.
- Garritsen FM, Roekevisch E, van der Schaft J, et al. Ten years experience with oral immunosuppressive treatment in adult patients with atopic dermatitis in two academic centers. J Eur Acad Dermatol Venereol. 2015;29:1905–12.
- van der Schaft J, Politiek K, van den Reek JM, et al. Drug survival for azathioprine and enteric-coated mycophenolate sodium in a long-term daily practice cohort of adult patients with atopic dermatitis. Br J Dermatol. 2016;175:199–202.
- Kuypers DR, Naesens M, Vermeire S, Vanrenterghem Y. The impact of uridine diphosphate-glucuronosyltransferase 1A9 (UGT1A9) gene promoter region single-nucleotide polymorphisms T-275A and C-2152T on early mycophenolic acid dose-interval exposure in de novo renal allograft recipients. Clin Pharmacol Ther. 2005;78:351–61.
- van Schaik RH, van Agteren M, de Fijter JW, et al. UGT1A9-275T > A/-2152C > T polymorphisms correlate with low MPA exposure and acute rejection in MMF/tacrolimus-treated kidney transplant patients. Clin Pharmacol Ther. 2009;86:319–27.
- Hanifin JM, Rajka G. Diagnostic features of atopic-dermatitis. Acta Dermatol –Venereol. 1980;92:44–7.
- Eichenfield LF, Lucky AW, Boguniewicz M, et al. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. J Am Acad Dermatol. 2002;46:495–504.
- Kuypers DR, de Jonge H, Naesens M, et al. Current target ranges of mycophenolic acid exposure and drug-related adverse events: a 5-year, open-label, prospective, clinical follow-up study in renal allograft recipients. Clin Therapeut. 2008;30:673–83.
- Levesque E, Delage R, Benoit-Biancamano MO, et al. The impact of UGT1A8, UGT1A9, and UGT2B7 genetic polymorphisms on the pharmacokinetic profile of mycophenolic acid after a single oral dose in healthy volunteers. Clin Pharmacol Ther. 2007;81:392–400.
- Fukuda T, Goebel J, Cox S, et al. UGT1A9, UGT2B7, and MRP2 genotypes can predict mycophenolic acid pharmacokinetic variability in pediatric kidney transplant recipients. Ther Drug Monitor. 2012;34:671–9.
- van der Schaft J, Politiek K, van den Reek JM, et al. Drug survival for ciclosporin A in a long-term daily practice cohort of adult patients with atopic dermatitis. Br J Dermatol. 2015;172:1621–7.
- Politiek K, van der Schaft J, Coenraads PJ, et al. Drug survival for methotrexate in a daily practice cohort of adult patients with severe atopic dermatitis. Br J Dermatol. 2016;174:201–3.
