Abstract
Approximately 70 years ago, the first topical dexpanthenol-containing formulation (Bepanthen™ Ointment) has been developed. Nowadays, various topical dexpanthenol preparations exist, tailored according to individual requirements. Topical dexpanthenol has emerged as frequently used formulation in the field of dermatology and skin care. Various studies confirmed dexpanthenol’s moisturizing and skin barrier enhancing potential. It prevents skin irritation, stimulates skin regeneration and promotes wound healing. Two main directions in the use of topical dexpanthenol-containing formulations have therefore been pursued: as skin moisturizer/skin barrier restorer and as facilitator of wound healing. This 70th anniversary paper reviews studies with topical dexpanthenol in skin conditions where it is most frequently used. Although discovered decades ago, the exact mechanisms of action of dexpanthenol have not been fully elucidated yet. With the adoption of new technologies, new light has been shed on dexpanthenol’s mode of action at the molecular level. It appears that dexpanthenol increases the mobility of stratum corneum molecular components which are important for barrier function and modulates the expression of genes important for wound healing. This review will update readers on recent advances in this field.
Introduction
Pantothenic acid, a member of the B complex vitamins (vitamin B5), was discovered in 1931 by Roger J. Williams (1893–1988) during his studies on microbial growth factors. The name pantothenic acid, given to this substance by Williams and Saunders in 1933, should indicate its wide spread occurrence in nature (Citation1,Citation2). In 1934, it was shown that pantothenic acid has a profound stimulating effect on cell proliferation in yeast (Citation3) which eventually led to the development of the first topical dexpanthenol preparation (Bepanthen™). Dexpanthenol is a stable alcoholic analog of pantothenic acid. Contrary to pantothenic acid, it is well absorbed through the skin (Citation4,Citation5). Bepanthen™ was first introduced as an ointment approximately 70 years ago (in 1944). When applied topically, dexpanthenol is readily absorbed and rapidly converted enzymatically to pantothenic acid, a constituent of coenzyme A (Citation6). Nowadays, different topical dexpanthenol preparations exist (cream, emollient, drops, gel, lotion, oil, ointment, solution and spray), tailored according to individual needs, ranging from pediatric to adult use. Dexpanthenol is also used as dissolving lozenge/pastille for mouth and throat irritations (Citation7). Coenzyme A catalyzes the synthesis of fatty acids and sphingolipids which are important for stratum corneum lipid layers (Citation7–10). Hence, pantothenic acid is essential for epithelia to maintain their physiological function (Citation11).
It has been shown that topical dexpanthenol acts like a moisturizer with barrier-improving properties; in addition, it exerts wound healing effects (e.g. Citation10,Citation12–17). Two main directions of its use have therefore been pursued: as skin moisturizer/skin barrier restorer and as facilitator of wound healing. It may be inferred that this molecule provides a dual action benefit for subjects in need for skin care and/or wound healing.
Based on a systematic literature search in the PubMed and Embase databases, this 70th anniversary paper reviews studies with topical dexpanthenol, with particular emphasis on its usefulness in different skin conditions. In addition, it will update readers on recent advances of dexpanthenol’s mechanism of action when used as a component of skin care products (Part I) and when used therapeutically (Part II). Double-blind controlled studies on topical dexpanthenol cited in Part I and Part II have been summarized in and , respectively.
Table 1. Double-blind controlled studies related to dexpanthenol’s use as skin care product.
Table 2. Double-blind controlled studies related to dexpanthenol’s topical use as facilitator of wound healing.
Part I – dexpanthenol as moisturizer withbarrier-improving properties
Impaired skin barrier plays a major role in various skin conditions like dry skin (as a condition itself), sensitive skin, seborrheic dermatitis, atopic dermatitis (AD) or contact dermatitis (Citation18,Citation19). Moisturization and restoration of the stratum corneum skin barrier are therefore important properties of any skin care product (Citation20). Dexpanthenol improves skin hydration when applied topically; this activity may be related to dexpanthenol’s hygroscopic properties and its capability to promote retention of moisture (Citation11,Citation20). The hydrating effect seems to be interrelated with its capacity to regenerate the epidermal barrier (Citation5). A recent experimental study with excised porcine skin showed that dexpanthenol increases the molecular mobility of several lipid and protein segments of the stratum corneum thereby generating properties of a hydrated skin also in dehydrated conditions. Specifically, it has been demonstrated that dexpanthenol interacts with lipid segments of the extracellular lamellae and protein residues in the corneocytes in stratum corneum and thus compensates for reduced hydration by retaining/increasing molecular fluidity (Citation21).
The mechanisms by which dexpanthenol restores and protects skin barrier function have not been fully elucidated. As the different layers of the skin undergo continual renewal, moisturizers provide an environment which promotes physiological processes (e.g. enzyme functioning) necessary for maintaining or restoring skin barrier function. In addition, topically applied substances may also penetrate deeper into the epidermis and interfere with the production of barrier lipids and the maturation of corneocytes (Citation20). In fact, it has been suggested that dexpanthenol promotes epidermal regeneration by enhancing epidermal differentiation and lipid synthesis (Citation19).
Role of topical dexpanthenol in atopic dermatitis and nappy rash
Atopic dermatitis
Deficient skin barrier function leading to increased transepidermal water loss (TEWL) and decreased stratum corneum hydration (dry skin) is a characteristic feature of AD (Citation22,Citation23). Clinical studies showed that the daily use of skin care products may prevent AD or can prolong the time between flare-ups (Citation24–26). Therefore, topical dexpanthenol has a clear role as skin care product during post-inflammatory AD maintenance stages due to its proven skin moisturization and skin barrier restoration potential.
In a double-blind, randomized controlled trial (RCT) in a total of 60 healthy subjects, topical dexpanthenol formulated in two different lipophilic vehicles at a concentration of 2.5% was administered to the skin. Twice-daily application over 7 d statistically significantly improved stratum corneum hydration and reduced TEWL compared with controls (Citation27). In the context of a randomized study in 20 healthy subjects, the same group evaluated a hydrophilic emulsion containing 2.5 or 5% dexpanthenol with respect to its effect on hydration and skin barrier repair using the repetitive washing test with sodium lauryl sulfate (SLS) (Citation28). Topical application of both dexpanthenol-containing emulsions statistically significantly improved stratum corneum hydration and skin barrier function compared with control. The effects were numerically more pronounced with the 5% dexpanthenol-containing emulsion. The results were in accordance with a similarly designed study which tested a 2.5% dexpanthenol-containing lotion in 20 healthy volunteers as to its effect on hydration and skin barrier repair using the repetitive washing test with SLS (Citation29).
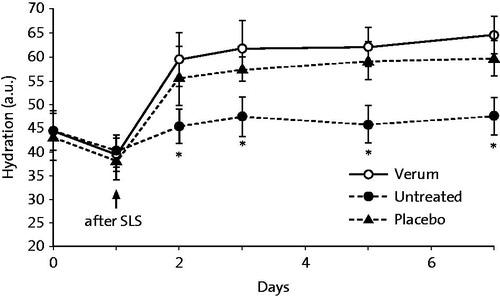
Proksch and Nissen (Citation10) showed that a 5% dexpanthenol-containing cream enhanced skin barrier repair and stratum corneum hydration in experimentally damaged human skin when applied twice-daily for 7 d as assessed by TEWL and corneometry, respectively ( and ).
Figure 1. Transepidermal water loss (TEWL) after SLS pretreatment and twice-daily application for 7 d of a dexpanthenol-containing preparation (cream), vehicle, or being left untreated. Permeability barrier disruption was induced by 5% SLS under occlusion by plastic chambers for 24 h. TEWL was measured by Tewameter. N = 20, *p < .05 (verum vs. vehicle). From Ref. (Citation10) with kind permission from Taylor & Francis Ltd. (http://www.tandfonline.com).
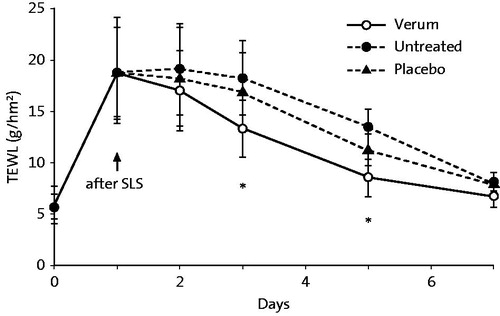
Figure 2. Stratum corneum hydration after SLS pretreatment and twice-daily application for 7 d of a dexpanthenol-containing preparation (cream), vehicle or being left untreated. Stratum corneum hydration was measured by Corneometer. N = 20, *p < .05 (verum vs. vehicle). a.u.=arbitrary units. From Ref. (Citation10) with kind permission from Taylor & Francis Ltd. (http://www.tandfonline.com).
Biro et al. (Citation30) conducted a double-blind RCT in 25 healthy subjects to investigate the efficacy of dexpanthenol in skin protection against irritation. The forearms of the subjects were treated with either an ointment containing 5% dexpanthenol or placebo twice-daily for 26 d. From days 15–22, SLS 2% was applied to these skin areas. Hydration of the stratum corneum remained essentially stable at the dexpanthenol-treated sites throughout the study, whereas corneometry results showed a decrease at the placebo-treated sites at the end of the SLS challenge period (p < .05). Moreover, topical dexpanthenol exhibited better protective effects against skin irritation than placebo. This observation was explained by dexpanthenol’s ability to preserve good hydration of the stratum corneum under the influence of an irritant agent, whereas placebo treatment failed to do so. The results were in agreement with an earlier single-blind study using an ointment in 35 female volunteers to investigate dexpanthenol’s protective effect against irritation induced by SLS (Citation31).
In a single-blind, placebo-controlled study, Camargo et al. (Citation32) evaluated the skin moisturizing efficacy of formulations containing 0.5, 1 or 5% dexpanthenol in a total of 40 healthy female subjects. Dexpanthenol-containing formulations (1.0 and 5.0%) produced significant decreases (p < .001) in TEWL after 15 and 30 d periods of daily application when compared with baseline and control site (vehicle only) values. Moreover, in skin washed with SLS, a significant reduction of TEWL was evident 2 h after application of formulations loaded with dexpanthenol.
Stettler et al. (Citation33) conducted two randomized, intra-individual comparison studies in a total of 43 healthy subjects to evaluate the skin moisturization and barrier restoration potential of a new topical dexpanthenol-containing emollient, and its effect on skin microflora. This skin care preparation has been developed for subjects with non-inflammatory dry and sensitive skin conditions, such as AD while in remission phase between flares. Study 1 showed that barrier restoration was more pronounced with the emollient as reflected by mean AUC for TEWL reduction from baseline (−168.36 vs. −123.38 g/m2/h, p = .023). Emollient use was also associated with statistically significant improvements in stratum corneum hydration and an increase in intercellular lipid lamellae length in the stratum corneum (increase from baseline on day 22: 120.61 vs. 35.85 nm/1000 nm2, p < .001). In study 2, the dexpanthenol emollient use had no negative impact on bacterial viability; a numerically higher proportion of subjects showed commensal bacteria.
In another set of two studies, Stettler et al. (Citation34) investigated the skin moisturizing effect of the dexpanthenol-containing emollient in healthy adults with dry skin and in healthy infants. Study 1 (N = 44) was a randomized, intra-individual comparison study while study 2 was a non-comparative trial (N = 65, age: 3–25 months). In study 1, application of emollient produced an increase in stratum corneum hydration as reflected by an enhanced electrical capacitance of the skin surface. In study 2, the dexpanthenol emollient was well tolerated by the infants and skin hydration significantly increased over the study course.
In studies with AD patients, the moisturizing and barrier-improving properties of topical dexpanthenol were well perceived by affected subjects (Citation35,Citation36).
Nappy rash
Nappy rash (irritant diaper dermatitis and napkin dermatitis) is a form of irritant contact dermatitis occurring in the diaper area, it is a common skin condition among infants (Citation37). The most crucial factor for developing nappy rash is prolonged skin contact with urine and feces which causes disruption of the skin barrier by maceration (softening and weakening of the stratum corneum with extraction of natural moisturizing factors), locally increased alkalinity leading to activation of fecal proteases and lipases, and friction (Citation38,Citation39). The key to successful nappy rash management is the implementation of preventive measures. It comprises gentle cleansing, immediate changing of diapers containing urine and feces, and regular application of a protective barrier preparation (Citation39–41). The application of barrier creams or ointments at each diaper change has been recommended as protective or preventive measure; they form a lipid film on the skin surface and protect it from contact with moisture and irritants (Citation39,Citation41). In fact, the use of barrier emollients at nappy changes is now standard practice (Citation42). In recognition of the increasing importance of the use of barrier skin care preparations as a measure in the prevention of nappy rash, an international expert panel of dermatologists and pediatricians agreed in 2012 on nine standards for the ideal nappy care preparation that is suitable for repeated application on the vulnerable skin of infants () (Citation39). The panel revised an earlier consensus published in 2004 (Citation43), increasing the previous seven standards to nine by adding the items “enhances natural protection of the skin” and “is pleasant to use”.
Table 3. The nine standards of an ideal topical nappy care preparationTable Footnotea.
Recently, a new dexpanthenol-containing water-in-oil ointment has been developed to be used topically in the diaper area of infants. Data from various in vitro and in vivo studies showed that this formulation meets the suggested standards for an ideal nappy care preparation (Citation44). In a double-blind RCT in 109 children predisposed to nappy rash (3–24 months), this formulation was equivalent to zinc oxide lipophilic paste in protecting infants form clinically relevant diaper rash when used for 28 d. According to pediatricians’ assessment, the overwhelming majority of subjects (about 96% for both dexpanthenol ointment and zinc oxide) were free of clinically relevant diaper rash during the study course (Citation44).
The usefulness of dexpanthenol-containing ointments in the management of nappy rash has also been shown by others (Citation45–48).
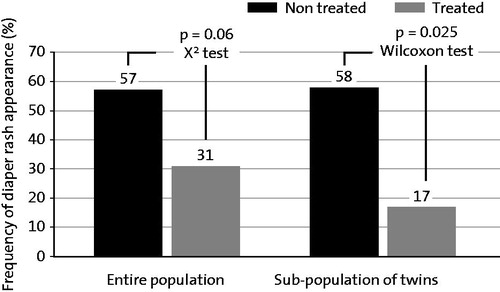
Putet et al. (Citation46) reported results from two clinical trials (on prevention and treatment) which showed that an ointment containing 5% dexpanthenol can help to manage nappy rash. The prevention study was an open, randomized trial in hospitalized newborns free from nappy rash at study inclusion with a double-blind evaluation of standardized photographs taken from treated skin areas at baseline and at study end (Citation45,Citation46). Subjects were randomized to receive either an ointment containing 5% dexpanthenol on the buttocks at each of the four diaper changes per day (N = 26) or no treatment (N = 28). The study population included 15 twins out of which 12 twins were allocated to different treatment groups. For both groups, the average study length was approximately 2 weeks. In the group treated with the ointment, 31% of subjects developed nappy rash over the study course compared with 57% of subjects in the untreated group. In the subpopulation of twins, the difference was even more pronounced ().
Figure 3. Frequency of nappy rash occurrence in newborns preventively treated with a dexpanthenol-containing ointment (5%) compared with untreated subjects. From Ref. (Citation46) with kind permission from Re´alite´s pe´diatriques.
Role of topical dexpanthenol in dermatologic conditions associated with diabetes
Proksch (Citation49) showed that two dexpanthenol-containing lotions suitable for skin care of diabetic feet and legs significantly improved skin hydration (as assessed by corneometry) when administered twice daily to diabetics with dry skin for 4 weeks. Both preparations were well accepted by study participants and improved subjective findings (tension, itching and dryness) in the treated areas. It was inferred that the tested preparations can help preventing foot infections in patients with diabetes mellitus.
Part II – dexpanthenol as facilitator of wound healing
The process of wound healing is often divided in three phases: inflammation, cell proliferation and matrix deposition. Tissue remodeling has an increased need for pantothenic acid because of the increased turnover of cells for wound repair (Citation14,Citation50,Citation51). It has been suggested that the reported beneficial effect of dexpanthenol on wound healing is the result of increased fibroblast proliferation and accelerated epithelialization (Citation17); both processes are important for the cure of both deep and superficial wounds (Citation7). This complies with in vitro findings gathered with dexpanthenol.
The dermal fibroblast possesses multilineage differentiation potential and plays a key role in tissue repair and thus cutaneous wound healing (Citation52,Citation53). The effects of dexpanthenol on human fibroblasts (e.g. enhanced proliferation, cellular migration, attachment of fibroblasts and collagen synthesis) have been shown in several in vitro studies (Citation14,Citation53–56). Although the beneficial influence of dexpanthenol on cell proliferation and wound healing has been well documented, its properties at the molecular level remained unclear for a long time. With the availability of novel molecular tools the situation changed.
Wiederholt et al. (Citation53) investigated the molecular mechanisms linked to the stimulatory effect of pantothenate on the proliferation of dermal fibroblasts in vitro. Gene expression was analyzed in human dermal fibroblasts cultivated with 20 μg/ml of pantothenate compared with untreated cells. Microarray analysis detected a significant upregulation of IL-6, IL-8, Id1, HMOX-1, HspB7 and CYP1B1 expression by pantothenate. As IL-6 and IL-8 are among the cytokines most strongly expressed during wound healing (Citation57), the upregulation of IL-6 and IL-8 expression in dermal fibroblasts may therefore contribute to the wound healing features of dexpanthenol-containing topicals (Citation53). To correlate these in vitro findings with the in vivo situation of wound healing, Heise et al. (Citation58) conducted a randomized, double-blind study in which the dexpanthenol-induced gene expression profile in punch biopsies of previously injured and dexpanthenol-treated skin in comparison to placebo-treated skin was analyzed at the molecular level. In samples treated topically with dexpanthenol, upregulation of IL-6, IL-1β, CYP1B1, CXCL1, CCL18 and KAP 4–2 gene expression was detected thereby suggesting a strong correlation between in vitro data assessed in cultured dermal fibroblasts and the in vivo situation. The favorable wound healing characteristics of dexpanthenol may therefore be mediated – at least in part – by a dexpanthenol-induced upregulation of the expression of genes (with enhanced mRNA levels) important for wound healing (Citation58). In a recent study, a novel human three-dimensional skin wound healing model was applied to investigate the dexpanthenol-mediated stimulatory effects on wound closure. Topical treatment of skin wounds with different dexpanthenol containing preparations clearly enhanced wound closure compared to untreated or vaseline-treated controls. Gene expression analysis showed increased mRNA expression of genes involved in wound healing (Citation16).
Role of topical dexpanthenol in the treatment of minor wounds
Models of superficial injury
Pugliese et al. (Citation59) conducted a double-blind RCT with a water-in-oil-emulsion containing 5% dexpanthenol in healthy adult volunteers. In all subjects, four standardized epidermal shave biopsy wounds were produced; three wounds were each treated daily for 5 d with either the dexpanthenol water-in-oil emulsion, a corresponding water-in-oil emulsion without dexpanthenol, or a first aid cream. The fourth wound remained untreated and served as additional control. Erythema, wound closure, wound volume and viscoelasticity were assessed using ultrasound and histological techniques. Epidermal wounds treated with the dexpanthenol emulsion showed a reduction in erythema and a more elastic and solid tissue regeneration. Histologically, in 10 of 15 subjects, the dexpanthenol preparation was most effective in improving the wound healing process.
Wollina and Kubicki (Citation15) investigated the potential of topical dexpanthenol to improve epidermal regeneration and wound healing by employing different in vivo models of minor skin trauma, including cryosurgery and suction blistering to induce superficial subepidermal wounds. The reduction of wound area was measured by TEWL. A faster healing was observed upon treatment with dexpanthenol compared with vehicle control.
Hartel et al. (Citation60) performed a RCT in 12 healthy subjects to explore the wound healing effect of water-filtered infrared-A using an acute wound model (superficial wounds generated by suction cup technique). The formation of the stratum corneum was measured by laser scanning microscopy. Four different treatments were administered over 10 d, including no therapy. The fastest stratum corneum formation was observed when water-filtered infrared-A irradiation was combined with the daily use of a dexpanthenol cream.
Cracked nipples
Cracked nipples may occur during the breastfeeding period. It is a painful condition and can lead to early cessation of breastfeeding despite the mothers wish to continue breastfeeding (Citation61). Dexpanthenol-containing ointments have a long history in nipple care during breastfeeding, particularly in the treatment of nipple cracks and fissures (Citation62,Citation63).
Dubecq and Detchart (Citation64) conducted a double-blind RCT in 60 nursing mothers with nipple fissures. Study participants were treated with an ointment containing 5% dexpanthenol or with placebo (vehicle only) for 1 week under hospitalized conditions. In three additional groups, the prophylactic effect of the dexpanthenol ointment was studied when administered for 1 week. These breastfeeding women received either the ointment (N = 25), vehicle only (N = 28), or were left untreated (N = 80). The proportion of subjects who experienced healing of their nipple fissures was significantly higher in the active group compared with placebo (68 vs. 34%, p < .05). In the prophylaxis study, 92% of subjects in the active group remained free from nipple fissures compared with 82% in the vehicle group, and 77.5% in the untreated group.
In the context of a single-blind RCT in 66 primiparous mothers with sore nipples, it was shown that a dexpanthenol-containing ointment had favorable effects on nipple pain when administered four times daily for 2 weeks. Over the entire study period, mean pain scores were lower with the ointment compared with control (only warm water and soap). On day 3, the difference in mean ± SEM pain scores reached statistical significance (1.27 ± 0.12 vs. 3.50 ± 0.14, p < .05) (Citation65). Similarly, Shanazi et al. demonstrated that topical dexpanthenol (5%) significantly reduced nipple pain compared with baseline in breastfeeding mothers suffering from traumatic nipples. In the group treated with dexpanthenol cream for 2 weeks (N = 42), the mean ± SD pain score on day 3 was 1.33 ± 0.65 compared with 3.07 ± 1.02 at baseline (p < .001). The improvement in nipple pain was associated with significantly reduced nipple trauma scores (Citation66).
Based on the results from a small observational study, Staubach et al. suggested that daily application of an ointment containing 5% dexpanthenol has the potential to alleviate symptoms associated with mammillary eczema (Citation67).
Postoperative use
A double-blind intra-individual comparative study investigated the wound healing properties of a 5% dexpanthenol-containing ointment in 35 patients undergoing skin grafting due to burns (Citation68). Mesh graft donor sites were treated with either the dexpanthenol ointment or vehicle for 14 d. An untreated area of healthy skin served as additional control. The skin area treated with the dexpanthenol ointment tended to return to a normal status sooner than with vehicle alone. From the 7th day onwards, the skin was more hydrated than the vehicle-treated area (p = .05) as assessed by clinical scores. In addition, the healing-associated pruritus ceased earlier (p = .06).
In a double-blind RCT, the potential of a dexpanthenol pastille containing 100 mg dexpanthenol was evaluated to alleviate pain and to improve wound healing following tonsillectomy (Citation69). In total, 120 pediatric patients were randomized to one of four groups (N = 30 each): surgical tonsillectomy technique 1 plus dexpanthenol pastille or placebo, or surgical tonsillectomy technique 2 plus dexpanthenol pastille or placebo. Study medication was administered three times a day. Postoperative throat pain and mucosal healing patterns were assessed at regular intervals over 2 weeks. Pain was assessed by visual analog scale and mucosal healing by the size of the post-tonsillectomy slough. Regardless of surgical technique, post-tonsillectomy throat pain was significantly less in the dexpanthenol groups than in the placebo groups (p < .05), and tonsillar wound healing was significantly better in the dexpanthenol groups than in the placebo groups (p < .05).
Role of topical dexpanthenol in scar management
Favorable effects of topical dexpanthenol preparations were also published in the daily care of scars resulting from skin transplantation and burns (Citation70,Citation71). This triggered the development of specific topical dexpanthenol formulations which also contain silicone. Topical silicone treatments have been recommended as first line therapy for prevention and treatment of red and hypertrophic scars (Citation72,Citation73). Recently, an international expert panel agreed that four groups would particularly benefit from the use of topical formulations containing silicone: burns patients, patients showing personal risks for hypertrophic or keloid scars (e.g. familial predisposition), patients with hypertrophic scars and small keloids, and patients likely to develop psychological distress in case hypertrophic/keloid scars occur. The formulation should be applied as early as possible after re-epithelialization to immature scars. Application on mature scars is possible but the effect may be smaller (Citation51).
Stettler and colleagues (Citation74) conducted an 8 week pilot study with a new anti-scar gel containing dexpanthenol and silicone in 34 healthy subjects with hypertrophic scars formed between 1 month and 1 year before study start. The gel was applied twice-daily, after having first massaged the scar with the integrated massage ball. After 8 weeks of treatment, the scars were significantly less vascularized, less pigmented, softer, thinner, flattened and more elastic. Skin hydration measured by corneometry increased while TEWL decreased over the study period (for both p < .0001) indicating a strengthened skin barrier of the scars. In addition, pain and itching scores decreased significantly from baseline assessments.
Conclusions
Approximately 70 years ago, the first topical dexpanthenol-containing formulation was commercialized as an ointment. Since then, various galenical forms have been developed which are widely used in the field of dermatology and skin care, and are associated with a high consumer satisfaction (Citation75,Citation76). Dexpanthenol, the stable alcoholic analog of pantothenic acid, shows good skin penetration and has moisturizing as well as skin barrier enhancing properties. It prevents skin irritation, stimulates skin regeneration and promotes wound healing. Topical dexpanthenol is therefore recommended for the treatment of superficial wounds and for skin care of different skin conditions. Although discovered decades ago, the exact mechanisms of action of dexpanthenol have not been fully elucidated yet. Recently, novel techniques enabled shedding new light on dexpanthenol’s mode of action. With these techniques, many favorable results from older studies can now be explained at the molecular level. This applies to dexpanthenol’s favorable effects on both barrier function as well as wound healing. It appears that dexpanthenol increases the molecular mobility of several lipid and protein segments of the stratum corneum thereby generating properties of a hydrated skin. For wound healing, dexpanthenol modulates the expression of certain genes. Hence, with the adoption of new technologies, the story of dexpanthenol continues, thereby providing further insight how this long-known molecule actually works.
Disclosure statement
Ehrhardt Proksch has participated in expert panels organized by Bayer Consumer Care AG. Raymond de Bony, Sonja Trapp and Stéphanie Boudon are employees of Bayer Consumer Care AG, Basel, Switzerland.
Additional information
Funding
References
- Kelly GS. Pantothenic acid. Monograph. Altern Med Rev. 2011;16:263–74.
- Lanska DJ. The discovery of niacin, biotin, and pantothenic acid. Ann Nutr Metab. 2012;61:246–53.
- Williams RJ, Saunders DH. The effects of inositol, crystalline vitamin B(1) and ”pantothenic acid”; on the growth of different strains of yeast . Biochem J. 1934;28:1887–93.
- Stüttgen G, Krause H. Die percutane absorption von Tritium-markiertem panthenol bei mensch und tier. Arch Klin Exp Derm. 1960;209:578–82.
- Wollina U, Kubicki J. Multiaktive eigenschaften von dexpanthenol-haltigen externa. Kosm Med. 2007;2:14–18.
- Abiko Y, Tomikawa M, Shimizu M. Enzymatic conversion of pantothenylalcohol to pantothenic acid. J Vitaminol (Kyoto). 1969;15:59–69.
- Proksch E, Jensen JM, Dexpanthenol. In Barel AO, Paye M, Maibach HI, ed. Handbook of Cosmetic Science and Technology. 2nd ed. New York, London: Taylor & Francis Group; 2006, p. 399–406.
- Proksch E, Holleran WM, Menon GK, et al. Barrier function regulates epidermal lipid and DNA synthesis. Br J Dermatol. 1993;128:473–82.
- Slyshenkov VS, Rakowska M, Moiseenok AG, Wojtczak L. Pantothenic acid and its derivatives protect Ehrlich ascites tumor cells against lipid peroxidation. Free Radic Biol Med. 1995;19:767–72.
- Proksch E, Nissen HP. Dexpanthenol enhances skin barrier repair and reduces inflammation after sodium lauryl sulphate-induced irritation. J Dermatolog Treat. 2002;13:173–8.
- Wollina U. Zur klinischen wirksamkeit von dexpanthenol. Kosm Med. 2001;4:180–4.
- Hosemann W, Wigand ME, Göde U, et al. Normal wound healing of the paranasal sinuses: clinical and experimental investigations. Eur Arch Otorhinolaryngol. 1991;248:390–4.
- Hosemann W, Göde U, Länger F, Wigand ME. Experimentelle untersuchungen zur wunderheilung in den nasennebenhöhlen. HNO. 1991;39:48–54.
- Weimann BI, Hermann D. Studies on wound healing: effects of calcium D-pantothenate on the migration, proliferation and protein synthesis of human dermal fibroblasts in culture. Int J Vitam Nutr Res. 1999;69:113–19.
- Wollina U, Kubicki J. Dexpanthenol supports healing of superficial wounds and injuries. Kosm Med. 2006;27:240–9.
- Marquardt Y, Amann PM, Heise R, et al. Characterization of a novel standardized human three-dimensional skin wound healing model using non-sequential fractional ultrapulsed CO2 laser treatments. Lasers Surg Med. 2015;47:257–65.
- Oguz A, Uslukaya O, Alabalık U, et al. Topical N-acetylcysteine improves wound healing comparable to dexpanthenol: an experimental study. Int Surg. 2015;100:656–61.
- Danby SG. Biological variation in skin barrier function: from A (atopic dermatitis) to X (xerosis). Curr Probl Dermatol. 2016;49:47–60.
- Giménez-Arnau A. Standards for the protection of skin barrier function. Curr Probl Dermatol. 2016;49:123–34.
- Lodén M. Treatments improving skin barrier function. Curr Probl Dermatol 2016;49:112–22.
- Björklund S, Pham QD, Jensen LB, et al. The effects of polar excipients transcutol and dexpanthenol on molecular mobility, permeability, and electrical impedance of the skin barrier. J Colloid Interface Sci. 2016;479:207–20.
- Proksch E. The role of emollients in the management of diseases with chronic dry skin. Skin Pharmacol Physiol. 2008;21:75–80.
- Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol. 2012;26:1045–60.
- Simpson EL, Berry TM, Brown PA, Hanifin JM. A pilot study of emollient therapy for the primary prevention of atopic dermatitis. J Am Acad Dermatol. 2010;63:587–93.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818–23.
- Gelmetti C, Metz M, Proksch E. Expert panel on best practices in atopic dermatitis management: outcome and recommendations. KOM Dermatol. 2015;10:1–4.
- Gehring W, Gloor M. Effect of topically applied dexpanthenol on epidermal barrier function and stratum corneum hydration. Results of a human in vivo study. Arzneimittelforschung. 2000;50:659–63.
- Gehring W, Gloor M. Der repetitive waschtest als modell zur beurteilung von hautschutzpräparaten am beispiel einer dexpanthenolhaltigen formulierung. Akt Dermatol. 2001;27:279–84.
- Gehring W, Gloor M. Der effekt von dexpanthenol bei experimentell geschädigter haut. Z Hautkr. 2001;76:212–18.
- Biro K, Thaçi D, Ochsendorf FR, et al. Efficacy of dexpanthenol in skin protection against irritation: a double-blind, placebo-controlled study. Contact Dermatitis. 2003;49:80–4.
- Goujon C, Alleaume B, de Bony R, Girard P. Randomized single-blind pilot comparison study of the efficacy and tolerability of Bepanthen® Ointment in subjects with bilateral dryness of the hands. Réal Thérap Dermatol. 1997;66:47–33.
- Camargo FB Jr, Gaspar LR, Maia Campos PM. Skin moisturizing effects of panthenol-based formulations. J Cosmet Sci. 2011;62:361–70.
- Stettler H, Kurka P, Lunau N, et al. A new topical panthenol-containing emollient: results from two randomized controlled studies assessing its skin moisturization and barrier restoration potential, and the effect on skin microflora. J Dermatolog Treat. 2017;28:173–80.
- Stettler H, Kurka P, Wagner C, et al. A new topical panthenol-containing emollient: skin-moisturizing effect following single and prolonged usage in healthy adults, and tolerability in healthy infants. J Dermatolog Treat. 2016;22:1–7. [Epub ahead of print]
- Distelmaier M, Gaffal E, Wenzel J, et al. Anwendungsbeobachtung bepanthol körperlotion plus bei atopischer haut. Kosm Med. 2007;4:182–5.
- Stettler H, Kurka P, Dominicus R, et al. Improved itch relief with new product formulation for topical treatment in patients with mild-to-moderate atopic dermatitis: results from an exploratory trial. KOM Dermatol. 2016;11:1–10.
- Klunk C, Domingues E, Wiss K. An update on diaper dermatitis. Clin Dermatol. 2014;32:477–87.
- Adam R. Skin care of the diaper area. Pediatr Dermatol. 2008;25:427–33.
- Atherton DJ, Proksch E, Schauber J, Stalder JF. Irritant diaper dermatitis: best practice management. SelfCare. 2015;6(S1):1–11.
- Atherton DJ. A review of the pathophysiology, prevention and treatment of irritant diaper dermatitis. Curr Med Res Opin. 2004;20:645–9.
- Stamatas GN, Tierney NK. Diaper dermatitis: etiology, manifestations, prevention, and management. Pediatr Dermatol. 2014;31:1–7.
- Atherton DJ. Understanding irritant napkin dermatitis. Int J Dermatol. 2016;55:7–9.
- Atherton DJ, Mills K. What can be done to keep babies' skin healthy? RCM Midwives. 2004;7:288–90.
- Sznurkowska K, Liberek A, Brzozowska Cieloch K, et al. Evaluation of a new cosmetic topical formulation in the management of irritant diaper dermatitis in infants. SelfCare. 2015;6(S1):12–24.
- Putet G, Guy B, Pages S, et al. An open pilot study on the effects of Bepanthen® ointment in the prevention of diaper dermatitis in premature and full-term infants. Réal Pédiatr. 2000;52:659–63.
- Putet G, Guy B, Andres P, et al. Effect of Bepanthen® ointment in the prevention and treatment of diaper rash on premature and full-term babies. Réal Pédiatr. 2001;63:33–8.
- Wolff HH, Kieser M. Hamamelis in children with skin disorders and skin injuries: results of an observational study. Eur J Pediatr. 2007;166:943–8.
- Al-Momani T, Tawalbeh A, Shatnawi M, et al. Napkin dermatitis in babies: characteristics and efficacy of panthenol treatment. WJPMR 2016;2:22–4.
- Proksch E. Dexpanthenol-haltige externa zur pflege trockener haut bei diabetikern. Kosm Med. 2008;6:22–5.
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46.
- Nast A, Carreras M, Thompson AR, et al. Scar management: using silicone-based products in primary health care. Wounds Int. 2016;7:23–7.
- Lorenz K, Sicker M, Schmelzer E, et al. Multilineage differentiation potential of human dermal skin-derived fibroblasts. Exp Dermatol. 2008;17:925–32.
- Wiederholt T, Heise R, Skazik C, et al. Calcium pantothenate modulates gene expression in proliferating human dermal fibroblasts. Exp Dermatol. 2009;18:969–78.
- Lacroix B, Didier E, Grenier JF. Effects of pantothenic acid on fibroblastic cell cultures. Res Exp Med (Berl). 1988;188:391–6.
- Hauptmann S, Schäfer H, Fritz A, Hauptmann P. Untersuchung der wachstumsbeeinflussenden wirkung von wundsalben an der zellkultur. Hautarzt. 1992;43:432–5.
- Hoeller Obrigkeit D, Oepen T, Jugert FK, et al. Xenobiotics in vitro: the influence of L-cystine, pantothenate, and miliacin on metabolic and proliferative capacity of keratinocytes. Cutan Ocul Toxicol. 2006;25:13–22.
- Takamiya M, Fujita S, Saigusa K, Aoki Y. Simultaneous detection of eight cytokines in human dermal wounds with a multiplex bead-based immunoassay for wound age estimation. Int J Legal Med. 2008;122:143–8.
- Heise R, Skazik C, Marquardt Y, et al. Dexpanthenol modulates gene expression in skin wound healing in vivo. Skin Pharmacol Physiol. 2012;25:241–8.
- Pugliese PT, Farina JC, Chautems Y. Efficacité du dexpanthénol dans la cicatrisation: etude en double-aveugle sur plaies chirurgicales. Evaluation par ultrasons et examens histologiques. Nouv Dermatol. 1995;14:130–8.
- Hartel M, Illing P, Mercer JB, et al. Therapy of acute wounds with water-filtered infrared-A (wIRA). GMS Krankenhaushyg Interdiszip. 2007;2:1–15.
- Morland-Schultz K, Hill PD. Prevention of and therapies for nipple pain: a systematic review. J Obstet Gynecol Neonatal Nurs. 2005;34:428–37.
- Grünberger V. Behandlung der Brustrhagaden mit Bepanthensalbe. Wien Med Wochenschr. 1948;98:150–1.
- Hofhansl W, Baumgarten K. Erfahrungen mit panthenol in der gynäkologie und der geburtshilfe. Wien Med Wochenschr. 1959;109:775–6.
- Dubecq JP, Detchart M. Etude d’un onguent pantothénique dans la prophylaxie et le traitement des crevasses du sein. Méd Prat. 1977;177:1–2.
- Kuşcu NK, Koyuncu F, Laçin S. Collagenase treatment of sore nipples. Int J Gynaecol Obstet. 2002;76:81–2.
- Shanazi M, Farshbaf Khalili A, Kamalifard M, et al. Comparison of the effects of lanolin, peppermint, and dexpanthenol creams on treatment of traumatic nipples in breastfeeding mothers. J Caring Sci. 2015;4:297–307.
- Staubach P, Meinert R, Grabbe S. Mamillenekzem in der Stillzeit: wie wirksam ist die therapie mit einer dexpanthenol-haltigen salbe? Hebamme. 2008;21:166–9.
- Girard P, Beraud A, Goujou C, et al. Effet de Bépanthène® onguent sur le modèle de cicatrisation du site de prélèvement de greffe: étude biométrologique, clinique et évaluation par le patient, en double aveugle contre véhicule. Nouv Dermatol. 1998;17:559–70.
- Celebi S, Tepe C, Yelken K, Celik O. Efficacy of dexpanthenol for pediatric post-tonsillectomy pain and wound healing. Ann Otol Rhinol Laryngol. 2013;122:464–7.
- Büttemeyer R, Vogt PM, Peter FW, Steinau HU. [Treatment of burns]. Med Monatsschr Pharm. 1995; 18:31–9.
- Grivet-Seyve M, Bellon C, Maares J, et al. Evaluation de l’effet hydratant de Bepanthen® burn relief foam spray contre comparateurs, dans le traitement de la brûlure du premier degré. Réal Thérap Dermatol. 2003;25:38–47.
- Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–71.
- Bloemen MC, van der Veer WM, Ulrich MM, et al. Prevention and curative management of hypertrophic scar formation. Burns. 2009;35:463–75.
- Stettler H, Kurka P, Kalentyeva J, et al. Clinical innovation: treatment with an anti-scar gel and massage ball improves physical parameters of hypertrophic scars. Wounds Int. 2016;7:18–23.
- Radtke MA, Lee-Seifert C, Rustenbach SJ, et al. Patientennutzen und anwendungsmerkmale der behandlung irritierter haut mit dexpanthenolhaltiger salbe. Hautarzt. 2009;60:414–19.
- Augustin M. Hoher patientennutzen mit dexpanthenol-haltiger salbe. DAZ. 2010;150:3031–3.
