Abstract
Purpose: A study was conducted with a new topical panthenol-containing emollient (NTP-CE) to investigate the efficacy and safety of a 3-month maintenance treatment in infants and children with stabilized mild atopic dermatitis (AD).
Methods: After the stabilization phase (up to 2 months) using a corticosteroid-free topical medical device, 108 subjects (aged 2–49 months) with a SCORing AD (SCORAD) on the target area of <5, were randomized to receive NTP-CE (N = 52) or reference emollient (N = 56) twice-daily for ∼3 months. SCORAD scores, occurrence of flares, and tolerability were assessed at regular intervals.
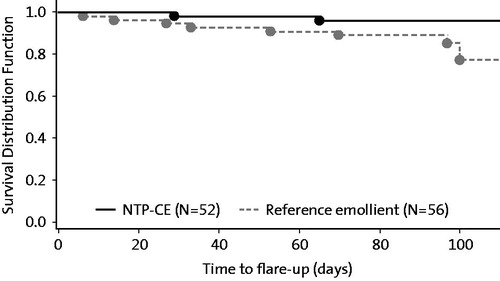
Results: In both groups, local SCORAD decreased during the study with a mean change from baseline (=end of stabilization phase) of −1.2 ± 1.3 (NTP-CE) and −1.0 ± 1.9. Kaplan–Meier analysis provided success rates (i.e. proportion of subjects without flares at study end) of 96 and 77% for the NTP-CE and reference group, respectively (p =.083, log-rank test). Mean time to flare-up was 47 days (range: 29–65) in the NTP CE group and 50 days (6–100). Study products were well tolerated.
Conclusions: Our results indicate that NTP-CE is efficacious and safe when used for maintenance treatment of mild childhood AD.
Introduction
Atopic dermatitis (AD) is an inflammatory, pruritic, chronic, relapsing skin disease which often manifests in infancy. In industrialized countries, 15–30% of children and 2–10% of adults are affected (Citation1,Citation2). It has been estimated that the onset of AD occurs during the first 12 months of life in 60% of affected subjects (Citation3). Xerosis is one of the main clinical features of AD and results from deficient skin barrier function leading to increased transepidermal water loss and decreased stratum corneum hydration (Citation4–6). This skin barrier dysfunction requires continued skin care thereby aiming at improvement of AD symptoms (e.g. itching and scratching) and prevention of acute flares. Recently, an expert panel reached consensus that the daily use of emollients has the potential to maintain skin hydration with reduced acute exacerbations of AD (Citation7). This is also reflected in current treatment guidelines which recommend long-term use of emollients for the maintenance of stable disease and prevention of flares (Citation5,Citation6). Another goal of emollient use in AD is its corticosteroid-sparing effect (Citation5). Although topical corticosteroids are the mainstay to address the inflammatory component of AD, their long-term use is associated with the risk of local and systemic adverse effects (Citation8,Citation9). Particularly in children, who have a greater body surface area to weight ratio with a higher likelihood for systemic absorption than adults, the long-term use of topical corticosteroids should be avoided because of the risk of systemic adverse effects (Citation6,Citation10). In addition, there have been reports that caregivers (usually mothers) are frequently reluctant to use corticosteroids until flares have progressed to more severe cases (Citation11,Citation12). Hence, there is a need for topical preparations enabling non-corticosteroidal management of pediatric AD.
A new topical panthenol-containing emollient (NTP-CE, Bepanthen® SensiDaily) (Bayer Consumer Care AG, Basel, Switzerland) has been developed for subjects with non-inflammatory dry and sensitive skin conditions, such as AD while in remission phase (Citation13,Citation14). Previous studies with NTP-CE in healthy adult subjects showed that its application is associated with significant improvements in skin barrier restoration, skin moisturization, and intercellular lipid layer organization in the stratum corneum. Results from Raman spectroscopy implied a NTP-CE-mediated relocation of the water molecules from upper to deeper layers of the stratum corneum leading to deep moisturization. NTP-CE also exerted favorable effects on the skin microflora (Citation13,Citation14). Moreover, in a study in healthy infants (aged 3–25 months) twice-daily application of NTP-CE for 4 weeks was well-tolerated and significantly increased skin hydration (Citation14). However, the efficacy of NTP-CE in the target population was not investigated.
In this study, we explored the efficacy and safety of a 3-month maintenance treatment with NTP-CE in infants and children with stabilized mild AD. Since for the run-in stabilization phase, also a corticosteroid-free topical preparation was used, this study investigated a regimen completely free of corticosteroids. Principal assessment criteria for the efficacy of NTP-CE were proportion of subjects with flares, time to flare-up, and SCORing AD (SCORAD) changes.
Methods
The trial was conducted in infants/children with acute flares of mild AD at 14 German centers (all were pediatricians in private practice) between December 2015 and September 2016. The study protocol was reviewed and approved by the local ethics committees, and written informed consent was obtained from the parents or legal guardians of the children. Study monitoring, data management, and analysis were performed under the auspices of Proinnovera GmbH, Münster, Germany.
Study design
This multicenter, prospective, exploratory trial consisted of two phases: an open-label stabilization phase (up to 2 months) and an investigator-blinded, randomized, parallel-group, maintenance phase (3 months). During the stabilization phase, all subjects were treated until the local SCORAD on the target area was <5. For that purpose, a corticosteroid-free topical medical device was administered known to provide a physical mode of action (Bepanthen® Itch Relief cream). Its composition and efficacy in the treatment of mild to moderate AD have been reported previously (Citation15). During the maintenance phase, the new cosmetic emollient Bepanthen® SensiDaily (Bayer Consumer Care AG) or the reference emollient (Stelatopia® Emollient Cream, Mustela; Laboratoires Expanscience, France) were applied. The latter is a topical preparation containing 2% sunflower oleodistillate that showed an efficacy in mild-to-moderate AD comparable to those of a topical corticosteroid (Citation16,Citation17). For the maintenance phase, subjects with stabilized mild AD were randomized to NTP-CE and reference group in a 1:1 fashion. Randomization was done according to a randomization list generated by a statistician not involved in the study conduct. For all sites, randomization was centrally monitored to assure that the three pre-specified age cohorts (≥1 month to <12 months; ≥12 to ≤24 months; >24 months to 4 years) were balanced between treatment groups.
At study entry, the target area was determined, defined as the AD lesion with the most severe symptoms. This lesion was continuously followed and evaluated during the trial. During the stabilization phase, the target and all other AD lesions were treated 2–3 times daily for a maximum duration of 2 months. During the maintenance phase, NTP-CE or reference emollient was to be applied twice-daily on the whole body for 3 months (or less in case of a flare-up). Test and reference emollients were distributed to the subjects’ parents by unblinded site personnel not involved in efficacy assessments. Approximately 5 g per application was to be used depending on subject’s age. Compliance was monitored by checking the number of applications documented in the diaries the parents had to complete.
Study visits took place at screening, baseline (Day 1) as well as on study Days 30 and/or 60 (depending on how quickly the target lesion reached a local SCORAD of <5). During the maintenance phase, visits at the study center were scheduled at monthly intervals on Days 30, 60 and 90. Application of study products was always done by the subject’s parents after they had been instructed accordingly. The parents were also advised to contact the study site if their child developed signs of a flare-up and to record the associated time point. Subjects not responding to treatment during the stabilization phase were withdrawn from the study for alternative treatments as per local practice. The same procedure was adopted for subjects completing the maintenance phase early due to flare-ups.
Subjects and assessments
Infants and children with acute AD flares, aged between 1 month and 4 years and having a skin type I–IV on the Fitzpatrick scale (Citation18), were to be enrolled. Subjects were required to have mild AD with a maximum SCORAD index of 25 (Citation19,Citation20) and a local SCORAD on the target area of ≥5. Subjects were excluded if they had any other skin disease at the target area that would interfere with clinical assessments, allergies to any ingredient of study products, a condition requiring intake of drugs interfering with the immune system within 30 days before/during the study (except routine vaccination), or a condition requiring administration of inhaled/topical corticosteroids within 14 days prior to/during the trial. Parents were instructed not to change the usual hygienic care or adjuvant therapy (e.g. probiotics or homeopathy) during the entire study. They were also not allowed to use other topical preparations on the target area during the study course.
Dermatological examinations and SCORAD calculations were performed by a pediatrician at each study visit and in case of flare-ups. Flare-up was defined as occurrence of a flare requiring alternative AD treatment and was judged by the investigator, a pediatrician. The local SCORAD was assessed on the target area and calculated as the sum of scores for six symptoms (erythema, edema/papulation, excoriation, lichenification, oozing/crusts, dryness). The intensity of each symptom was graded on a four-point scale ranging from 0 to 3 (0 = absent, 1 = mild, 2 = moderate, 3 = severe). Thus, the values of the local SCORAD could range between 0 (minimum) and 18 (maximum). Incidence and severity of systemic/local adverse events (AEs) were assessed by investigators at every visit, which included the evaluation of diaries the parents had to complete. In that diary, the parents also rated the intensity of pruritus and sleeplessness over the last 24 h on a numerical scale ranging from 0 to 10 (0 = absent, 10 = worst imaginable condition) at regular intervals during the study (every 3 days during first 2 weeks, then weekly). Finally, subjects’ parents had to complete a quality of life (QoL) questionnaire every week to determine the QoL of study participants. The QoL questionnaire was newly assembled from QoL questionnaires previously used in such type of studies. The questionnaire specified the following parameters: scratching behavior, onset of falling asleep, number of wake-ups during night time, and duration of continuous sleep.
Statistical evaluation
All statistical analyzes were performed using SAS software, version 9.3 (SAS Institute, Cary, NC, USA). For both study phases, the full analysis set (FAS; all subjects who received at least one application of study product and had at least one post-baseline efficacy assessment in the respective phase) and safety population (all subjects who received at least one application of study product in the respective phase) were analyzed. For the stabilization phase, changes from baseline were calculated for SCORAD index, local SCORAD, pruritus and sleeplessness scores, and QoL parameters. Missing efficacy data at end of study phase were imputed by using the last observation carried forward method. The changes from baseline for SCORAD assessments were statistically analyzed using the paired t-test at a significance level of 0.05. For the maintenance phase, changes from baseline (=end of the stabilization phase) were calculated for the same parameters. Missing efficacy data were imputed by using the worst available assessment taking the last previous and the first subsequent visit into consideration. For statistical evaluation, an analysis of covariance (ANCOVA) model was applied with treatment, age cohort, and baseline value as covariates. Based on this model, point estimates [least square (LS) means] with two-sided 95% confidence intervals (CIs) were calculated for the changes from baseline in each group. Differences between groups (NTP-CE versus reference emollient) and corresponding two-sided 95% CIs were determined based on LS means. In addition, for local SCORAD values, the area under the curve (AUC) normalized to a 30-day administration period was calculated and subjected to a between-group comparison.
The proportion of subjects with a flare-up during the maintenance phase was compared between groups using Fisher’s exact test. Two-sided Wilson score-based 95% CIs were calculated for differences between proportions. Taking age cohorts into consideration, differences between groups (NTP-CE versus reference emollient) were analyzed by means of the Cochran–Mantel–Haenszel test. The time to flare-up was estimated by means of Kaplan–Meier methods and compared between groups by the Peto’s log-rank test. Further efficacy variables documented in the diary and AEs were evaluated descriptively.
Given the exploratory nature of the study, no formal sample size calculation was performed. For the same reason, no primary and secondary variables were defined and no adjustment for multiple testing was made. It was planned to enroll 135 subjects in the stabilization phase of the study. Assuming a drop-out rate of 25%, it was expected that a minimum of 100 subjects will be randomized to NTP-CE or reference emollient in the maintenance phase. Based on historical data, it was inferred that scientifically meaningful results can be obtained with the selected sample size (Citation12,Citation17,Citation21).
Results
Run-in stabilization phase
A total of 136 infants and children (56 girls, 80 boys) between 1 and 51 months of age (mean: 19.2 months) were enrolled and included in both the FAS and safety population, among them 49 subjects younger than 1 year. Mean ± standard deviation (SD) SCORAD index and local SCORAD at baseline was assessed at 20.4 ± 3.4 and 5.6 ± 0.9, respectively. Most subjects (N = 108, 79.4%) completed the stabilization phase as responder (i.e. the local SCORAD score on the target area decreased to <5); 14 subjects (10.3%) completed as non-responder. Overall, 14 subjects (10.3%) discontinued this study phase prematurely, among them five responders. The reasons were occurrence of AE (N = 9; mostly local AEs at the treatment area), non-adherence to the study protocol (N = 4), and withdrawal of consent (N = 1).
During the run-in stabilization phase, the mean ± SD duration of study product application was 34.6 ± 16.9 days. SCORAD index and local SCORAD decreased on average by 6.9 ± 11.5 and 2.5 ± 2.8 from baseline, respectively (for both p < .0001). Based on diary records, the intensity of pruritus continuously decreased during the stabilization phase while sleeplessness scores remained essentially stable. Associated QoL scores showed similar trends (data not shown). The study product was well tolerated. None of the infants/children experienced a systemic AE considered to be study product-related by the investigators. In eight subjects (5.9%), local skin reactions were observed. These study participants discontinued the trial upon which the AE resolved.
Maintenance phase
All 108 subjects completing the stabilization phase as responder entered the maintenance phase and were randomized to receive NTP-CE (N = 52) or reference emollient (N = 56). Infants and children (44 girls, 64 boys) between 2 and 49 months of age were enrolled and included in both the FAS and safety population. Demographic and AD characteristics were well balanced between groups (). Mean SCORAD scores were low and virtually identical in both groups reflecting the fact that only subjects with stabilized mild AD were included.
Table 1. Demographic and AD characteristics of subjects enrolled in the maintenance phase of the study at baselineTable Footnotea.
Most subjects (N = 94, 87.0%) finished the maintenance phase in remission. Ten subjects (9.3%) showed flares and completed the study before Day 90. Four study participants discontinued this study phase prematurely (NTP-CE: 3 of 52; Reference: 1 of 56). The reasons were non-adherence to the study protocol (N = 2), loss to follow-up (N = 1), and withdrawal of consent (N = 1). During the maintenance phase, the mean ± SD duration of study product application was 88.6 ± 18.5 days in the NTP-CE group and 84.3 ± 24.4 days in the reference group.
SCORAD results
In both groups, local SCORAD decreased during the observation period (). At study end, the mean ± SD change from baseline (=end of the stabilization phase) was −1.2 ± 1.3 (NTP-CE) and −1.0 ± 1.9 (Reference). Local SCORAD scores improved somewhat better with NTP-CE (−63%) compared with the reference emollient (−50%). For the same observation period, the applied ANCOVA model yielded similar results with an adjusted change from baseline of −1.25 (95% CI: −1.67 to −0.83) for NTP-CE and −0.94 (95% CI: −1.34 to −0.53) for Reference. Although the difference between groups favored NTP-CE, it did not reach statistical significance (p = .286).
Table 2. Local SCORAD scores during maintenance phase.
Mean ± SD AUC0–30d for local SCORAD scores was 36.12 ± 30.76 and 41.61 ± 29.93 for NTP-CE and Reference, respectively (n.s.), thereby supporting the results from single point measurements (i.e. changes from baseline). Among the individual items of local SCORAD, reductions of erythema and dryness from baseline were most pronounced in both the NTP-CE group [−0.36 (95% CI: −0.51 to −0.20) and −0.62 (95% CI: −0.78 to −0.45)] and the reference group [−0.33 (95% CI: −0.48 to −0.18) and −0.49 (95% CI: −0.65 to −0.34)]. Between-group differences were not statistically significant (ANCOVA). The other local SCORAD items showed no considerable changes during the maintenance study phase.
Similar to the local SCORAD, the mean ± SD decrease in the SCORAD index was more pronounced with NTP-CE (−4.7 ± 7.3; −51%) compared with the reference emollient (−3.9 ± 8.7; −42%) at study end. ANCOVA analysis revealed an adjusted change from baseline of −4.80 (95% CI: −6.72 to −2.88) for NTP-CE and −3.98 (95% CI: −5.83 to −2.14) for Reference. Although the observed between-group difference again favored NTP-CE, statistical significance was not reached (p=.542).
Analyses by age group showed no age dependence of observed SCORAD results.
Flare-ups
Simple proportions of evaluable patients with flares were 4.0% (2 of 50) and 14.5% (8 of 55) in the NTP-CE and reference group, respectively, resulting in a difference of −10.5% (95% CI: −23.64 to 2.72%) and a statistical trend in favor of NTP-CE (p=.097). Numerically, 3.6 times more subjects developed a flare-up in the reference group. A comparable result was obtained when comparing treatment groups within age cohorts (p =.070) indicating a consistent effect across age groups.
Kaplan–Meier analysis provided success rates (i.e. proportion of subjects without flares at study end) of 96 and 77% for the NTP-CE and reference group, respectively, again with a statistical trend in favor of NTP-CE (p=.083, ). For patients with flares, mean time to flare-up was 47 days (range: 29–65 days) in the NTP-CE group and 50 days (6–100 days) in the Reference group.
Pruritus and sleeplessness
Based on available diary records, the intensity of pruritus further decreased during the maintenance study phase in both groups. For NTP-CE, the mean ± SD score decreased from 1.3 ± 1.7 at baseline to 0.7 ± 1.4 at study end while for the reference emollient the score decreased from 1.0 ± 1.3 to 0.8 ± 1.8. In both groups, sleeplessness scores remained essentially stable and at a low levels (almost all ≤1.0) during the observation period.
Quality of life
In both groups, most items of the QoL questionnaire showed no relevant change during the maintenance phase of the study. For instance, the average maximum duration of continuous sleep per night remained long in both groups (≥7.7 h). Regarding scratching behavior, the proportion of evaluable subjects without scratching increased from baseline to study end without notable between-group differences (NTP-CE: from 52.1 to 72.7%; Reference: from 44.0 to 73.3%). This observation was most likely a reflection of reduced pruritus.
Safety
NTP-CE and Reference applications were well tolerated during the maintenance study phase. None of the infants/children experienced a systemic or local AE considered to be related to study products by the investigators. No subject was withdrawn for safety reasons.
Discussion
In the context of the development of NTP-CE, this multicenter study explored the efficacy and safety of a 3-month maintenance treatment with NTP-CE in infants and children after their AD disease (being mild in nature) had been stabilized. During the uncontrolled run-in stabilization phase, a corticosteroid-free topical preparation was used; during the parallel-group maintenance phase, the effects of NTP-CE were compared against a well-established active control (reference emollient). The findings of this two-phase study can be summarized as follows: (i) a high proportion of subjects (79%) responded during the stabilization phase with significant improvements in SCORAD scores; (ii) upon twice-daily application of NTP-CE to subjects with stabilized AD for ∼3 months (maintenance phase), SCORAD index and local SCORAD decreased further with erythema and dryness showing the greatest reductions; (iii) in stabilized subjects, NTP-CE prevented flare-ups and was able to maintain the achieved remission in 96% of subjects; (iv) during NTP-CE use, pruritus and scratching behavior improved continuously; (v) twice-daily NTP-CE application for ∼3 months was well-tolerated by AD infants/children as young as 1 month; and (vi) NTP-CE was at least as efficacious and safe as the reference emollient.
The results of our study are in accordance with other studies showing that the daily use of skin care products may prevent AD or reduces flare-ups (Citation22–26). The previously proven skin moisturization and skin barrier restoration potential of NTP-CE is considered responsible for the high success rate of flare prevention recorded in our study (Citation13,Citation14). This hypothesis is supported by the observed associated improvements in SCORAD scores and some subjective parameters. Particularly, the reductions of dryness, pruritus, and scratching behavior are considered directly related to improved skin hydration. It needs emphasis that these favorable effects on disease parameters were observed although the associated scores were already low at baseline (i.e. start of maintenance phase) and NTP-CE has been designed for maintenance of healthy skin rather than amelioration of disease symptoms. No effect of NTP-CE on sleep quality was seen in our study. However, the sleep of the population studied can be influenced by many factors (e.g. teething) and may not serve as reliable efficacy parameter for skin care products.
Results from healthy volunteer studies suggested that the mechanism by which NTP-CE exerts its effects involves stratum corneum replenishment with lipids and restoration of intercellular lamellar lipid structures (Citation13,Citation14). Specifically, it was shown that NTP-CE application on skin induces a structural change of lipid arrangement within the stratum corneum which is associated with an increased ceramide 3, cholesterol and free fatty acids content, and an increased length of intercellular lipid lamellae (Citation13). In this context, it appears of particular interest that stratum corneum in AD reveals a reduced ceramide content (Citation27). Based on the results of our study, it can be inferred that the effects of NTP-CE assessed in healthy subjects translate into a beneficial clinical outcome in AD patients.
The frequency of flare-ups we observed during application of the reference emollient is consistent with a previous study with the reference preparation in AD infants/children when normalized to the same observation period (Citation17). In addition, the decrease in SCORAD index during treatment with active control is in good agreement with a similarly designed study investigating an emollient in the maintenance therapy of childhood AD (Citation26). Finally, the decrease in local SCORAD scores during the stabilization phase is in accordance with an earlier study in AD subjects which tested the same preparation (Citation15). All these observations validate the efficacy results of our study. In terms of safety, all study products administered in our study were well tolerated thereby corroborating results from other studies (Citation13–15,Citation17).
A limitation of our study is the absence of a placebo arm in the maintenance study phase. Hence, we could not account for spontaneous resolution or seasonal fluctuation of AD disease activity over the 3-month observation period. However, it was not possible to control for all ingredients considered important for the skin moisturizing and barrier restoration effects of NTP-CE. In addition, to withhold emollient application for several months in the population studied was considered unethical (Citation26). Another limitation is the restriction to recruit only subjects with mild AD. Therefore, study results cannot easily be extrapolated to subjects with higher disease activity. Subjects with mild AD were selected for our study because cosmetic emollients are the main primary treatment in that population (Citation6,Citation28).
Basic petrolatum-based emollients are generally less costly than emollients with complex formulas (Citation29,Citation30). In the absence of comparative health economic studies, it remains unclear whether the special additives in NTP-CE (e.g. ceramide 3) translate into better clinical outcomes and thus differences in cost-effectiveness compared with petrolatum-based emollients. However, based on previous studies with non-steroidal topical barrier-strengthening moisturizers (Citation29,Citation31), it may be inferred that NTP-CE is a cost-effective option for AD patients while in remission phase.
Conclusions
Our results indicate that NTP-CE is efficacious and safe when used for maintenance treatment of mild childhood AD. In stabilized pediatric AD subjects, NTP-CE usage was at least as efficacious as a reference emollient in improving SCORAD scores (NTP-CE: −63%; Reference: −50% for local SCORAD scores) and delaying the time to acute flare-ups. In fact, NTP-CE was able to maintain the achieved remission in a high proportion of subjects (NTP-CE: 96%; Reference: 77%, Kaplan–Meier analysis). NTP-CE is an emollient and therefore not to be used as topical preparation for the anti-inflammatory treatment of active AD. However, our study suggests that NTP-CE can effectively be integrated as skin care product in a corticosteroid-free regimen for maintenance of stable disease and prevention of flares in the setting of mild AD disease.
Acknowledgements
It is confirmed that we complied with all mandatory health and safety procedures in the course of conducting the work reported in our article.
Drafting of the article was done by Edgar A. Mueller, 3P Consulting; the authors were responsible for critical revisions of the article and for important intellectual content.
We acknowledge that study participants cannot be identified via this article; we have fully anonymized them.
Disclosure statement
H.S., P.K. and A.M.-B. are employees of Bayer Consumer Care AG, Basel, Switzerland. The other authors report no conflicts of interest.
The study was funded by Bayer Consumer Care AG, Basel, Switzerland.
References
- Williams H, Flohr C. How epidemiology has challenged 3 prevailing concepts about atopic dermatitis. J Allergy Clin Immunol. 2006;118:209–13.
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–94.
- Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4:22–42.
- Proksch E. The role of emollients in the management of diseases with chronic dry skin. Skin Pharmacol Physiol. 2008;21:75–80.
- Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol. 2012;26:1045–60.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116–32.
- Gelmetti C, Metz M, Proksch E. Expert panel on best practices in atopic dermatitis management: outcome and recommendations. KOM Dermatol. 2015;10:1–4.
- Lyons JJ, Milner JD, Stone KD. Atopic dermatitis in children: clinical features, pathophysiology, and treatment. Immunol Allergy Clin North Am. 2015;35:161–83.
- Silverberg JI, Nelson DB, Yosipovitch G. Addressing treatment challenges in atopic dermatitis with novel topical therapies. J Dermatolog Treat. 2016;27:568–76.
- Sathishkumar D, Moss C. Topical therapy in atopic dermatitis in children. Indian J Dermatol. 2016;61:656.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29–35.
- Weber TM, Samarin F, Babcock MJ, et al. Steroid-free over-the-counter eczema skin care formulations reduce risk of flare, prolong time to flare, and reduce eczema symptoms in pediatric subjects with atopic dermatitis. J Drugs Dermatol. 2015;14:478–85.
- Stettler H, Kurka P, Lunau N, et al. A new topical panthenol-containing emollient: Results from two randomized controlled studies assessing its skin moisturization and barrier restoration potential, and the effect on skin microflora. J Dermatolog Treat. 2017;28:173–80.
- Stettler H, Kurka P, Wagner C, et al. A new topical panthenol-containing emollient: Skin-moisturizing effect following single and prolonged usage in healthy adults, and tolerability in healthy infants. J Dermatolog Treat. 2017;28:251–7.
- Stettler H, Kurka P, Dominicus R, et al. Improved itch relief with new product formulation for topical treatment in patients with mild-to-moderate atopic dermatitis: results from an exploratory trial. KOM Dermatol. 2016;11:1–10.
- Msika P, De Belilovsky C, Piccardi N, et al. New emollient with topical corticosteroid-sparing effect in treatment of childhood atopic dermatitis: SCORAD and quality of life improvement. Pediatr Dermatol. 2008;25:606–12.
- De Belilovsky C, Roo-Rodriguez E, Baudouin C, et al. Natural peroxisome proliferator-activated receptor-alpha agonist cream demonstrates similar therapeutic response to topical steroids in atopic dermatitis. J Dermatolog Treat. 2011;22:359–65.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–71.
- Kunz B, Oranje AP, Labrèze L, et al. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology (Basel). 1997;195:10–19.
- Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007;157:645–8.
- Wirén K, Nohlgård C, Nyberg F, et al. Treatment with a barrier-strengthening moisturizing cream delays relapse of atopic dermatitis: a prospective and randomized controlled clinical trial. J Eur Acad Dermatol Venereol. 2009;23:1267–72.
- Simpson EL, Berry TM, Brown PA, Hanifin JM. A pilot study of emollient therapy for the primary prevention of atopic dermatitis. J Am Acad Dermatol. 2010;63:587–93.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818–23.
- Horimukai K, Morita K, Narita M, et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol. 2014;134:824–30.
- Åkerström U, Reitamo S, Langeland T, et al. Comparison of moisturizing creams for the prevention of atopic dermatitis relapse: a randomized double-blind controlled multicentre clinical trial. Acta Derm Venereol. 2015;95:587–92.
- Mengeaud V, Phulpin C, Bacquey A, et al. An innovative oat-based sterile emollient cream in the maintenance therapy of childhood atopic dermatitis. Pediatr Dermatol. 2015;32:208–15.
- Chamlin SL, Kao J, Frieden IJ, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002;47:198–208.
- Bianchi P, Theunis J, Casas C, et al. Effects of a new emollient-based treatment on skin microflora balance and barrier function in children with mild atopic dermatitis. Pediatr Dermatol. 2016;33:165–71.
- Tang MB, Leong KF, Ou LS, et al. Cost-effectiveness study of pediatric atopic dermatitis in Asia: atopiclair vs. regular emollient (AD-ATOP). J Drugs Dermatol. 2015;14:169–75.
- Xu S, Immaneni S, Hazen GB, et al. Cost-effectiveness of prophylactic moisturization for atopic dermatitis. JAMA Pediatr. 2017;171:e163909.
- Norrlid H, Hjalte F, Lundqvist A, et al. Cost-effectiveness of maintenance treatment with a barrier-strengthening moisturizing cream in patients with atopic dermatitis in Finland, Norway and Sweden. Acta Derm Venereol. 2016;96:173–6.