Abstract
Topical treatments in dermatology can be long, complex and lead to nonadherence and nonpersistence to prescribed treatment. Clinical efficacy observed in randomized clinical trials (RCT) may therefore be reduced in real-world clinical practice. The objective of this study was to analyze patient-reported treatment adherence, treatment satisfaction and health-related quality of life (HRQoL) with topical treatments of actinic keratosis (AK) in routine clinical practice in Denmark and Sweden. Adult patients prescribed field-directed topical AK treatments with diclofenac gel, imiquimod or ingenol mebutate per routine clinical practice were eligible for the observational RAPID-ACT study. Data were collected through physician and patient questionnaires that included validated instruments to measure treatment satisfaction (TSQM-9), treatment adherence (MMAS) and HRQoL (EQ-5D-5L, EQ-VAS, AKQoL). In total, 446 patients from Denmark and Sweden were included. Ingenol mebutate patients reported a higher satisfaction with treatment effectiveness compared to patients treated with diclofenac (p = .006) while no other differences in treatment satisfaction could be determined. Treatment adherence was generally high, but higher for ingenol mebutate compared to both diclofenac (p < .001) and imiquimod (p = .007), possibly due to shorter treatment duration. No differences in improved HRQoL were found. More research is needed about the link between treatment adherence and real-world effectiveness.
Introduction
Actinic keratosis (AK) is a common skin condition caused by cumulative sun exposure (Citation1). Diagnosis is based on histology of clinically suspect lesions, but various imaging technologies are being tested as diagnostic aids (Citation2–6). Some AK lesions spontaneously regress (Citation7), while a minority may progress to squamous cell carcinoma (SCC) (Citation8–10). Single lesions most often appear as a consequence of field cancerization in a generally sun exposed area of the skin (Citation2). AK prevalence has been estimated to between 1.4% and 25% of the population (Citation11–14) and known risk factors are age, cumulative sun exposure, Fitzpatrick skin type and previous AK diagnosis (Citation15,Citation16). Current guidelines mostly recommend active treatment of AK, both to reduce symptoms and to lower the risk of developing SCC (Citation17–19), although Danish guidelines accept “no treatment” as a valid treatment option (Citation20).
AK has shown to impair health-related quality of life (HRQoL) (Citation21,Citation22). AK lesions are often red and scaly and may cause itching and bleeding. As AK lesions are caused by cumulative sun exposure they often develop in visible skin areas (e.g. face, scalp and hands). Patients also fear that their AK lesion may develop into NMSC (Citation23). These factors may influence a person’s HRQoL. Yet, knowledge about the impact of AK and AK treatments on patients’ HRQoL is limited (Citation22,Citation24–26).
There are many treatment options available for AK and in addition to targeted therapy such as cryotherapy, multiple field-directed treatments are listed in the Danish and Swedish treatment guidelines (Citation17,Citation20). Targeted therapy targets only single visible AK lesions, and recurrence rates are therefore high (Citation27,Citation28). Field-directed treatments, on the other hand, target both visible and nonvisible multiple lesions and are therefore often used for areas of field cancerization (Citation27). Field-directed treatments include photodynamic therapy (PDT) and topical treatments applied to the skin by the patient. Treatment regimens of topical AK treatments may last from 2 to 90 days with varying dosing complexity (Citation29).
All AK therapies may cause local skin reactions (LSRs), such as blistering, inflammation, erythema, ulceration, burning and pain (Citation27). LSRs are common and often last throughout the treatment duration and persist 2 to 4 weeks after treatment completion (Citation30). In some cases, patients need treatment-free periods due to severe LSRs.
Topical treatments in dermatology are often challenging due to prolonged and complex treatment regimens and can often lead to nonadherence and nonpersistence to prescribed treatment (Citation31–33). Clinical efficacy observed in randomized clinical trials (RCT) may therefore be reduced in real-world clinical practice (Citation32,Citation34). While RCTs assess the safety and efficacy of a drug, whether it can work under ideal condition; observational studies are used to assess the effectiveness of drugs, that is, whether it works in a heterogenous population in clinical practice. As treatment adherence may influence the effectiveness of a drug, knowledge about adherence of drugs is important to inform decisions on treatment prioritizations and clinical guidelines.
In 2014 and 2015, an observational study of topical field treatment of AK, the Real-Life Topical Field Treatment of Actinic Keratosis (RAPID-ACT, NCT02362152), was conducted in Sweden, Denmark, United Kingdom, the Netherlands and Canada. The present paper studies Danish and Swedish data from the RAPID-ACT. The objective of this study was to analyze patient-reported treatment adherence, treatment satisfaction and HRQoL in patients with topical treatment for AK in routine clinical practice in Denmark and Sweden using a subset of the RAPID-ACT data.
Materials and methods
Study design
Adult patients diagnosed with AK and prescribed field-directed topical treatments with diclofenac gel, imiquimod 3.75% or 5% or ingenol mebutate 150 μg/g or 500 μg/g by their physician, were eligible for the RAPID-ACT study in Denmark and Sweden (). In the observational RAPID-ACT trial, each physician selected the treatment per routine clinical practice.
Table 1. Included topical treatments for actinic keratosis.
Physicians reported baseline patient characteristics, while patients reported outcomes in terms of treatment satisfaction, adherence, HRQoL and resource utilization (the latter is not analyzed in this paper). Patients filled out questionnaires at the baseline visit and were given a follow-up questionnaire to fill out 3 weeks (21 days) after completed treatment. The study did not include any protocol-driven follow-up visits.
The RAPID-ACT trial received ethical approval from the Ethical Board in Stockholm, Sweden, in December 2014 (Dnr 2014/2026–31/4). Ethical approval is not required for non-interventional studies in Denmark. Patients were enrolled at 18 sites in Denmark and 20 sites in Sweden.
Study population
This paper includes the Danish and Swedish population of the RAPID-ACT trial. The Danish and Swedish populations can be assumed to have similar geographical, demographical and genetic conditions and could therefore be combined in a pooled analysis in terms of treatment outcomes. Patients who did not state the type of AK treatment and patients with AK lesions on more than one body part were excluded to enable analysis by treatment and by body part.
Outcomes measures
The RAPID-ACT questionnaires included a set of validated instruments that measure treatment satisfaction, treatment adherence and HRQoL. The Treatment Satisfaction Questionnaire for Medication (TSQM)-9 includes nine questions that assess patients’ treatment satisfaction by providing score on three scales; effectiveness, convenience and global satisfaction (Citation35). The scores of each scale range from 0 to 100, where a higher score indicates a greater satisfaction. The TSQM questionnaire is widely used in a variety of disease areas (Citation36,Citation37).
The Morisky Medication Adherence Scale (MMAS) is a four-item patient questionnaire that estimates treatment adherence (Citation38,Citation39). Questions are scored as “Yes”=0 and “No”=1. The items are summed to give a range of scores from 0 to 4, where 0 is interpreted as “High Adherence,” 1–2 as “Medium Adherence” and 3–4 as “Low Adherence.”
The EuroQol-5 dimension (EQ-5D) is a generic standardized instrument to measure HRQoL (Citation40). The instrument includes five dimensions; mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The EQ-5D-5L version includes five response levels; no problems, slight problems, moderate problems, severe problems, unable to/extreme problems. An utility weight value set for England from 2016 (Citation41) was used to calculate the EQ-5D-5L index scores. The EuroQol Visual Analogue Scale (EQ-VAS) is a subsection of the EQ-5D instrument that asks respondents to indicate their current health state on a scale from 0 (worst imaginable health state) to 100 (best imaginable health state). The result of primary interest, with both the EQ-5D-5L and the EQ-VAS instruments, is the difference in HRQoL between baseline and follow-up, that is, whether the treatment results in improved HRQoL. Therefore, patients who did not complete the follow-up questionnaire were excluded from these analyses.
Recently, a disease specific HRQoL index called the Actinic Keratosis Quality of Life Questionnaire (AKQoL) was developed (Citation24). This questionnaire reflects how sun-damaged skin affects HRQoL by focusing on psychological aspects. The AKQoL includes nine questions regarding personal daily life, personal view of quality of life, social life, emotional life and control of life (Citation24). Each question is scored on a 4-point scale; “a lot/all the time”=3, “quite a lot/often”=2, “some/sometimes”=1, “rarely/not at all”=0. By summing the score of each question the total score range from 0 to 27, where a higher score implies a larger HRQoL impairment (Citation24).
Trial subjects were also asked whether they experienced LSRs or not.
Statistical analyses
t-Tests were used to test differences between two large samples while Kruskal–Wallis tests were used to test differences in groups (presented in tables). When differences were found within a group, Mann–Whitney/Wilcoxon tests – suitable for tests of two smaller samples – were used to test differences between individual treatments (presented in text upon statistically significant differences).
Since strength-specific sample sizes were small, analyses were conducted by pooling treatment strengths. Mann–Whitney/Wilcoxon tests were then used in a subgroup analysis to test differences between treatments strengths of imiquimod (3.75% vs. 5%) and ingenol mebutate (150 μg/g vs. 500 μg/g) in TSQM-9, MMAS, AKQoL, EQ-5D-5L, EQ-VAS and frequency of LSR. A significance level of 5% (p≤.05) was applied for statistical significance in all cases.
Results
A total of 446 patients were included in the analysis. Patient and lesion characteristics are presented in .
Table 2. Patient and lesion characteristics.
Treatment satisfaction and adherence
Treatment satisfaction is presented in and . Ingenol mebutate patients reported a higher satisfaction with treatment effectiveness compared to patients treated with diclofenac (p = .006) and the difference between diclofenac and imiquimod was borderline significant (p = .061), while the difference between imiquimod and ingenol mebutate was not statistically significant (p = .285).
Figure 1. Treatment satisfaction in terms of treatment effectiveness, treatment convenience and global treatment satisfaction based on the TSQM-9 instrument.
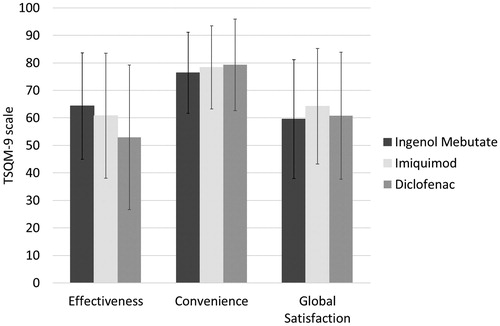
Table 3. Patient-reported TSQM-9 score at follow-up, MMAS scores at follow-up, AKQoL index at baseline and EQ-5D-5L & EQ-VAS at baseline as well as follow-up and the difference there between.
Treatment adherence was generally high (). Ingenol mebutate patients reported better treatment adherence compared to both diclofenac (p < .001) and imiquimod patients (p = .007).
Health-related quality of life
AKQoL was measured at baseline. Diclofenac patients reported a higher HRQoL impairment in terms of AKQoL compared to patients treated with imiquimod (p = .048) or ingenol mebutate (p = .017). No statistically significant differences were found between imiquimod and ingenol mebutate at baseline (p = .667). The EQ-5D-5L and the EQ-VAS instruments showed no statistically significant differences in HRQoL improvement from baseline to follow-up between treatment groups ().
Local skin reactions
LSRs were less common in patients treated with diclofenac (42%) compared to those treated with imiquimod (79%; p < .001) or ingenol mebutate (89%; p < .001) and less common with imiquimod compared to ingenol mebutate (p = .015).
Subgroup analysis
In total, 45 patients were treated with imiquimod 3.75%, 102 patients with imiquimod 5%, 241 with ingenol mebutate 150 μg/g and 18 were treated with ingenol mebutate 500 μg/g. No statistically significant differences were found between treatment strengths for either imiquimod or ingenol mebutate (data not shown).
Discussion
In this real-world study of Danish and Swedish AK patients, ingenol mebutate-treated patients reported a higher satisfaction with treatment effectiveness compared to patients treated with diclofenac. No other differences in treatment satisfaction were found. Self-reported treatment adherence was high in all groups, the highest among ingenol mebutate patients. No differences could be detected in HRQoL improvement from baseline and after treatment with either EQ-5D-5L or EQ-VAS. LSRs were most common with ingenol mebutate followed by imiquimod, and least common with diclofenac.
The strength of observational studies is that they provide additional information that complement RCTs, as it captures adherence and patient perceived effectiveness in a real-world setting. The three treatment populations were similar in terms of patient and background characteristics, except for the fraction of female patients and national differences in choice of treatment, which is subject to guideline differences. Product label differences may explain treatment groups differences in number of lesions, average lesion size and number of prescribed treatment cycles, that is, diclofenac has a general indication for “AK lesions,” ingenol mebutate and imiquimod 5% are explicitly indicated for a 25 cm2 area, while imiquimod 3.75% is indicated for a treatment area no larger than “full” face/scalp (Citation29).
A limitation in this study is the small sample sizes for different treatment strengths which required a pooled analysis. Another limitation is that the real-world effectiveness was not evaluated by a physician. However, patient-reported effectiveness was assessed as a part of the TSQM questionnaire which can be used as a proxy for clinical effectiveness in AK treatment (Citation21).
It may be argued that the short treatment duration of ingenol mebutate affects treatment adherence. A recent literature review on adherence to topical AK therapies found that several studies report that long and complex treatment regimens contribute to decreased adherence (Citation31–33). A UK survey of persons with AK treated during the last 12 months found nonadherence rates of 52% for treatment durations of 3–4 weeks and of 72% for 6–12 weeks of treatment. The results were supported by a web-based survey of patient adherence and persistence of topical AK therapies in Germany, France and the United Kingdom (Citation42).
Treatment-induced HRQoL improvements were small and similar for all treatment groups in the present study. A previous US study on treatment-induced changes in HRQoL among AK patients and compared cryotherapy followed by topical treatment with either ingenol mebutate or vehicle (Citation43). The baseline EQ-5D and EQ-VAS estimates of the present study were in line with those of the US study, while the US study implied somewhat larger HRQoL gains from treatment (0.033 with EQ-5D and 3.5 with EQ-VAS). This may be due to the longer follow-up in the US study or the inclusion of cryotherapy.
Our AKQoL estimates were in line with those of a recent Danish study, whereas our EQ-5D-5L and EQ-VAS estimates were somewhat higher (Citation22). The authors of the Danish study argued that the various HRQoL instruments are complementary in AK as they measure different aspects of HRQoL (Citation22). This is in line with the reasoning in a literature review of the use of EQ-5D in economic evaluations in dermatology, where the authors suggest that although the EQ-5D is broad enough to allow comparison between different diseases, it may not be specific enough to capture important aspects of HRQoL in dermatology (Citation44). Therefore, it is important to use both generic, dermatology- and disease specific HRQoL measures in dermatologic conditions such as AK (Citation22).
In conclusion, topical AK treatment with ingenol mebutate indicates greater treatment adherence compared to diclofenac and imiquimod. Furthermore, patient reported (TSQM) effectiveness were higher for patients with ingenol mebutate than diclofenac. More research is needed about the association between adherence and real-world effectiveness of AK treatments.
Acknowledgements
This work was supported by LEO Pharma AB.
Disclosure statement
HN, JN and GRT are employees at IHE and declare no conflict of interest. HH is an employee at LEO Pharma AB and IM was an employee at LEO Pharma at the time of the study.
HT and KS were national investigator in the RAPID-ACT study sponsored by LEO Pharma AB. HT holds a seat at the scientific board of LEO Pharma AB and has received travel grants from Desitin Pharma. GBEJ has received honoraria from AbbVie, Coloplast, Pfizer, Pierre Fabre, MSD, Novartis and UCB for participation on advisory boards, and grants from Abbvie, Leo Pharma, Actelion, Janssen-Cilag, Regeneron, Sanofi-Aventis and Novartis for participation as an investigator, research funding from Abbvie, Leo Pharma and Novartis and speaker honoraria from AbbVie, Galderma, Leo Pharma and MSD.
References
- Feldman SR, Fleischer AB. Jr., Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87:201–7.
- van der Geer S, Kleingeld PA, Snijders CC, et al. Development of a non-melanoma skin cancer detection model. Dermatology (Basel). 2015;230:161–9.
- Ulrich M, Alarcon I, Malvehy J, Puig S. In vivo reflectance confocal microscopy characterization of field-directed 5-fluorouracil 0.5%/salicylic acid 10% in actinic keratosis. Dermatology (Basel). 2015;230:193–8.
- Friis KB, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of actinic keratosis – a systematic review. Photodiagnosis Photodyn Ther. 2017.
- Boone MA, Suppa M, Marneffe A, et al. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J Eur Acad Dermatol Venereol. 2016;30:1714–25.
- Olsen J, Themstrup L, De Carvalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44–9.
- Marks R, Foley P, Goodman G, et al. Spontaneous remission of solar keratoses: the case for conservative management. Br J Dermatol. 1986;115:649–55.
- Dodson JM, DeSpain J, Hewett JE, Clark DP. Malignant potential of actinic keratoses and the controversy over treatment. A patient-oriented perspective. Arch Dermatol. 1991;127:1029–31.
- Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42:23–4.
- Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet. 1988;1:795–7.
- Frost CA, Green AC. Epidemiology of solar keratoses. Br J Dermatol. 1994;131:455–64.
- Harvey I, Frankel S, Marks R, et al. Non-melanoma skin cancer and solar keratoses. I. Methods and descriptive results of the South Wales Skin Cancer Study. Br J Cancer. 1996;74:1302–7.
- Memon AA, Tomenson JA, Bothwell J, Friedmann PS. Prevalence of solar damage and actinic keratosis in a Merseyside population. Br J Dermatol. 2000;142:1154–9.
- Naldi L, Chatenoud L, Piccitto R, et al. Prevalence of actinic keratoses and associated factors in a representative sample of the Italian adult population: Results from the Prevalence of Actinic Keratoses Italian Study, 2003. Arch Dermatol. 2006;142:2004–722.
- Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42:4–7.
- Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2016.
- Swedish Society for Dermatology and Venereology [Swedish: Svenska Sällskapet för Dermatologisk Kirurgi och Onkologi (SDKO)]. SDKO:s Riktlinjer för handläggning av skivepitelcancer och basalcellscancer - Reviderad version 2016. 2016 [August 25, 2016]. Available from: http://ssdv.se/images/pdf/SDKOs_Riktlinjer_for_SCC__BCC_2016.pdf.
- Werner RN, Stockfleth E, Connolly SM, et al. Evidence- and consensus-based (S3) Guidelines for the Treatment of Actinic Keratosis - International League of Dermatological Societies in cooperation with the European Dermatology Forum – Short version. J Eur Acad Dermatol Venereol. 2015;29:2069–79.
- Dirschka T, Gupta G, Micali G, et al. Real-world approach to actinic keratosis management: practical treatment algorithm for office-based dermatology. J Dermatolog Treat. 2016;1–12.
- Dansk Dermatologisk Selskab (DDS). DDS AK guidelines - Guidelines vedrørende behandling af aktiniske keratoser Udarbejdet for Dansk Dermatologisk Selskab. 2014. Available from: http://www.dds.nu/wp-content/uploads/2014/09/2014_DDS-guidelines-AK_Godkendt_2014-09-28.pdf. Accessed March 13, 2017.
- Augustin M, Tu JH, Knudsen KM, et al. Ingenol mebutate gel for actinic keratosis: the link between quality of life, treatment satisfaction, and clinical outcomes. J Am Acad Dermatol. 2015;72:816–21.
- Tennvall GR, Norlin JM, Malmberg I, et al. Health related quality of life in patients with actinic keratosis–an observational study of patients treated in dermatology specialist care in Denmark. Health Qual Life Outcomes. 2015;13:111.
- Esmann S, Jemec GB. Management of actinic keratosis patients: a qualitative study. J Dermatolog Treat. 2007;18:53–8.
- Esmann S, Vinding GR, Christensen KB, Jemec GB. Assessing the influence of actinic keratosis on patients' quality of life: the AKQoL questionnaire. Br J Dermatol. 2013;168:277–83.
- Gibbons E, Casanas I, Comabella C, Fitzpatrick R. A structured review of patient-reported outcome measures for patients with skin cancer, 2013. Br J Dermatol. 2013;168:1176–86.
- Hanke WC, Norlin JM, Mark Knudsen K, et al. Quality of life in treatment of AK: treatment burden of ingenol mebutate gel is small and short lasting. J Dermatolog Treat. 2016;27:450–5.
- Berlin JM. Current and emerging treatment strategies for the treatment of actinic keratosis. Clinical, cosmetic and investigational dermatology. CCID. 2010;3:119.
- Krawtchenko N, Roewert‐Huber J, et al. A randomised study of topical 5% imiquimod vs. topical 5‐fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1‐year follow‐up. Br J Dermatol. 2007;157:34–40.
- FASS. Available from: www.fass.se. Accessed June 3, 2016.
- Jim On SC, Knudsen KM, Skov T, Lebwohl M. Relationship between severity of the local skin reactions and the rate of local skin reaction resolution in patients treated with ingenol mebutate gel. Clin Cosmet Investig Dermatol. 2016;9:211–16.
- Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: A literature review. J Dermatolog Treat. 2016;27:538–45.
- Shergill B, Zokaie S, Carr AJ. Non-adherence to topical treatments for actinic keratosis. Patient Prefer Adherence. 2013;8:35–41.
- Erntoft S, Norlin JM, Pollard C, Diepgen TL. Patient-reported adherence and persistence to topical treatments for actinic keratosis: a longitudinal diary study. Br J Dermatol. 2016;175:1094–6.
- Ceilley RI, Jorizzo JL. Current issues in the management of actinic keratosis. J Am Acad Dermatol. 2013;68:S28–S38.
- Bharmal M, Payne K, Atkinson MJ, et al. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36.
- Atkinson MJ, Kumar R, Cappelleri JC, Hass SL. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health. 2005;8:S9–s24.
- Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12.
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care. 1986;24:67–74.
- Morisky DE, Malotte CK, Choi P, et al. A patient education program to improve adherence rates with antituberculosis drug regimens. Health Educ Q. 1990;17:253–67.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337–43.
- Devlin N, Shah K, Feng Y, et al. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England. Office of Health Economics (OHE) Research Paper. 2016.
- Erntoft S, Norlin JM, Pollard C, Diepgen TL. Patient-reported adherence and persistence to topical treatments for actinic keratosis: a longitudinal diary study. Br J Dermatol 2016;175:1094–6.
- Hanke WC, Norlin JM, Knudsen KM, et al. Quality of life in treatment of AK: treatment burden of ingenol mebutate gel is small and short lasting. J Dermatolog Treat. 2016;27:450–5.
- Pereira FR, Basra MK, Finlay AY, Salek MS. The role of the EQ-5D in the economic evaluation of dermatological conditions and therapies. Dermatology (Basel). 2012;225:45–53.
